Abstract
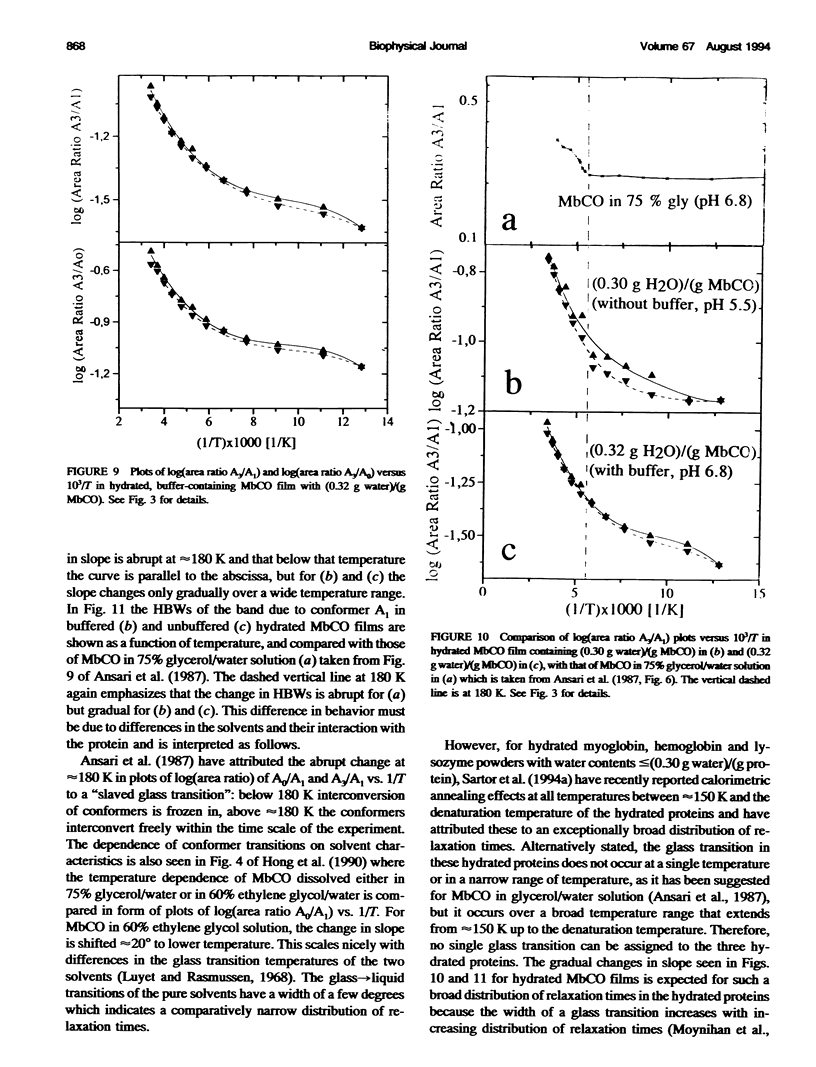
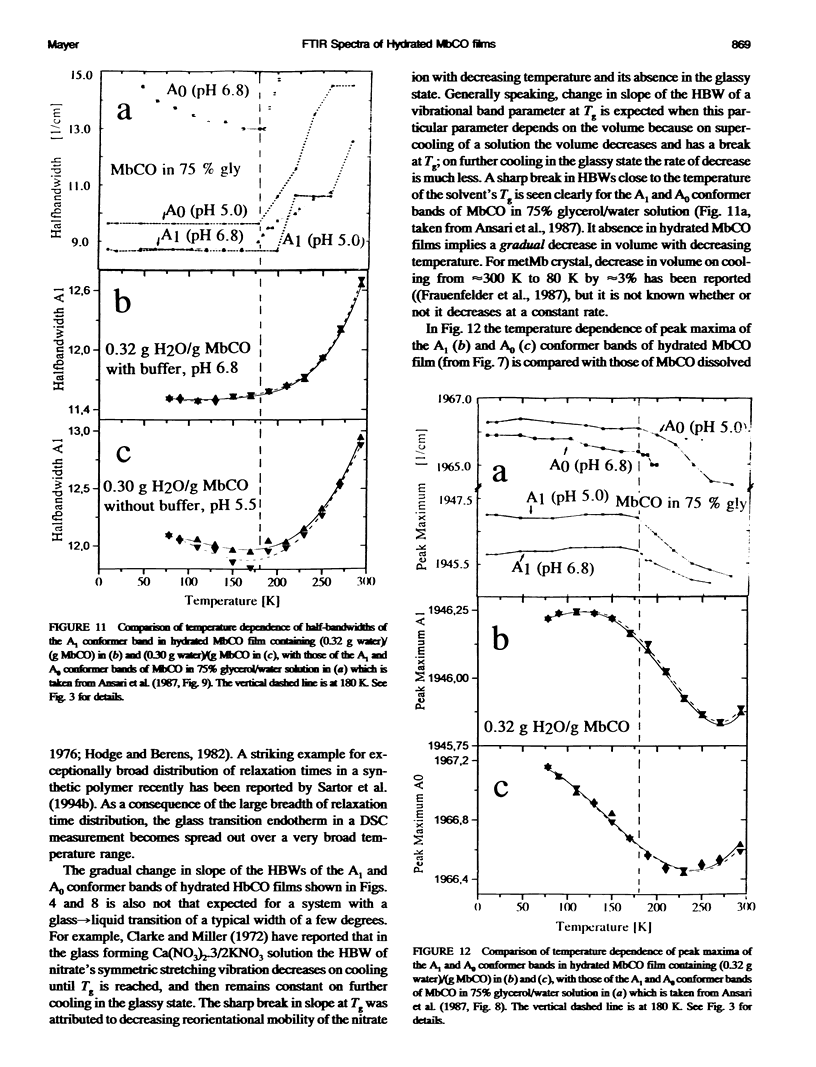
Two hydrated carbonyl myoglobin (MbCO) films, one containing (0.30 g water)/(g MbCO) from MbCO solution in water at pH 5.5 and the other (0.32 g water)/(gMbCO) from 0.1 M potassium phosphate buffer solution at pH 6.8, were studied by FTIR spectroscopy from 293 K to 78 K at selected temperatures on cooling and reheating. Above approximately 180 K the general trend in temperature dependence of half-bandwidths, peak maxima, and band area ratios of the A1 and A3 conformer bands is similar to those reported by Ansari et al. (1987. Biophys. J. 26:337) for MbCO in 75% glycerol/water solution, but abrupt changes in slopes at approximately 180-200 K and freezing-in of conformer populations, which could be taken as indicator for glass transition of the solvent or the protein, are absent for the hydrated MbCO films. This is interpreted in terms of an exceptionally broad distribution of relaxation times, and is in accord with conclusions from recent calorimetric annealing studies of hydrated protein powders (Sartor et al. 1994. Biophys. J. 66:249). Exchange between the three A conformers does not stop at approximately 180-200 K but occurs over the whole temperature region studied. These results are then discussed with respect to MbCO's behavior in the glass-->liquid transition region of glass-forming solvents, and it is concluded that, in analogy to the behavior of low-molecular-weight compounds with a distribution of rapidly interconverting conformers, freezing-in of MbCO's A conformer populations by the solvent should not be mistaken for a glass transition of MbCO.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansari A., Berendzen J., Braunstein D., Cowen B. R., Frauenfelder H., Hong M. K., Iben I. E., Johnson J. B., Ormos P., Sauke T. B. Rebinding and relaxation in the myoglobin pocket. Biophys Chem. 1987 May 9;26(2-3):337–355. doi: 10.1016/0301-4622(87)80034-0. [DOI] [PubMed] [Google Scholar]
- Ansari A., Jones C. M., Henry E. R., Hofrichter J., Eaton W. A. The role of solvent viscosity in the dynamics of protein conformational changes. Science. 1992 Jun 26;256(5065):1796–1798. doi: 10.1126/science.1615323. [DOI] [PubMed] [Google Scholar]
- Astl G., Mayer E. Alkali cation effect on carbonyl-hemoglobin's and -myoglobin's conformer populations when exposed to freeze-concentration of their phosphate-buffered aqueous solutions. Biochim Biophys Acta. 1991 Oct 25;1080(2):155–159. doi: 10.1016/0167-4838(91)90143-n. [DOI] [PubMed] [Google Scholar]
- Beece D., Eisenstein L., Frauenfelder H., Good D., Marden M. C., Reinisch L., Reynolds A. H., Sorensen L. B., Yue K. T. Solvent viscosity and protein dynamics. Biochemistry. 1980 Nov 11;19(23):5147–5157. doi: 10.1021/bi00564a001. [DOI] [PubMed] [Google Scholar]
