Abstract
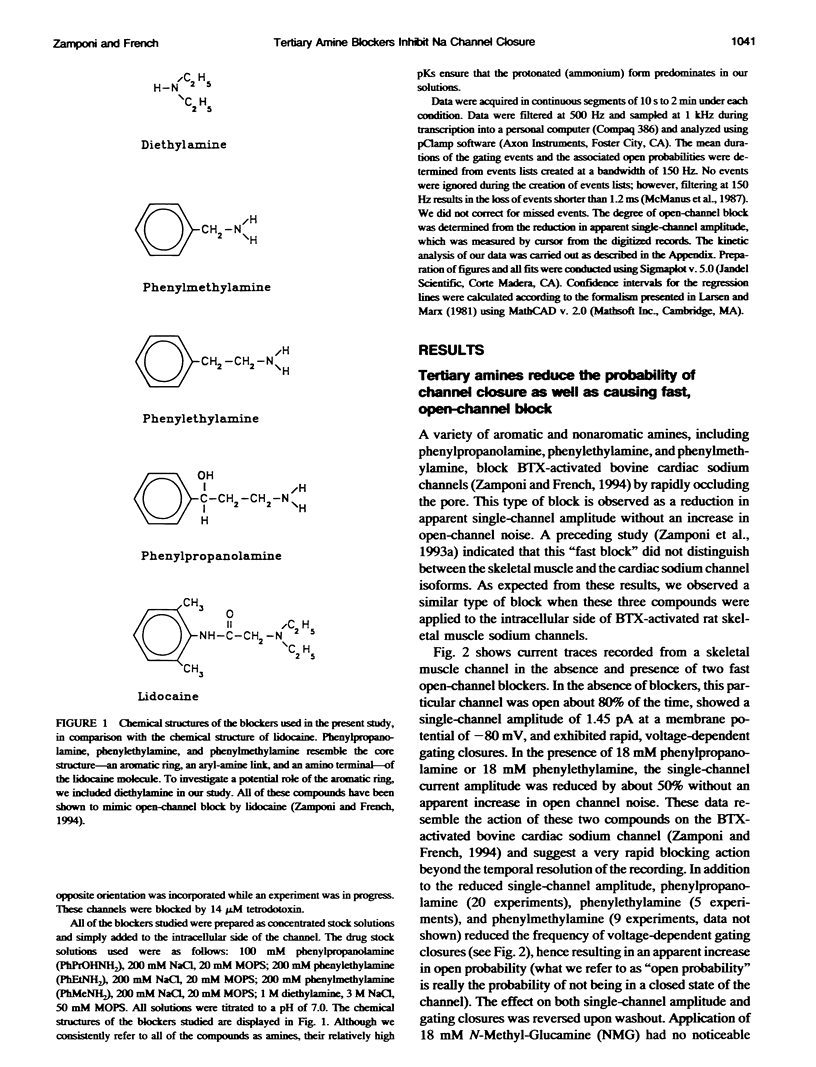
