Abstract
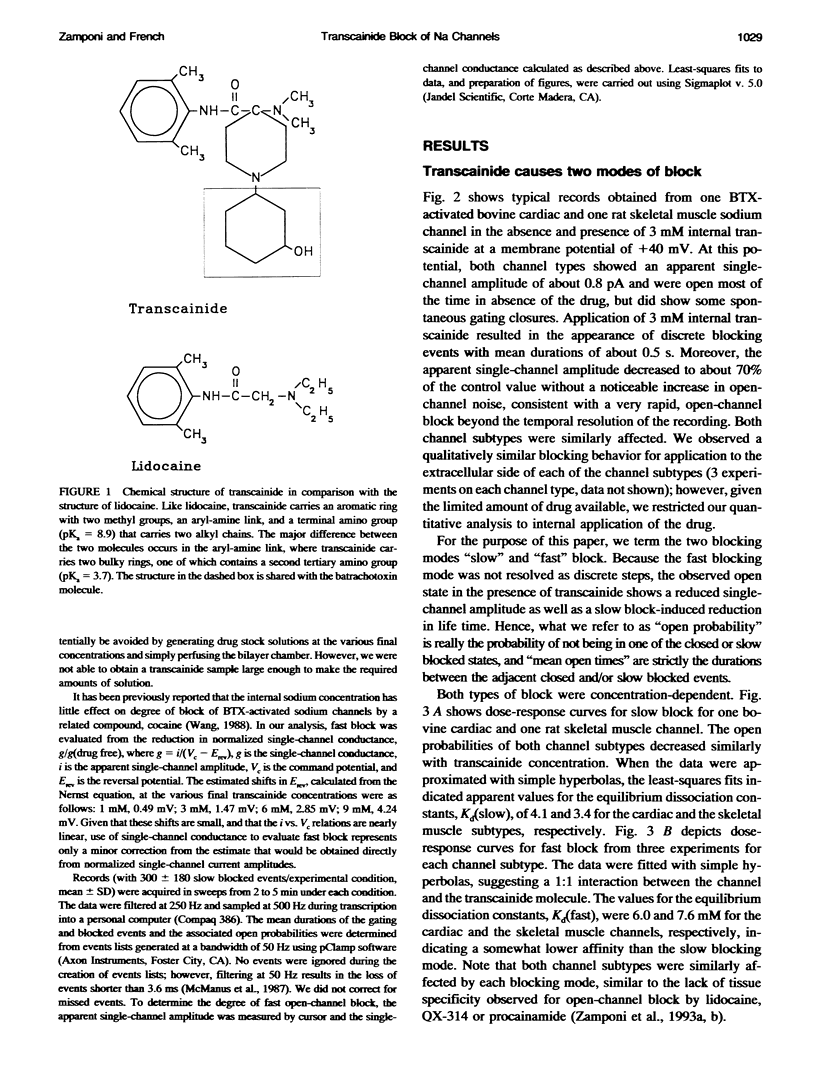
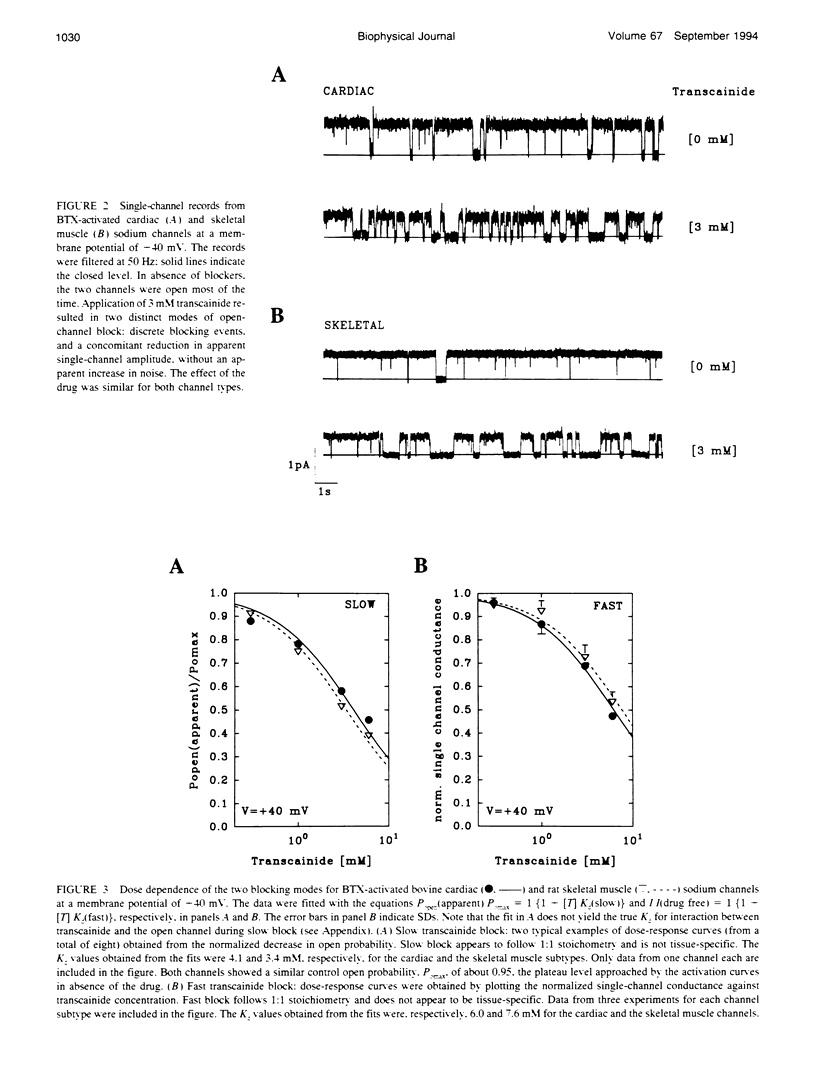
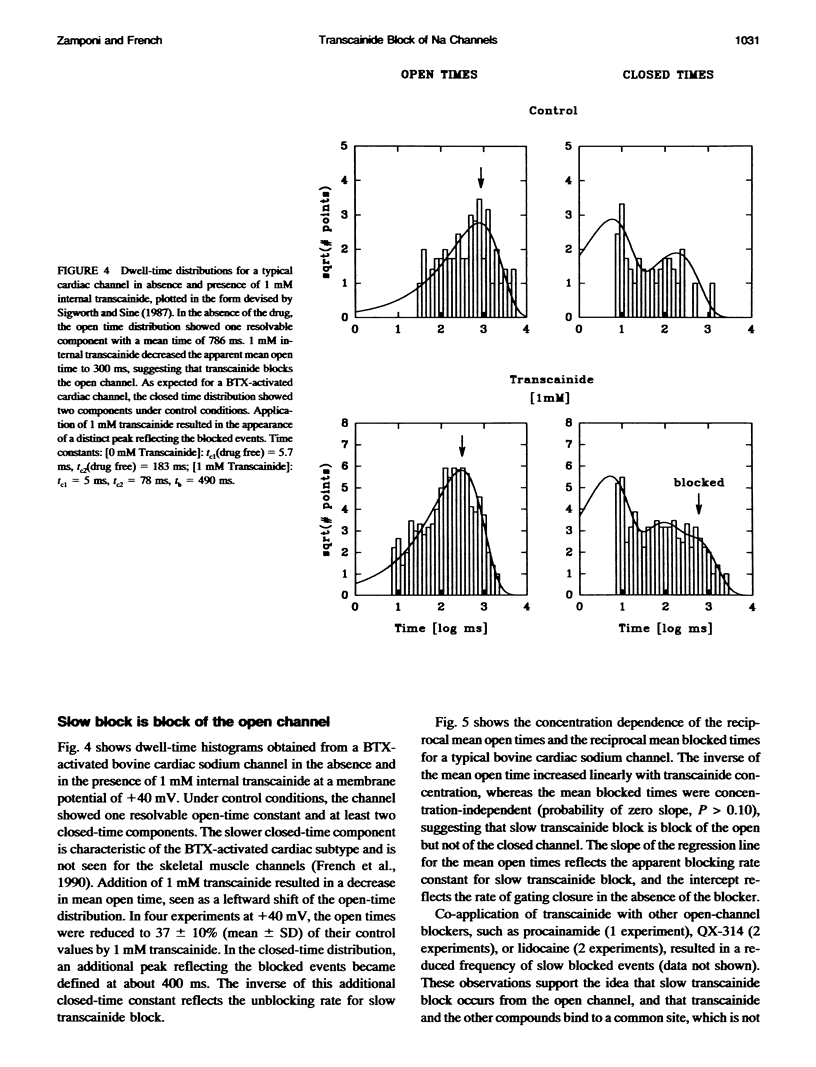
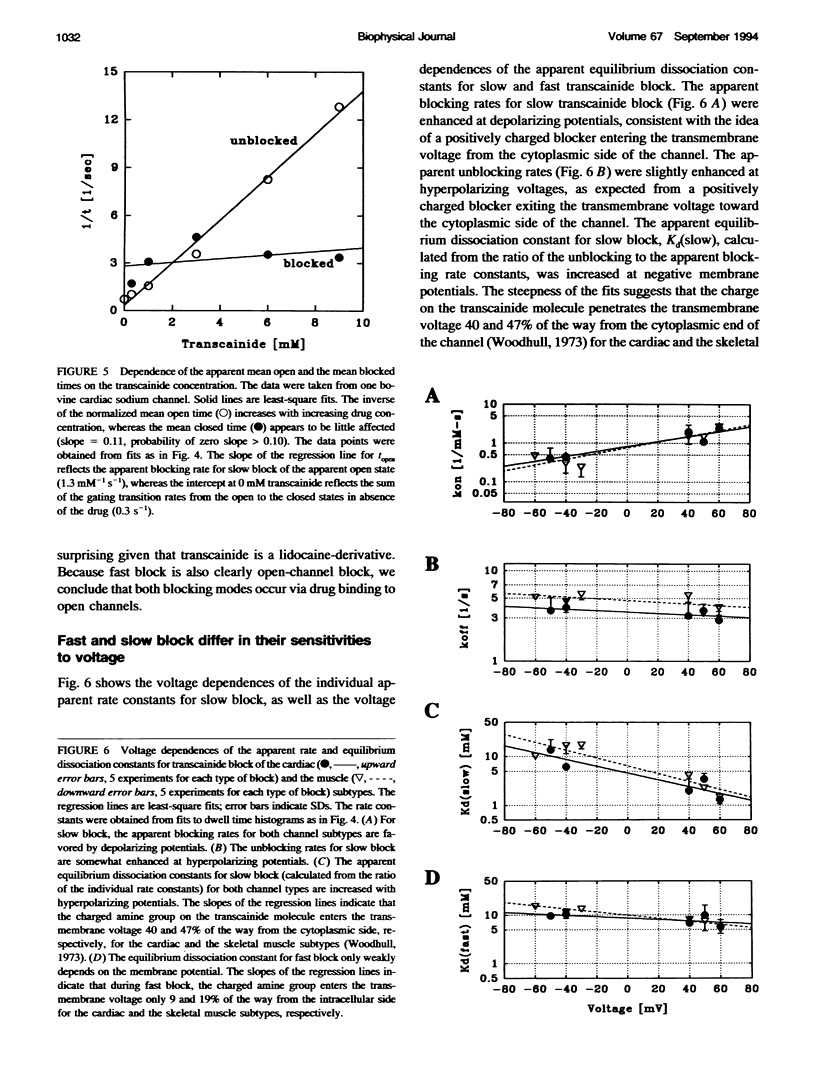
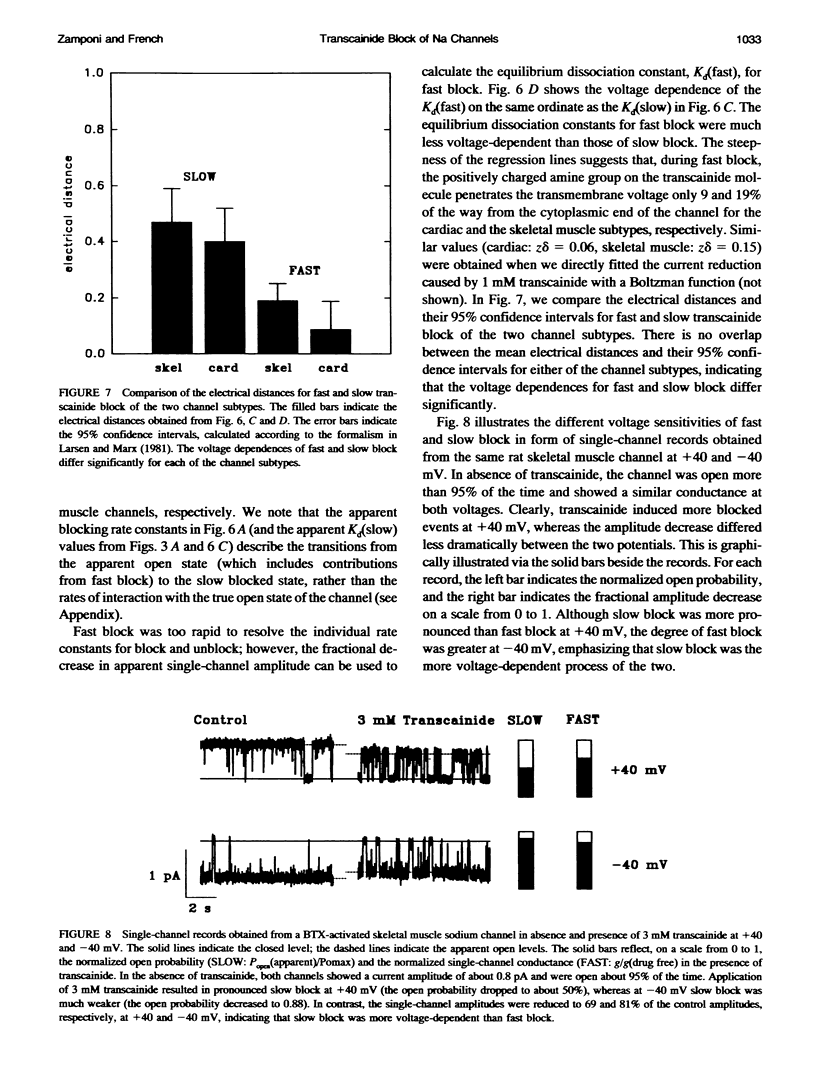
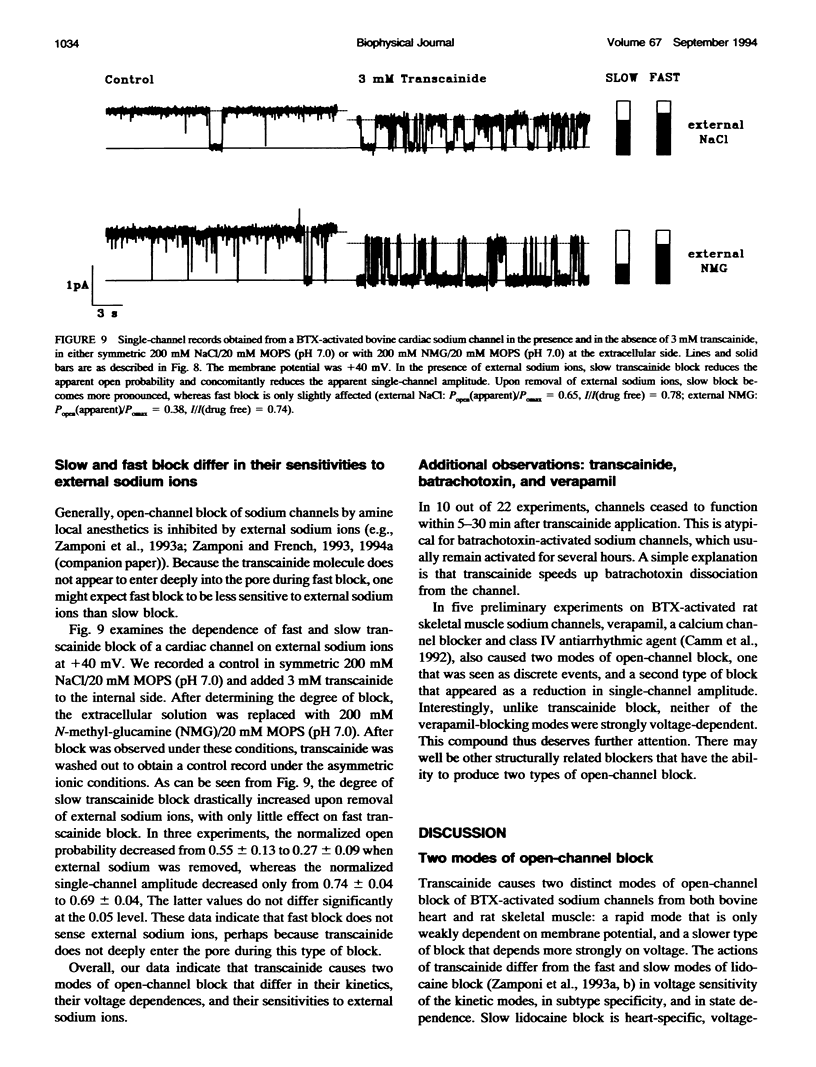
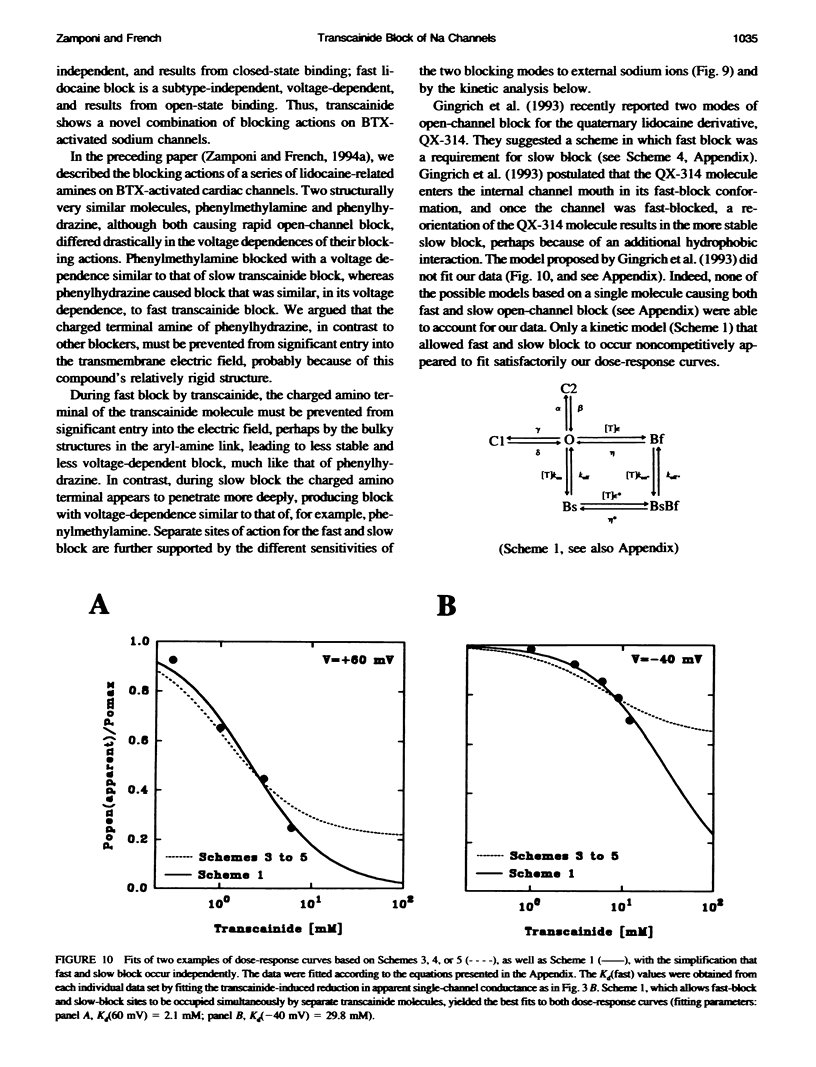
Transcainide, a complex derivative of lidocaine, blocks the open state of BTX-activated sodium channels from bovine heart and rat skeletal muscle in two distinct ways. When applied to either side of the membrane, transcainide caused discrete blocking events a few hundred milliseconds in duration (slow block), and a concomitant reduction in apparent