Abstract
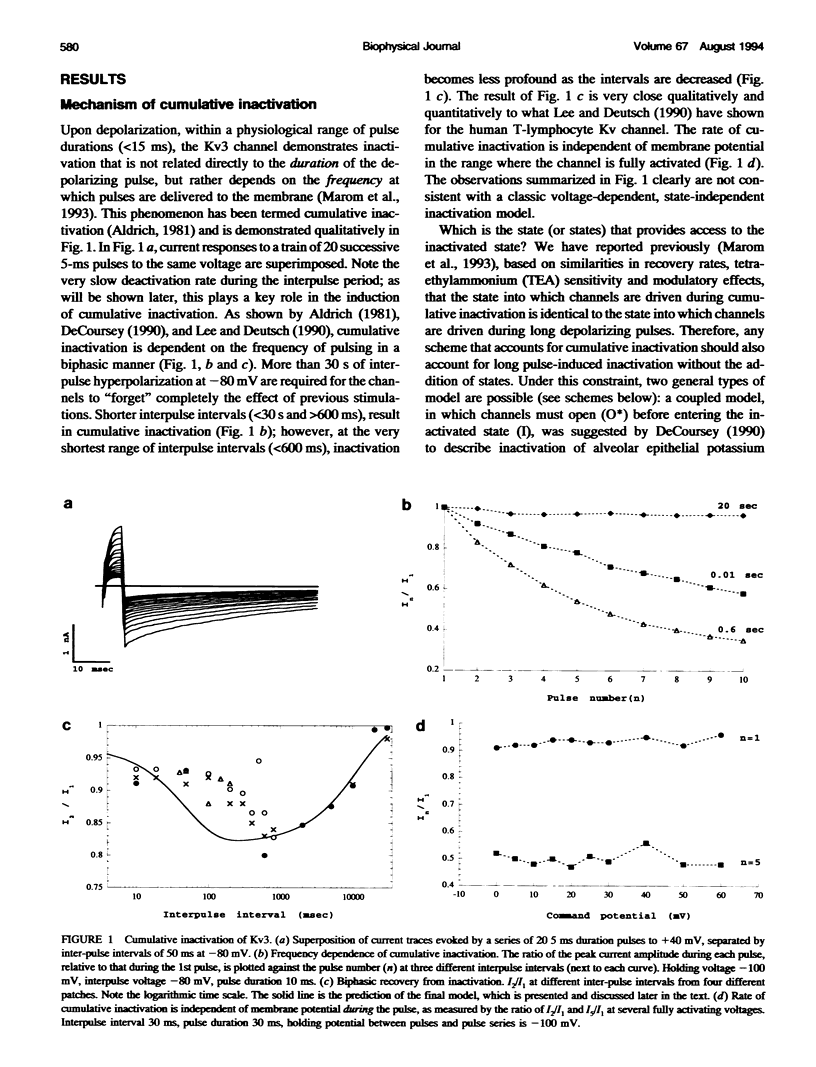
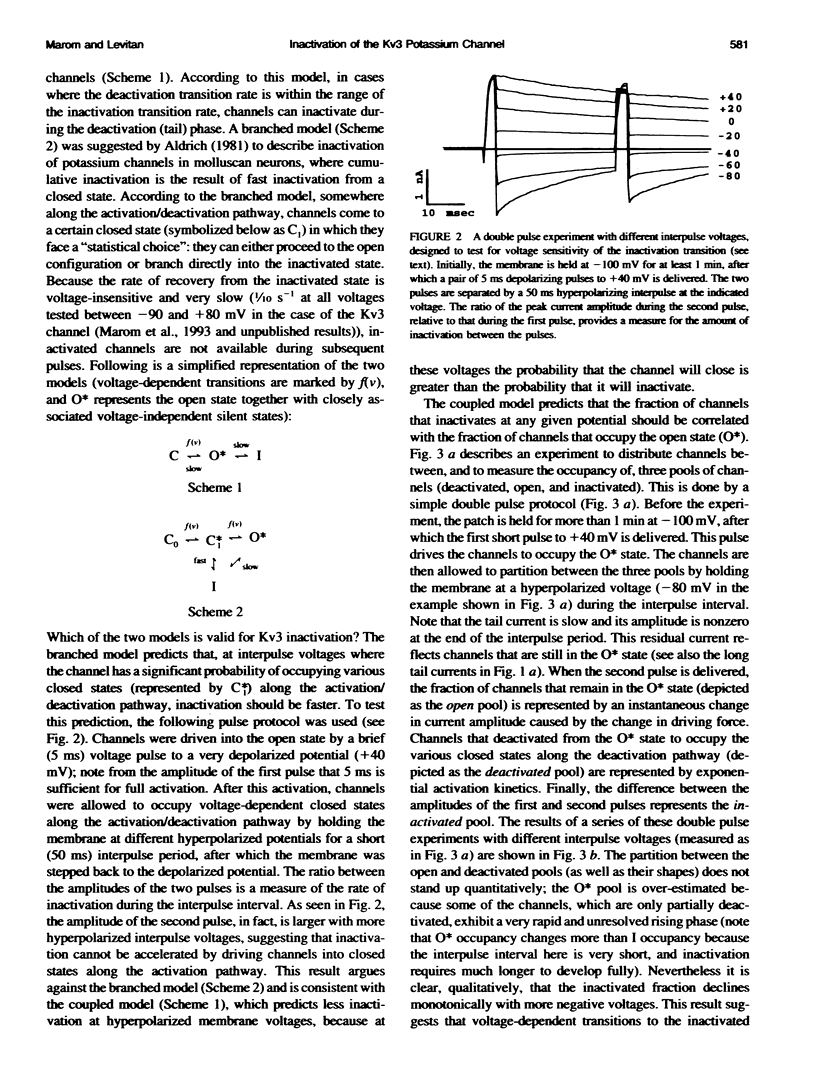
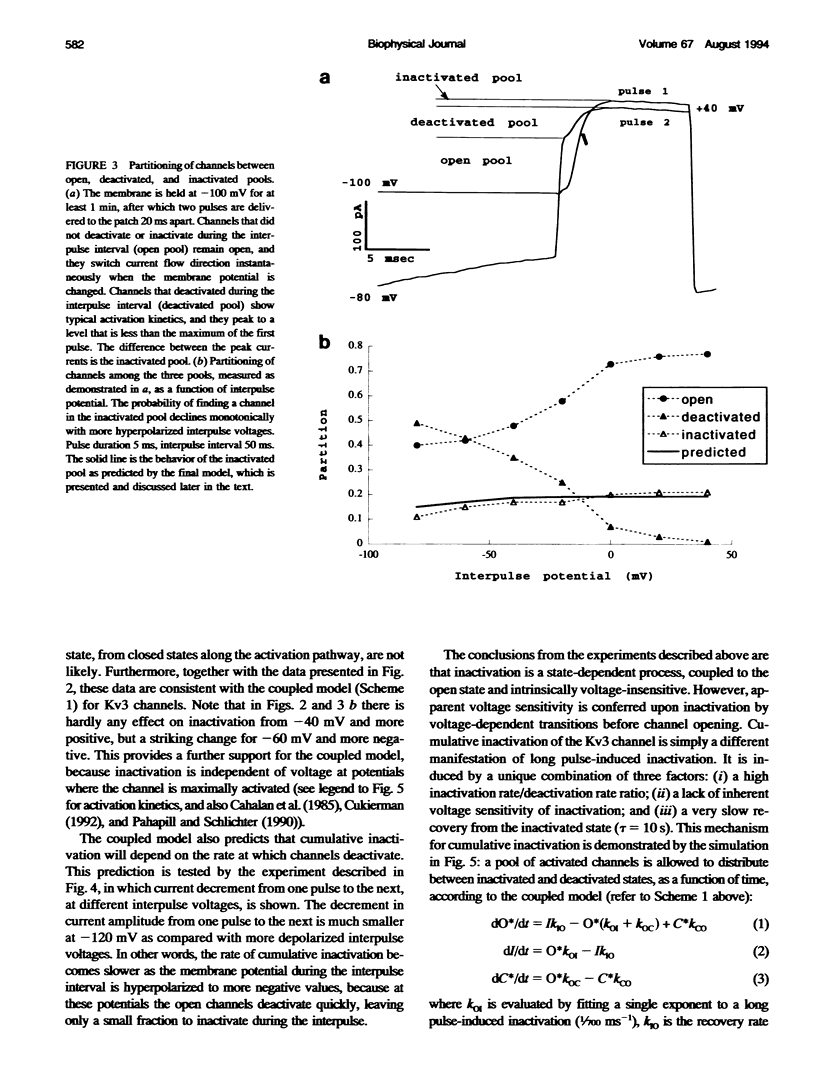
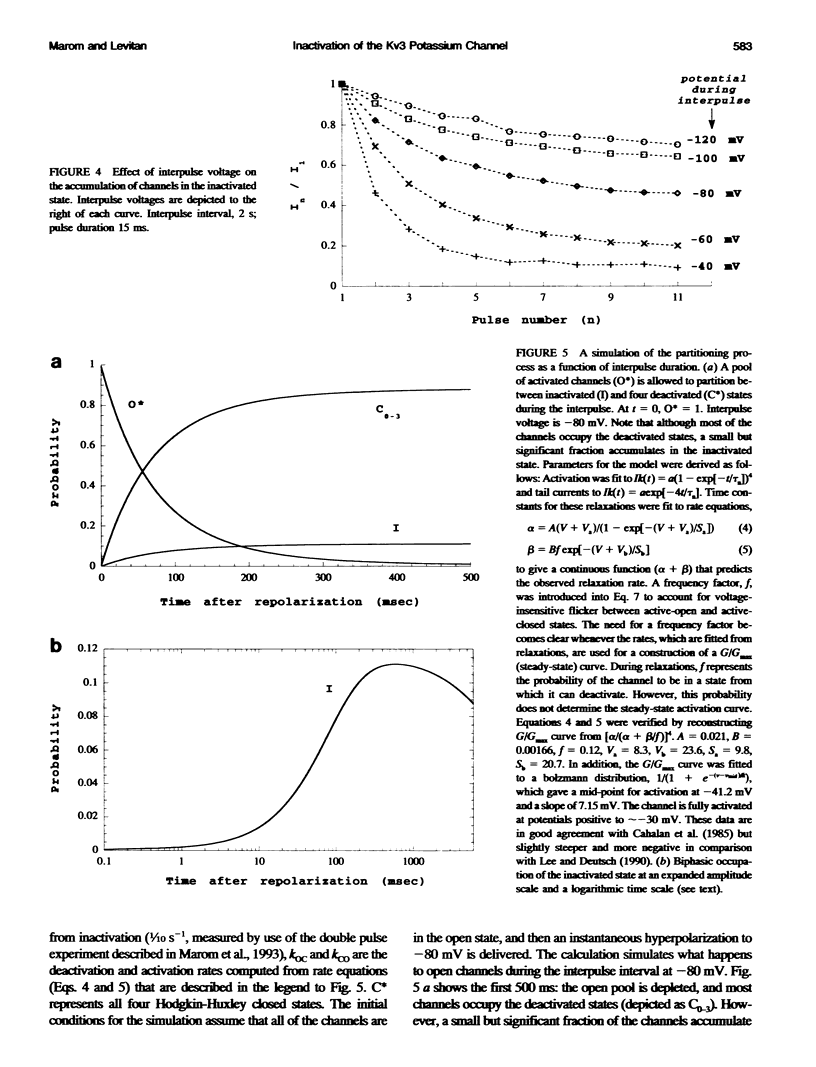
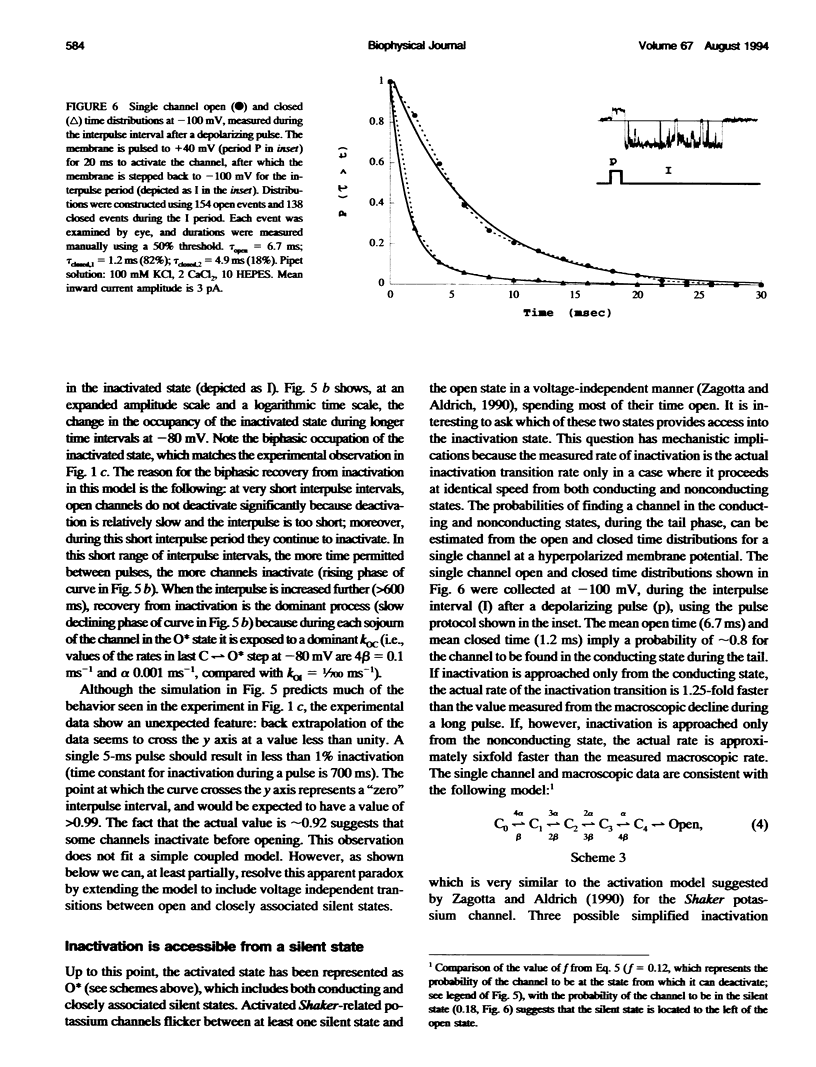
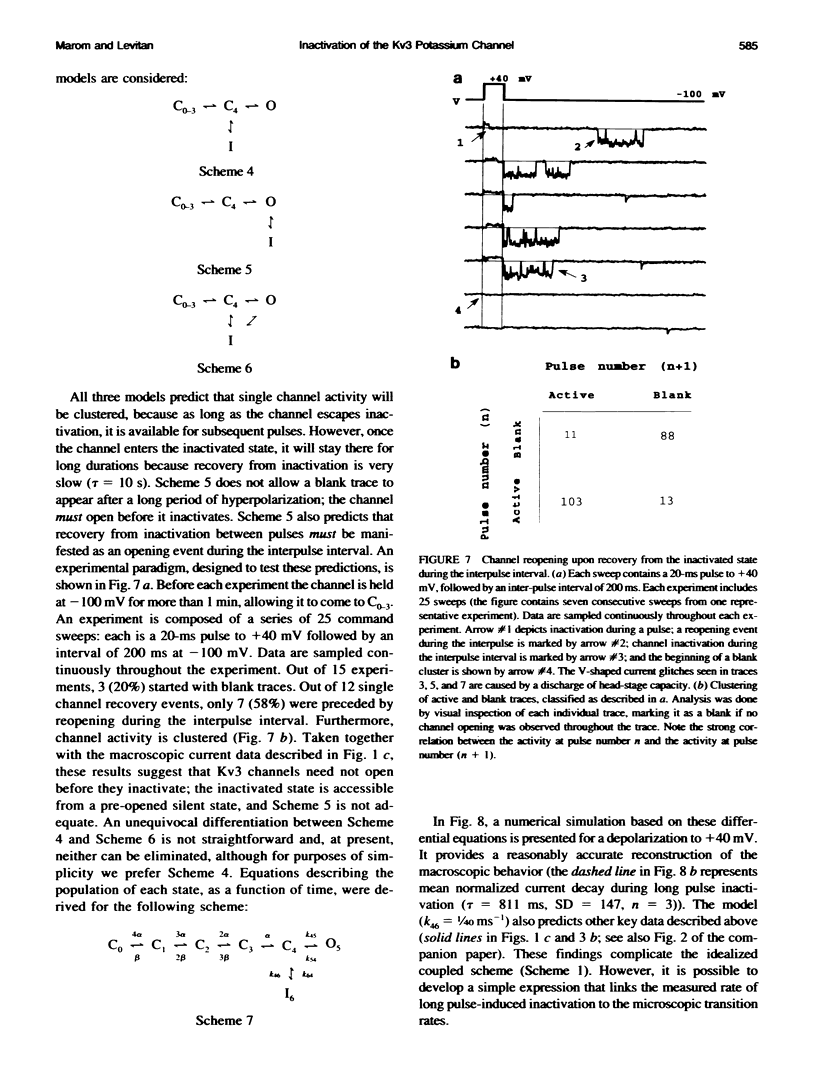
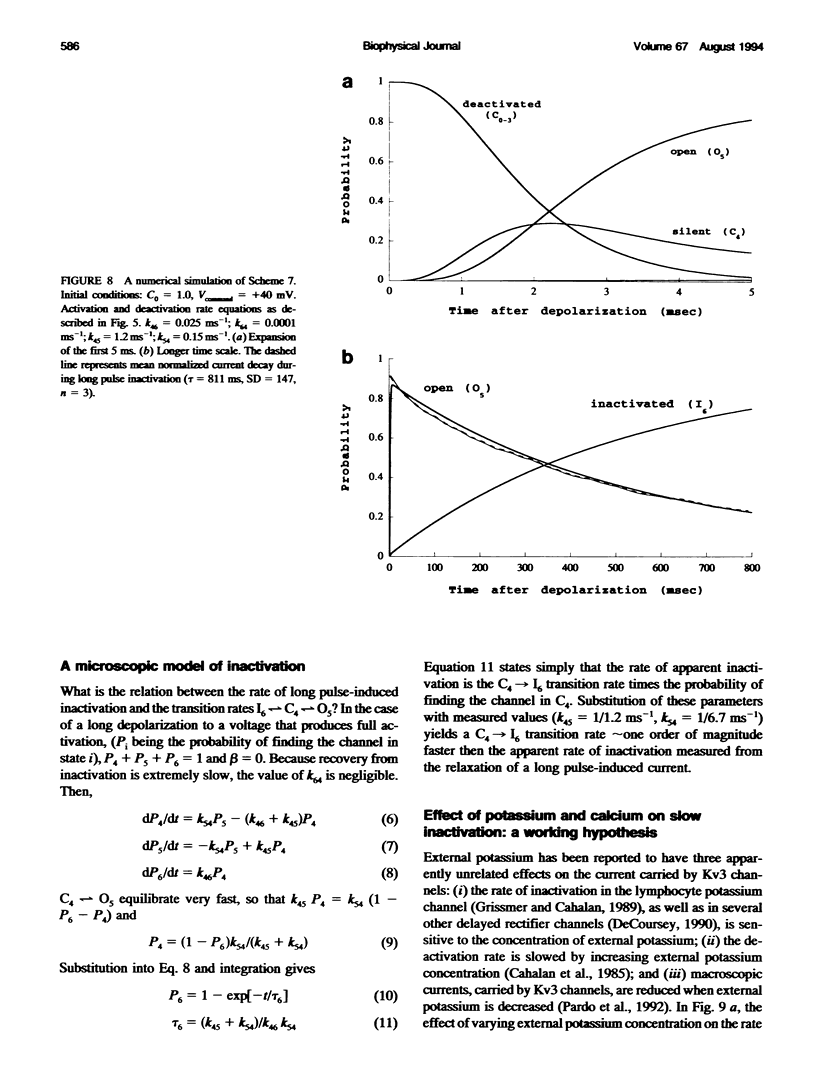
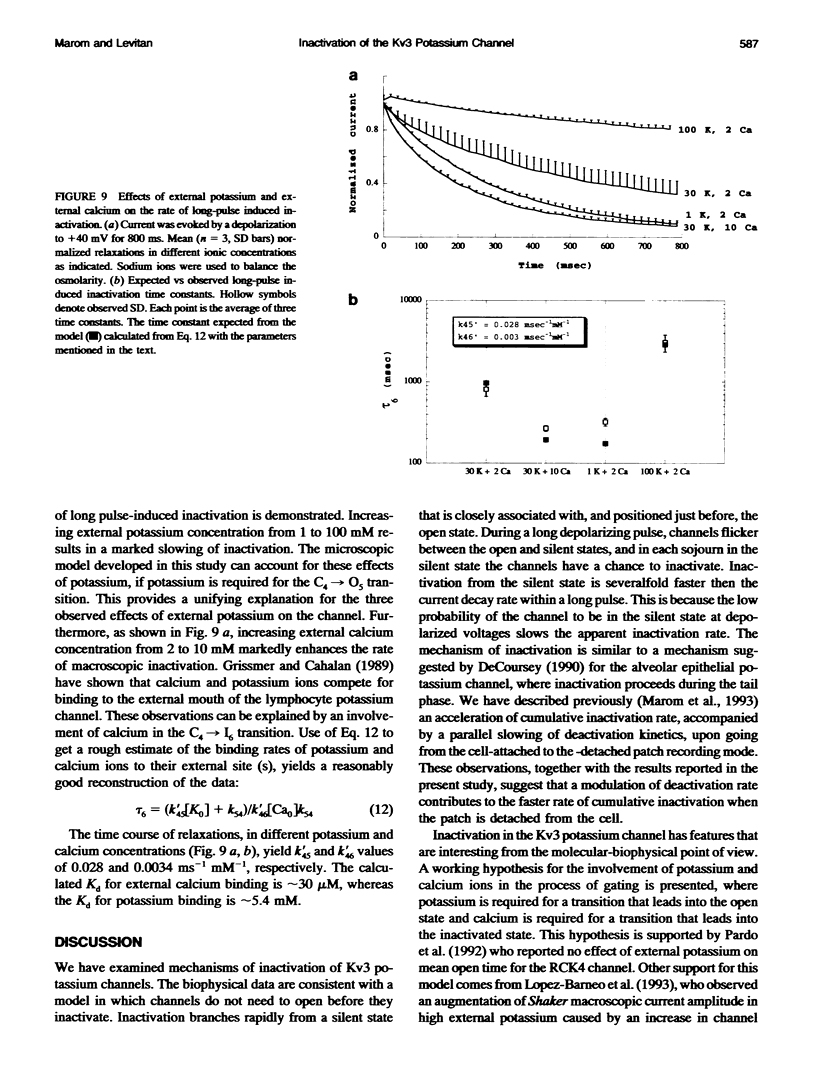
Inactivation of Kv3 (Kv1.3) delayed rectifier potassium channels was studied in the Xenopus oocyte expression system. These channels inactivate slowly during a long depolarizing pulse. In addition, inactivation accumulates in response to a series of short depolarizing pulses (cumulative inactivation), although no significant inactivation occurs within each short pulse. The extent of cumulative inactivation does not depend on the voltage during the depolarizing pulse, but it does vary in a biphasic manner as a function of the interpulse