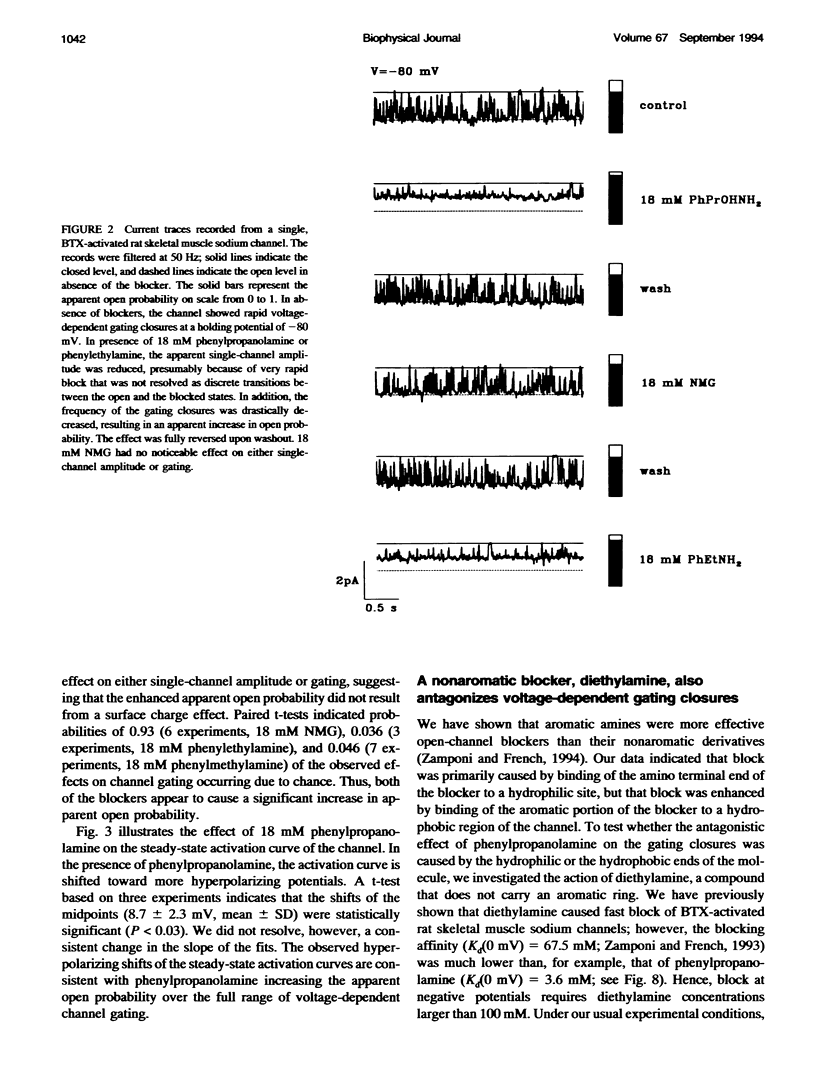
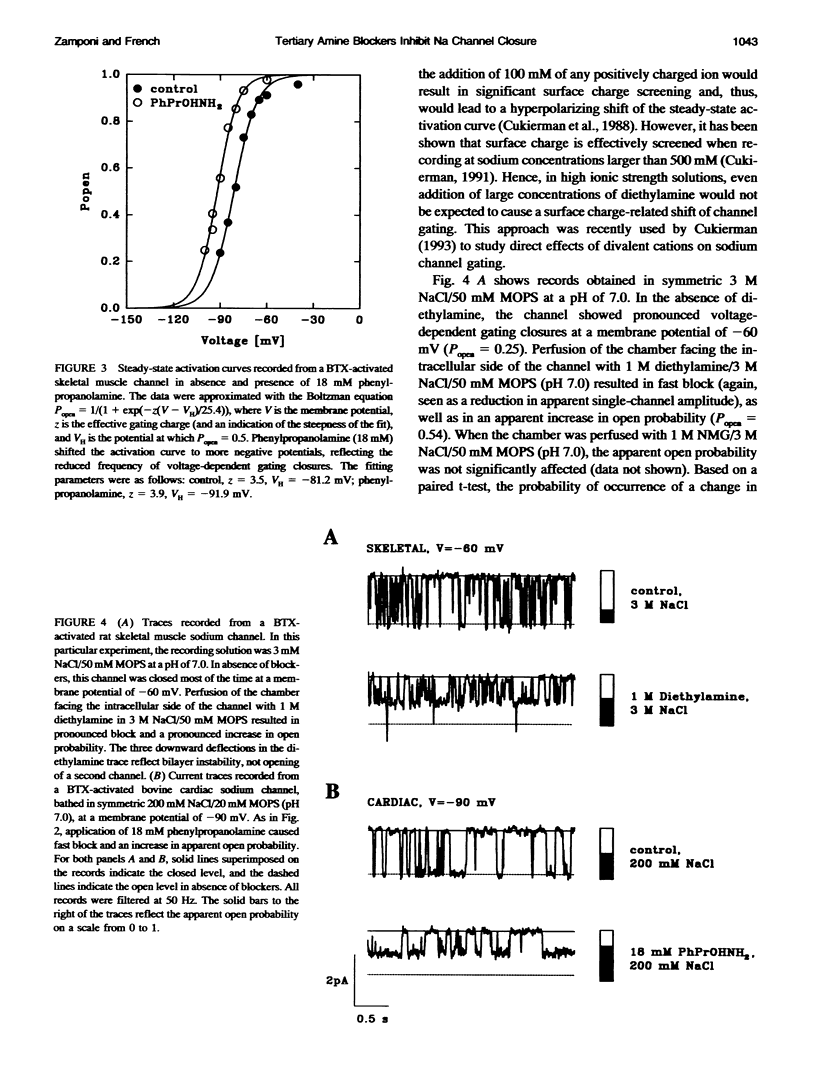
