Abstract
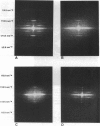
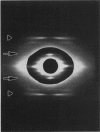
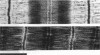
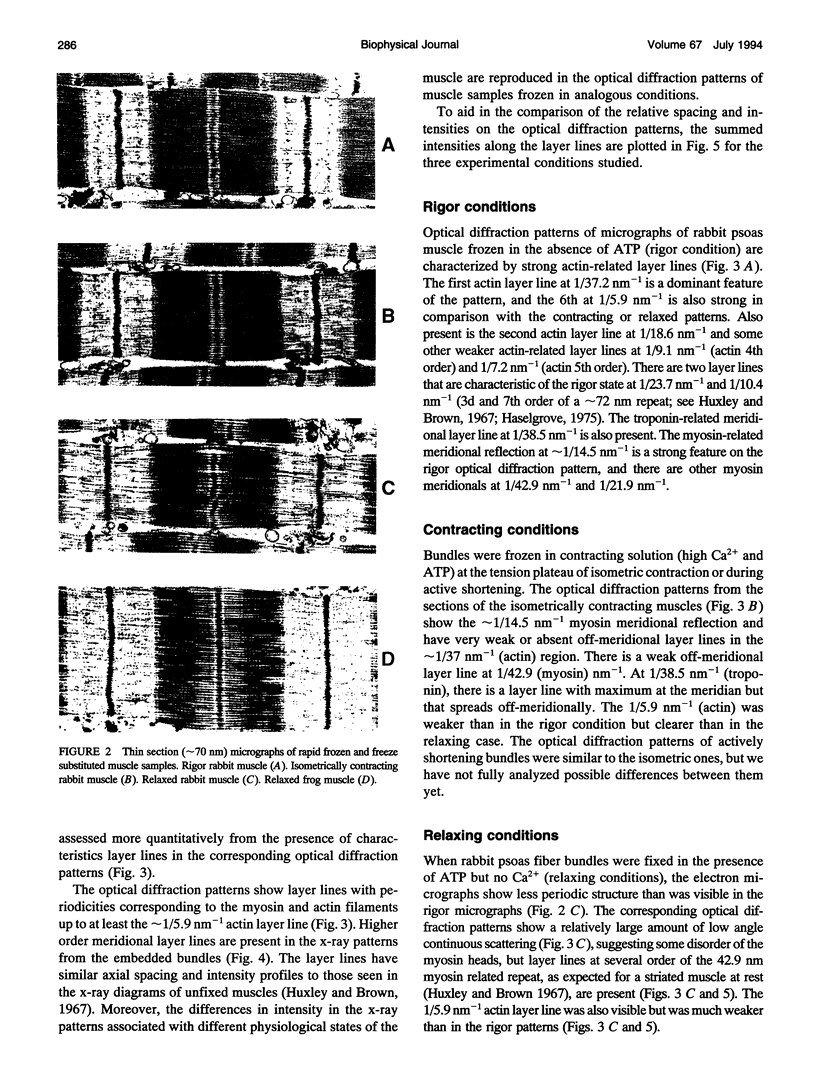
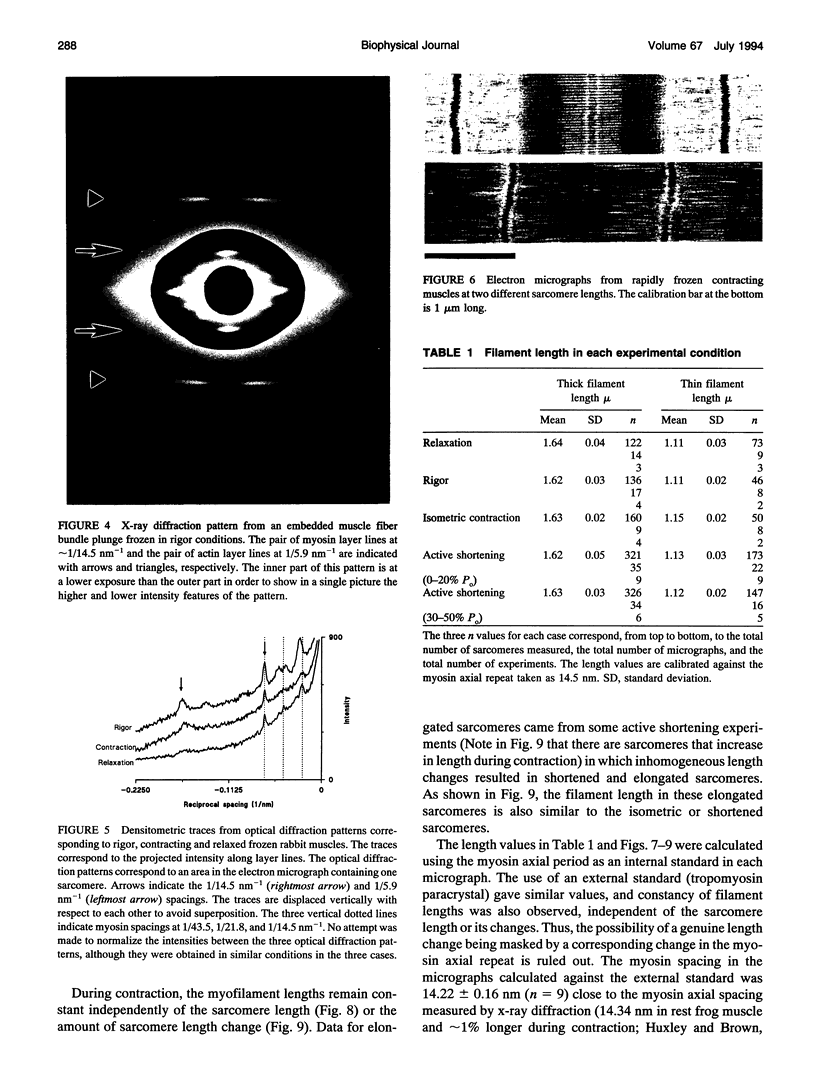
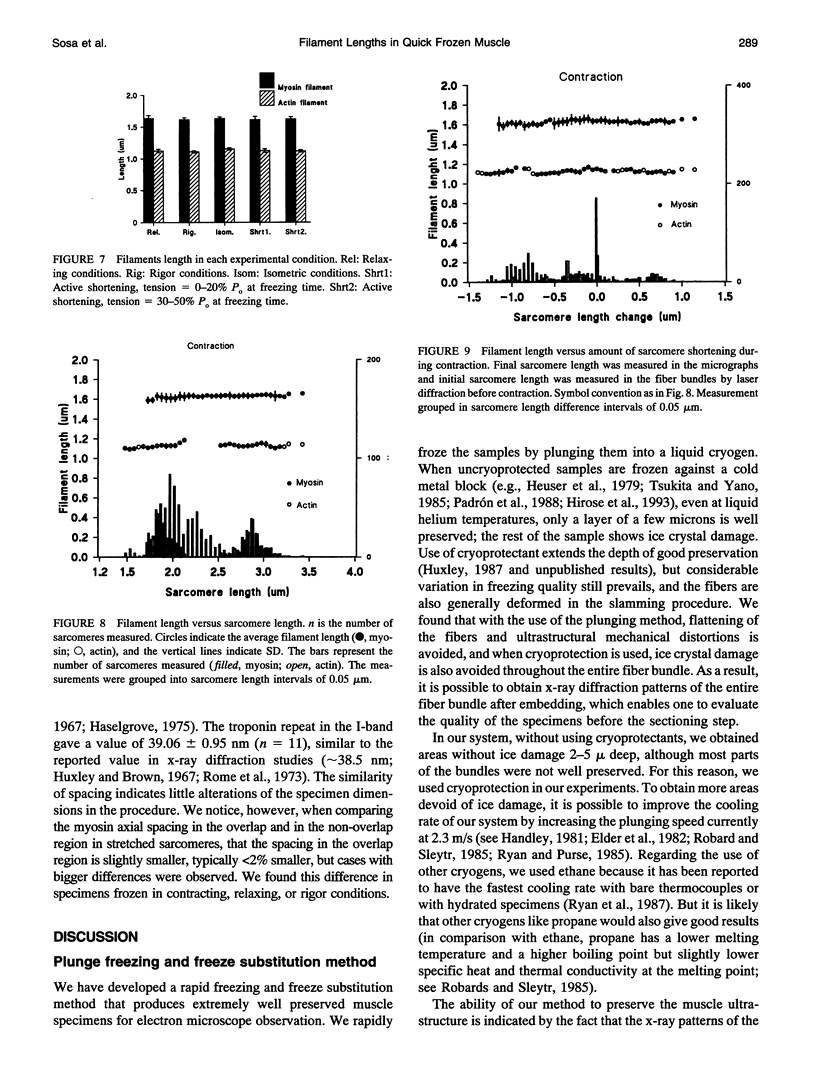
We have set up a system to rapidly freeze muscle fibers during contraction to investigate by electron microscopy the ultrastructure of active muscles. Glycerinated fiber bundles of rabbit psoas muscles were frozen in conditions of rigor, relaxation, isometric contraction, and active shortening. Freezing was carried out by plunging the bundles into liquid ethane. The frozen bundles were then freeze-substituted, plastic-embedded, and sectioned for electron microscopic observation. X-ray diffraction patterns of the embedded bundles and optical diffraction patterns of the micrographs resemble the x-ray diffraction patterns of unfixed muscles, showing the ability of the method to preserve the muscle ultrastructure. In the optical diffraction patterns layer lines up to 1/5.9 nm-1 were observed. Using this method we have investigated the myofilament lengths and concluded that there are no major changes in length in either the actin or the myosin filaments under any of the conditions explored.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown L. M., Hill L. Mercuric chloride in alcohol and chloroform used as a rapidly acting fixative for contracting muscle fibres. J Microsc. 1982 Mar;125(Pt 3):319–336. doi: 10.1111/j.1365-2818.1982.tb00348.x. [DOI] [PubMed] [Google Scholar]
- Cohen C., Longley W. Tropomyosin paracrystals formed by divalent cations. Science. 1966 May 6;152(3723):794–796. doi: 10.1126/science.152.3723.794. [DOI] [PubMed] [Google Scholar]
- Craig R., Alamo L., Padrón R. Structure of the myosin filaments of relaxed and rigor vertebrate striated muscle studied by rapid freezing electron microscopy. J Mol Biol. 1992 Nov 20;228(2):474–487. doi: 10.1016/0022-2836(92)90836-9. [DOI] [PubMed] [Google Scholar]
- DE VILLAFRANCA G. W. The A and IB and lengths in stretched or contracted horseshoe crab skeletal muscle. J Ultrastruct Res. 1961 Apr;5:109–115. doi: 10.1016/s0022-5320(61)90008-9. [DOI] [PubMed] [Google Scholar]