- Brown W. E., 3rd, Sutcliffe J. W., Pulsinelli P. D. Multiple internal reflectance infrared spectra of variably hydrated hemoglobin and myoglobin films: effects of globin hydration on ligand conformer dynamics and reactivity at the heme. Biochemistry. 1983 Jun 7;22(12):2914–2923. doi: 10.1021/bi00281a021. [DOI] [PubMed] [Google Scholar]
- Caughey W. S., Shimada H., Choc M. G., Tucker M. P. Dynamic protein structures: infrared evidence for four discrete rapidly interconverting conformers at the carbon monoxide binding site of bovine heart myoglobin. Proc Natl Acad Sci U S A. 1981 May;78(5):2903–2907. doi: 10.1073/pnas.78.5.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doster W., Bachleitner A., Dunau R., Hiebl M., Lüscher E. Thermal properties of water in myoglobin crystals and solutions at subzero temperatures. Biophys J. 1986 Aug;50(2):213–219. doi: 10.1016/S0006-3495(86)83455-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doster W., Cusack S., Petry W. Dynamical transition of myoglobin revealed by inelastic neutron scattering. Nature. 1989 Feb 23;337(6209):754–756. doi: 10.1038/337754a0. [DOI] [PubMed] [Google Scholar]
- Doster W, Cusack S, Petry W. Dynamic instability of liquidlike motions in a globular protein observed by inelastic neutron scattering. Phys Rev Lett. 1990 Aug 20;65(8):1080–1083. doi: 10.1103/PhysRevLett.65.1080. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Gratton E. Protein dynamics and hydration. Methods Enzymol. 1986;127:207–216. doi: 10.1016/0076-6879(86)27017-2. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Hartmann H., Karplus M., Kuntz I. D., Jr, Kuriyan J., Parak F., Petsko G. A., Ringe D., Tilton R. F., Jr, Connolly M. L. Thermal expansion of a protein. Biochemistry. 1987 Jan 13;26(1):254–261. doi: 10.1021/bi00375a035. [DOI] [PubMed] [Google Scholar]
- Goldanskii V. I., Krupyanskii Y. F. Protein and protein-bound water dynamics studied by Rayleigh scattering of Mössbauer radiation (RSMR). Q Rev Biophys. 1989 Feb;22(1):39–92. doi: 10.1017/s003358350000336x. [DOI] [PubMed] [Google Scholar]
- Hanania G. I., Yeghiayan A., Cameron B. F. Absorption spectra of sperm-whale ferrimyoglobin. Biochem J. 1966 Jan;98(1):189–192. doi: 10.1042/bj0980189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M. K., Braunstein D., Cowen B. R., Frauenfelder H., Iben I. E., Mourant J. R., Ormos P., Scholl R., Schulte A., Steinbach P. J. Conformational substates and motions in myoglobin. External influences on structure and dynamics. Biophys J. 1990 Aug;58(2):429–436. doi: 10.1016/S0006-3495(90)82388-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iben IE, Braunstein D, Doster W, Frauenfelder H, Hong MK, Johnson JB, Luck S, Ormos P, Schulte A, Steinbach PJ. Glassy behavior of a protein. Phys Rev Lett. 1989 Apr 17;62(16):1916–1919. doi: 10.1103/PhysRevLett.62.1916. [DOI] [PubMed] [Google Scholar]