single-channel amplitude, presumably because of rapid block beyond the temporal resolution of our recordings (fast block). We quantitatively analyzed block from the cytoplasmic side. Both modes of block occurred via binding of the drug to the open channel, approximately followed 1:1 stoichiometry, and were similar for both channel subtypes. For slow block, the blocking rate increased, and the unblocking rate decreased with depolarization, yielding an overall enhancement of block at positive potentials, and suggesting a blocking site at an apparent electrical distance about 45% of the way from the cytoplasmic end of the channel (z delta approximately 0.45). In contrast, the fast blocking mode was only slightly enhanced by depolarization (z delta approximately 0.15). Phenomenologically, the bulky and complex transcainide molecule combines the almost voltage-insensitive blocking action of phenylhydrazine (Zamponi and French, 1994a (companion paper)) with a slow open-channel blocking action that shows a voltage dependence typical of simpler amines. Only the slower blocking mode was sensitive to the removal of external sodium ions, suggesting that the two types of block occur at distinct sites. Dose-response relations were also consistent with independent binding of transcainide to two separate sites on the channel.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett P. B., Stroobandt R., Kesteloot H., Hondeghem L. M. Sodium channel block by a potent, new antiarrhythmic agent, transcainide, in guinea pig ventricular myocytes. J Cardiovasc Pharmacol. 1987 Jun;9(6):661–667. doi: 10.1097/00005344-198706000-00004. [DOI] [PubMed] [Google Scholar]
- Garber S. S., Miller C. Single Na+ channels activated by veratridine and batrachotoxin. J Gen Physiol. 1987 Mar;89(3):459–480. doi: 10.1085/jgp.89.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
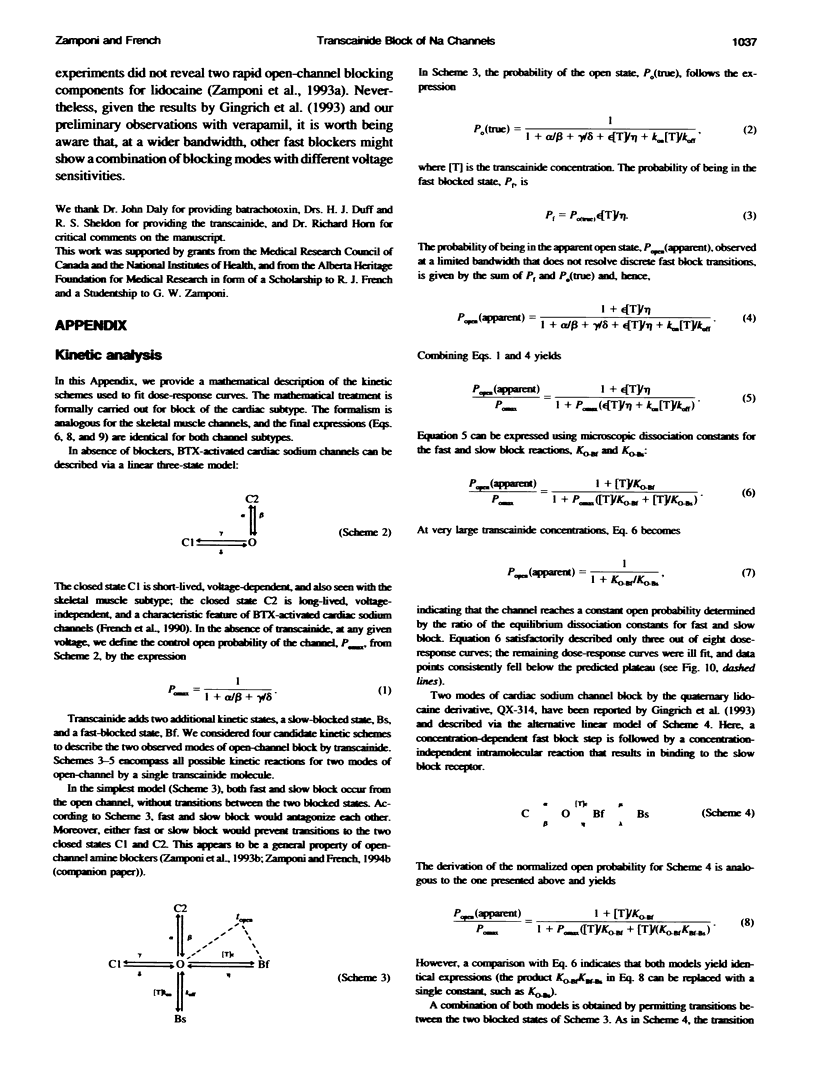