- Dewey M. M., Levine R. J., Colflesh D. E. Structure of Limulus striated muscle. The contractile apparatus at various sarcomere lengths. J Cell Biol. 1973 Sep;58(3):574–593. doi: 10.1083/jcb.58.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood A. B., Wood D. S., Bock K. L., Sorenson M. M. Chemically skinned mammalian skeletal muscle. I. The structure of skinned rabbit psoas. Tissue Cell. 1979;11(3):553–566. doi: 10.1016/0040-8166(79)90062-4. [DOI] [PubMed] [Google Scholar]
- Elder H. Y., Gray C. C., Jardine A. G., Chapman J. N., Biddlecombe W. H. Optimum conditions for cryoquenching of small tissue blocks in liquid coolants. J Microsc. 1982 Apr;126(Pt 1):45–61. doi: 10.1111/j.1365-2818.1982.tb00356.x. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F., NIEDERGERKE R. Measurement of the striations of isolated muscle fibres with the interference microscope. J Physiol. 1958 Dec 30;144(3):403–425. doi: 10.1113/jphysiol.1958.sp006110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F., NIEDERGERKE R. Structural changes in muscle during contraction; interference microscopy of living muscle fibres. Nature. 1954 May 22;173(4412):971–973. doi: 10.1038/173971a0. [DOI] [PubMed] [Google Scholar]
- HUXLEY H., HANSON J. Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature. 1954 May 22;173(4412):973–976. doi: 10.1038/173973a0. [DOI] [PubMed] [Google Scholar]
- Handley D. A., Alexander J. T., Chien S. The design and use of a simple device for rapid quench-freezing of biological samples. J Microsc. 1981 Mar;121(Pt 3):273–282. doi: 10.1111/j.1365-2818.1981.tb01224.x. [DOI] [PubMed] [Google Scholar]
- Haselgrove J. C. X-ray evidence for conformational changes in the myosin filaments of vertebrate striated muscle. J Mol Biol. 1975 Feb 15;92(1):113–143. doi: 10.1016/0022-2836(75)90094-7. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Dennis M. J., Jan Y., Jan L., Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979 May;81(2):275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K., Lenart T. D., Murray J. M., Franzini-Armstrong C., Goldman Y. E. Flash and smash: rapid freezing of muscle fibers activated by photolysis of caged ATP. Biophys J. 1993 Jul;65(1):397–408. doi: 10.1016/S0006-3495(93)81061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K., Wakabayashi T. Thin filaments of rabbit skeletal muscle are in helical register. J Mol Biol. 1988 Dec 5;204(3):797–801. doi: 10.1016/0022-2836(88)90371-3. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967 Dec 14;30(2):383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Faruqi A. R., Bordas J., Koch M. H., Milch J. R. The use of synchrotron radiation in time-resolved X-ray diffraction studies of myosin layer-line reflections during muscle contraction. Nature. 