- Luyet B., Rasmussen D. Study by differential thermal analysis of the temperatures of instability of rapidly cooled solutions of glycerol, ethylene glycol, sucrose and glucose. Biodynamica. 1968;10(210):167–191. [PubMed] [Google Scholar]
- Parak F. Correlation of protein dynamics with water mobility: Mössbauer spectroscopy and microwave absorption methods. Methods Enzymol. 1986;127:196–206. doi: 10.1016/0076-6879(86)27016-0. [DOI] [PubMed] [Google Scholar]
- Pethig R. Protein-water interactions determined by dielectric methods. Annu Rev Phys Chem. 1992;43:177–205. doi: 10.1146/annurev.pc.43.100192.001141. [DOI] [PubMed] [Google Scholar]
- Poole P. L., Finney J. L. Solid-phase protein hydration studies. Methods Enzymol. 1986;127:284–293. doi: 10.1016/0076-6879(86)27023-8. [DOI] [PubMed] [Google Scholar]
- Post F., Doster W., Karvounis G., Settles M. Structural relaxation and nonexponential kinetics of CO-binding to horse myoglobin. Multiple flash photolysis experiments. Biophys J. 1993 Jun;64(6):1833–1842. doi: 10.1016/S0006-3495(93)81554-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothgeb T. M., Gurd F. R. Physical methods for the study of myoglobin. Methods Enzymol. 1978;52:473–486. doi: 10.1016/s0076-6879(78)52052-1. [DOI] [PubMed] [Google Scholar]
- Rupley J. A., Careri G. Protein hydration and function. Adv Protein Chem. 1991;41:37–172. doi: 10.1016/s0065-3233(08)60197-7. [DOI] [PubMed] [Google Scholar]
- Sartor G., Mayer E., Johari G. P. Calorimetric studies of the kinetic unfreezing of molecular motions in hydrated lysozyme, hemoglobin, and myoglobin. Biophys J. 1994 Jan;66(1):249–258. doi: 10.1016/S0006-3495(94)80774-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settles M., Post F., Müller D., Schulte A., Doster W. Solvent damping of internal processes in myoglobin studied by specific heat spectroscopy and flash photolysis. Biophys Chem. 1992 Jun;43(2):107–116. doi: 10.1016/0301-4622(92)80026-2. [DOI] [PubMed] [Google Scholar]
- Smith J., Kuczera K., Karplus M. Dynamics of myoglobin: comparison of simulation results with neutron scattering spectra. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1601–1605. doi: 10.1073/pnas.87.4.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srajer V., Reinisch L., Champion P. M. Investigation of laser-induced long-lived states of photolyzed MbCO. Biochemistry. 1991 May 21;30(20):4886–4895. doi: 10.1021/bi00234a008. [DOI] [PubMed] [Google Scholar]
- Steinbach P. J., Ansari A., Berendzen J., Braunstein D., Chu K., Cowen B. R., Ehrenstein D., Frauenfelder H., Johnson J. B., Lamb D. C. Ligand binding to heme proteins: connection between dynamics and function. Biochemistry. 1991 Apr 23;30(16):3988–4001. doi: 10.1021/bi00230a026. [DOI] [PubMed] [Google Scholar]