- Gingrich K. J., Beardsley D., Yue D. T. Ultra-deep blockade of Na+ channels by a quaternary ammonium ion: catalysis by a transition-intermediate state? J Physiol. 1993 Nov;471:319–341. doi: 10.1113/jphysiol.1993.sp019903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X. T., Uehara A., Ravindran A., Bryant S. H., Hall S., Moczydlowski E. Kinetic basis for insensitivity to tetrodotoxin and saxitoxin in sodium channels of canine heart and denervated rat skeletal muscle. Biochemistry. 1987 Dec 1;26(24):7546–7556. doi: 10.1021/bi00398a003. [DOI] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977 Apr;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger B. K., Worley J. F., 3rd, French R. J. Single sodium channels from rat brain incorporated into planar lipid bilayer membranes. Nature. 1983 May 12;303(5913):172–175. doi: 10.1038/303172a0. [DOI] [PubMed] [Google Scholar]
- McManus O. B., Blatz A. L., Magleby K. L. Sampling, log binning, fitting, and plotting durations of open and shut intervals from single channels and the effects of noise. Pflugers Arch. 1987 Nov;410(4-5):530–553. doi: 10.1007/BF00586537. [DOI] [PubMed] [Google Scholar]
- Postma S. W., Catterall W. A. Inhibition of binding of [3H]batrachotoxinin A 20-alpha-benzoate to sodium channels by local anesthetics. Mol Pharmacol. 1984 Mar;25(2):219–227. [PubMed] [Google Scholar]
- Quandt F. N., Narahashi T. Modification of single Na+ channels by batrachotoxin. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6732–6736. doi: 10.1073/pnas.79.21.6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J., Sine S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987 Dec;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi G. W., French R. J. Open-channel block by internally applied amines inhibits activation gate closure in batrachotoxin-activated sodium channels. Biophys J. 1994 Sep;67(3):1040–1051. doi: 10.1016/S0006-3495(94)80569-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