duration. Furthermore, the rate of cumulative inactivation is influenced by changing the rate of deactivation. These data are consistent with a model in which Kv3 channel inactivation is a state-dependent and voltage-independent process. Macroscopic and single channel experiments indicate that inactivation can occur from a closed (silent) state before channel opening. That is, channels need not open to inactivate. The transition that leads to the inactivated state from the silent state is, in fact, severalfold faster then the observed inactivation of current during long depolarizing pulses. Long pulse-induced inactivation appears to be slow, because its rate is limited by the probability that channels are in the open state, rather than in the silent state from which they can inactivate. External potassium and external calcium ions alter the rates of cumulative and long pulse-induced inactivation, suggesting that antagonistic potassium and calcium binding steps are involved in the normal gating of the channel.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich R. W. Inactivation of voltage-gated delayed potassium current in molluscan neurons. A kinetic model. Biophys J. 1981 Dec;36(3):519–532. doi: 10.1016/S0006-3495(81)84750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Matteson D. R. The role of calcium ions in the closing of K channels. J Gen Physiol. 1986 May;87(5):817–832. doi: 10.1085/jgp.87.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch A. E., Hurst R. S., North R. A., Adelman J. P., Kavanaugh M. P. Current inactivation involves a histidine residue in the pore of the rat lymphocyte potassium channel RGK5. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1384–1390. doi: 10.1016/0006-291x(91)91726-s. [DOI] [PubMed] [Google Scholar]