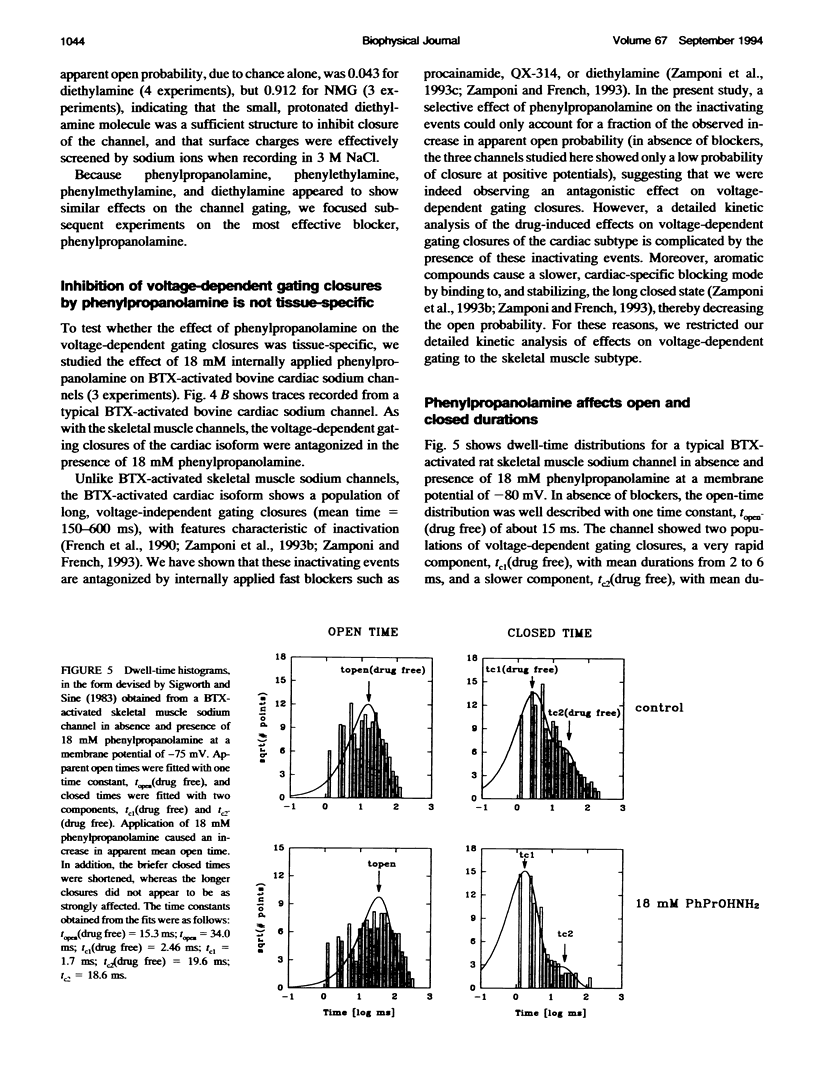