1980 Mar 13;284(5752):140–143. doi: 10.1038/284140a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Kress M. Crossbridge behaviour during muscle contraction. J Muscle Res Cell Motil. 1985 Apr;6(2):153–161. doi: 10.1007/BF00713057. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. The crossbridge mechanism of muscular contraction and its implications. J Exp Biol. 1985 Mar;115:17–30. doi: 10.1242/jeb.115.1.17. [DOI] [PubMed] [Google Scholar]
- Jones G. J. On estimating freezing times during tissue rapid freezing. J Microsc. 1984 Dec;136(Pt 3):349–360. doi: 10.1111/j.1365-2818.1984.tb00546.x. [DOI] [PubMed] [Google Scholar]
- Kress M., Huxley H. E., Faruqi A. R., Hendrix J. Structural changes during activation of frog muscle studied by time-resolved X-ray diffraction. J Mol Biol. 1986 Apr 5;188(3):325–342. doi: 10.1016/0022-2836(86)90158-0. [DOI] [PubMed] [Google Scholar]
- Levine R. J., Woodhead J. L., King H. A. The effect of calcium activation of skinned fiber bundles on the structure of Limulus thick filaments. J Cell Biol. 1991 May;113(3):573–583. doi: 10.1083/jcb.113.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara I., Yagi N., Hashizume H. Use of an X-ray television for diffraction of the frog striated muscle. Nature. 1975 Jun 26;255(5511):728–729. doi: 10.1038/255728a0. [DOI] [PubMed] [Google Scholar]
- Maéda Y., Popp D., McLaughlin S. M. Cause of changes in the thin filament-associated reflexions on activation of frog muscle--myosin binding or conformational change of actin. Adv Exp Med Biol. 1988;226:381–390. [PubMed] [Google Scholar]
- McDowall A. W., Hofmann W., Lepault J., Adrian M., Dubochet J. Cryo-electron microscopy of vitrified insect flight muscle. J Mol Biol. 1984 Sep 5;178(1):105–111. doi: 10.1016/0022-2836(84)90233-x. [DOI] [PubMed] [Google Scholar]
- Moisescu D. G. Kinetics of reaction in calcium-activated skinned muscle fibres. Nature. 1976 Aug 12;262(5569):610–613. doi: 10.1038/262610a0. [DOI] [PubMed] [Google Scholar]
- Moor H. Recent progress in the freeze-etching technique. Philos Trans R Soc Lond B Biol Sci. 1971 May 27;261(837):121–131. doi: 10.1098/rstb.1971.0042. [DOI] [PubMed] [Google Scholar]