- Cahalan M. D., Chandy K. G., DeCoursey T. E., Gupta S. A voltage-gated potassium channel in human T lymphocytes. J Physiol. 1985 Jan;358:197–237. doi: 10.1113/jphysiol.1985.sp015548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman S. Characterization of K+ currents in rat malignant lymphocytes (Nb2 cells). J Membr Biol. 1992 Mar;126(2):147–157. doi: 10.1007/BF00231913. [DOI] [PubMed] [Google Scholar]
- DeCoursey T. E. State-dependent inactivation of K+ currents in rat type II alveolar epithelial cells. J Gen Physiol. 1990 Apr;95(4):617–646. doi: 10.1085/jgp.95.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass J., Osborne P. B., Cai Y. C., Wilkinson M., Christie M. J., Adelman J. P. Characterization and functional expression of a rat genomic DNA clone encoding a lymphocyte potassium channel. J Immunol. 1990 Jun 15;144(12):4841–4850. [PubMed] [Google Scholar]
- Grissmer S., Cahalan M. TEA prevents inactivation while blocking open K+ channels in human T lymphocytes. Biophys J. 1989 Jan;55(1):203–206. doi: 10.1016/S0006-3495(89)82793-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissmer S., Dethlefs B., Wasmuth J. J., Goldin A. L., Gutman G. A., Cahalan M. D., Chandy K. G. Expression and chromosomal localization of a lymphocyte K+ channel gene. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9411–9415. doi: 10.1073/pnas.87.23.9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré E., Attali B., Romey G., Lesage F., Barhanin J., Lazdunski M. Different types of K+ channel current are generated by different levels of a single mRNA. EMBO J. 1992 Jul;11(7):2465–2471. doi: 10.1002/j.1460-2075.1992.tb05311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W. N., Aldrich R. W. Two types of inactivation in Shaker K+ channels: effects of alterations in the carboxy-terminal region. Neuron. 1991 Oct;7(4):547–556. doi: 10.1016/0896-6273(91)90367-9. [DOI] [PubMed] [Google Scholar]
- Koren G., Liman E. R., Logothetis D. E., Nadal-Ginard B., Hess P. Gating mechanism of a cloned potassium channel expressed in frog oocytes and mammalian cells. Neuron. 1990 Jan;4(1):39–51. doi: 10.1016/0896-6273(90)90442-i. [DOI] [PubMed] [Google Scholar]
- Lee S. C., Deutsch C. Temperature dependence of K(+)-channel properties in human T lymphocytes. Biophys J. 1990 Jan;57(1):49–62. doi: 10.1016/S0006-3495(90)82506-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Barneo J., Hoshi T., Heinemann S. H., Aldrich R. W. Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channels. Receptors Channels. 1993;1(1):61–71. [PubMed] [Google Scholar]
- Marom S., Abbott L. F. Modeling state-dependent inactivation of membrane currents. Biophys J. 1994 Aug;67(2):515–520. doi: 10.1016/S0006-3495(94)80518-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marom S., Goldstein S. A., Kupper J., Levitan I. B. Mechanism and modulation of inactivation of the Kv3 potassium channel. Receptors Channels. 1993;1(1):81–88. [PubMed] [Google Scholar]
- Pardo L. A., Heinemann S. H., Terlau H., Ludewig U., Lorra C., Pongs O., Stühmer W. Extracellular K+ specifically modulates a rat brain K+ channel. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2466–2470. doi: 10.1073/pnas.89.6.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahamimoff R., Edry-Schiller J., Ginsburg S. A long closed state of the synaptosomal bursting potassium channel confers a statistical memory. J Neurophysiol. 1992 Dec;68(6):2260–2263. doi: 10.1152/jn.1992.68.6.2260. [DOI] [PubMed] [Google Scholar]