- PAGE S. G., HUXLEY H. E. FILAMENT LENGTHS IN STRIATED MUSCLE. J Cell Biol. 1963 Nov;19:369–390. doi: 10.1083/jcb.19.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padrón R., Alamo L., Craig R., Caputo C. A method for quick-freezing live muscles at known instants during contraction with simultaneous recording of mechanical tension. J Microsc. 1988 Aug;151(Pt 2):81–102. doi: 10.1111/j.1365-2818.1988.tb04616.x. [DOI] [PubMed] [Google Scholar]
- Padrón R., Craig R. Disorder induced in nonoverlap myosin cross-bridges by loss of adenosine triphosphate. Biophys J. 1989 Nov;56(5):927–933. doi: 10.1016/S0006-3495(89)82738-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padrón R., Granados M., Alamo L., Guerrero J. R., Craig R. Visualization of myosin helices in sections of rapidly frozen relaxed tarantula muscle. J Struct Biol. 1992 May-Jun;108(3):269–276. doi: 10.1016/1047-8477(92)90027-8. [DOI] [PubMed] [Google Scholar]
- Periasamy A., Burns D. H., Holdren D. N., Pollack G. H., Trombitás K. A-band shortening in single fibers of frog skeletal muscle. Biophys J. 1990 Apr;57(4):815–828. doi: 10.1016/S0006-3495(90)82601-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack G. H. The cross-bridge theory. Physiol Rev. 1983 Jul;63(3):1049–1113. doi: 10.1152/physrev.1983.63.3.1049. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen B. F., Stock A. M., Ringe D., Petsko G. A. Crystalline ribonuclease A loses function below the dynamical transition at 220 K. Nature. 1992 Jun 4;357(6377):423–424. doi: 10.1038/357423a0. [DOI] [PubMed] [Google Scholar]
- Reedy M. K., Goody R. S., Hofmann W., Rosenbaum G. Co-ordinated electron microscopy and X-ray studies of glycerinated insect flight muscle. I. X-ray diffraction monitoring during preparation for electron microscopy of muscle fibres fixed in rigor, in ATP and in AMPPNP. J Muscle Res Cell Motil. 1983 Feb;4(1):25–53. doi: 10.1007/BF00711957. [DOI] [PubMed] [Google Scholar]
- Reedy M. K., Reedy M. C. Rigor crossbridge structure in tilted single filament layers and flared-X formations from insect flight muscle. J Mol Biol. 1985 Sep 5;185(1):145–176. doi: 10.1016/0022-2836(85)90188-3. [DOI] [PubMed] [Google Scholar]
- Rome E. M., Hirabayashi T., Perry S. V. X-ray diffraction of muscle labelled with antibody to troponin-C. Nat New Biol. 1973 Aug 1;244(135):154–155. doi: 10.1038/newbio244154a0. [DOI] [PubMed] [Google Scholar]
- Ryan K. P., Purse D. H. Plunge-cooling of tissue blocks: determinants of cooling rates. J Microsc. 1985 Oct;140(Pt 1):47–54. doi: 10.1111/j.1365-2818.1985.tb02659.x. [DOI] [PubMed] [Google Scholar]
- Ryan K. P., Purse D. H., Robinson S. G., Wood J. W. The relative efficiency of cryogens used for plunge-cooling biological specimens. J Microsc. 1987 Jan;145(Pt 1):89–96. doi: 10.1111/j.1365-2818.1987.tb01318.x. [DOI] [PubMed] [Google Scholar]
- Salmon E. D., DeRosier D. A surveying optical diffractometer. J Microsc. 1981 Sep;123(Pt 3):239–247. doi: 10.1111/j.1365-2818.1981.tb02468.x. [DOI] [PubMed] [Google Scholar]
- Schutt C. E., Lindberg U. Actin as the generator of tension during muscle contraction. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):319–323. doi: 10.1073/pnas.89.1.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilton R. F., Jr, Dewan J. C., Petsko G. A. Effects of temperature on protein structure and dynamics: X-ray crystallographic studies of the protein ribonuclease-A at nine different temperatures from 98 to 320 K. Biochemistry. 1992 Mar 10;31(9):2469–2481. doi: 10.1021/bi00124a006. [DOI] [PubMed] [Google Scholar]
- Trus B. L., Steven A. C., McDowall A. W., Unser M., Dubochet J., Podolsky R. J. Interactions between actin and myosin filaments in skeletal muscle visualized in frozen-hydrated thin sections. Biophys J. 1989 Apr;55(4):713–724. doi: 10.1016/S0006-3495(89)82870-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S., Yano M. Actomyosin structure in contracting muscle detected by rapid freezing. Nature. 1985 Sep 12;317(6033):182–184. doi: 10.1038/317182a0. [DOI] [PubMed] [Google Scholar]
- Van Harreveld A., Trubatch J., Steiner J. Rapid freezing and electron microscopy for the arrest of physiological processes. J Microsc. 1974 Mar;100(2):189–198. doi: 10.1111/j.1365-2818.1974.tb03928.x. [DOI] [PubMed] [Google Scholar]
- Vibert P. J., Haselgrove J. C., Lowy J., Poulsen F. R. Structural changes in actin-containing filaments of muscle. J Mol Biol. 1972 Nov 28;71(3):757–767. doi: 10.1016/s0022-2836(72)80036-6. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K., Tanaka H., Saito H., Moriwaki N., Ueno Y., Amemiya Y. Dynamic X-ray diffraction of skeletal muscle contraction: structural change of actin filaments. Adv Biophys. 1991;27:3–13. doi: 10.1016/0065-227x(91)90004-w. [DOI] [PubMed] [Google Scholar]
- Wakabayashi T., Akiba T., Hirose K., Tomioka A., Tokunaga M., Suzuki M., Toyoshima C., Sutoh K., Yamamoto K., Matsumoto T. Temperature-induced change of thick filament and location of the functional sites of myosin. Adv Exp Med Biol. 1988;226:39–48. [PubMed] [Google Scholar]