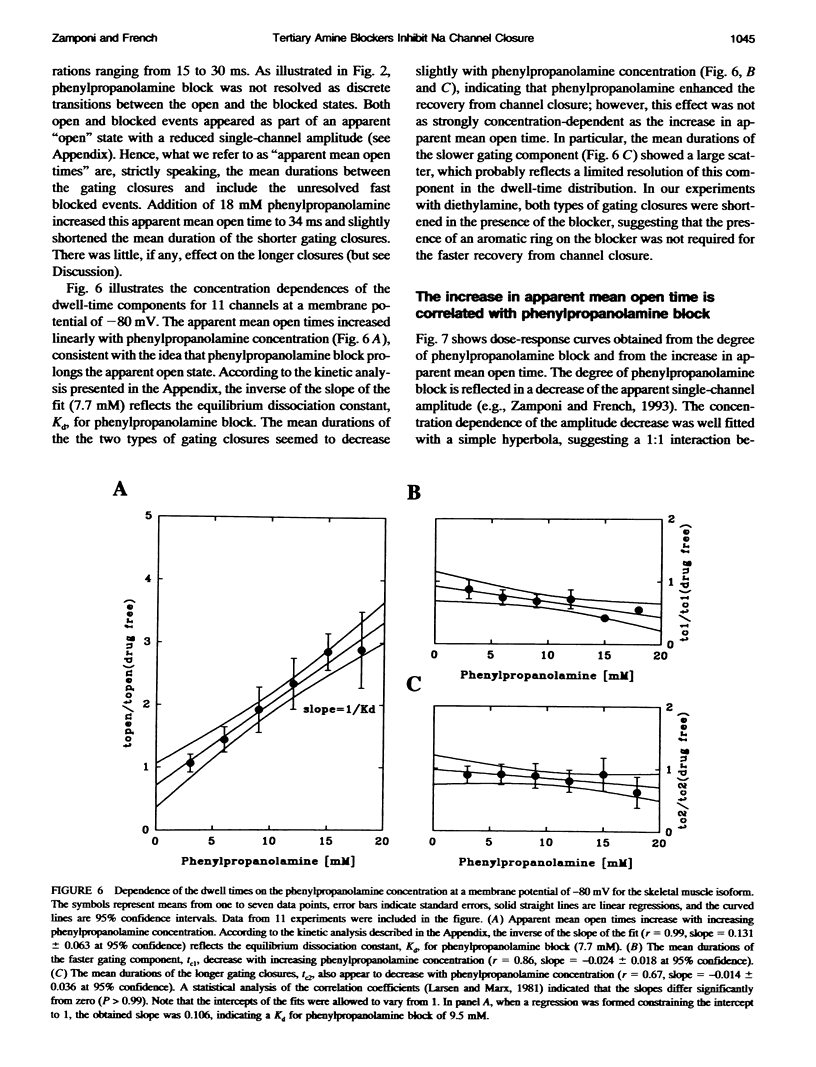
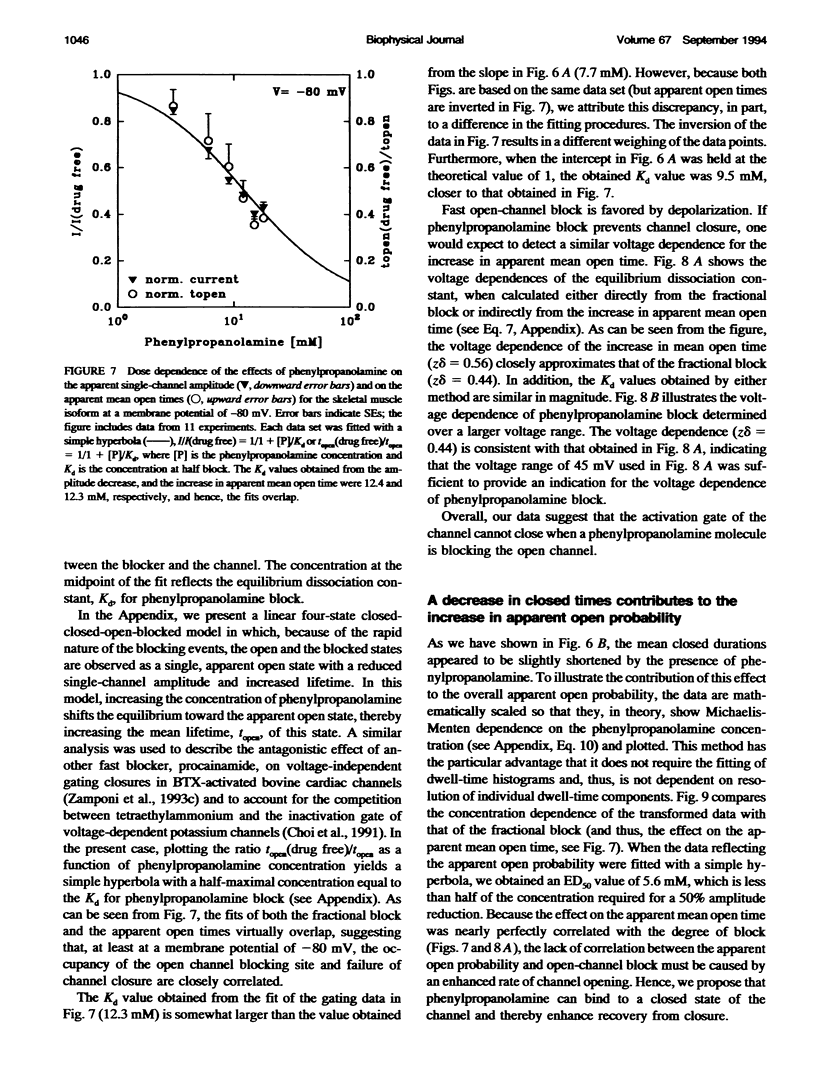
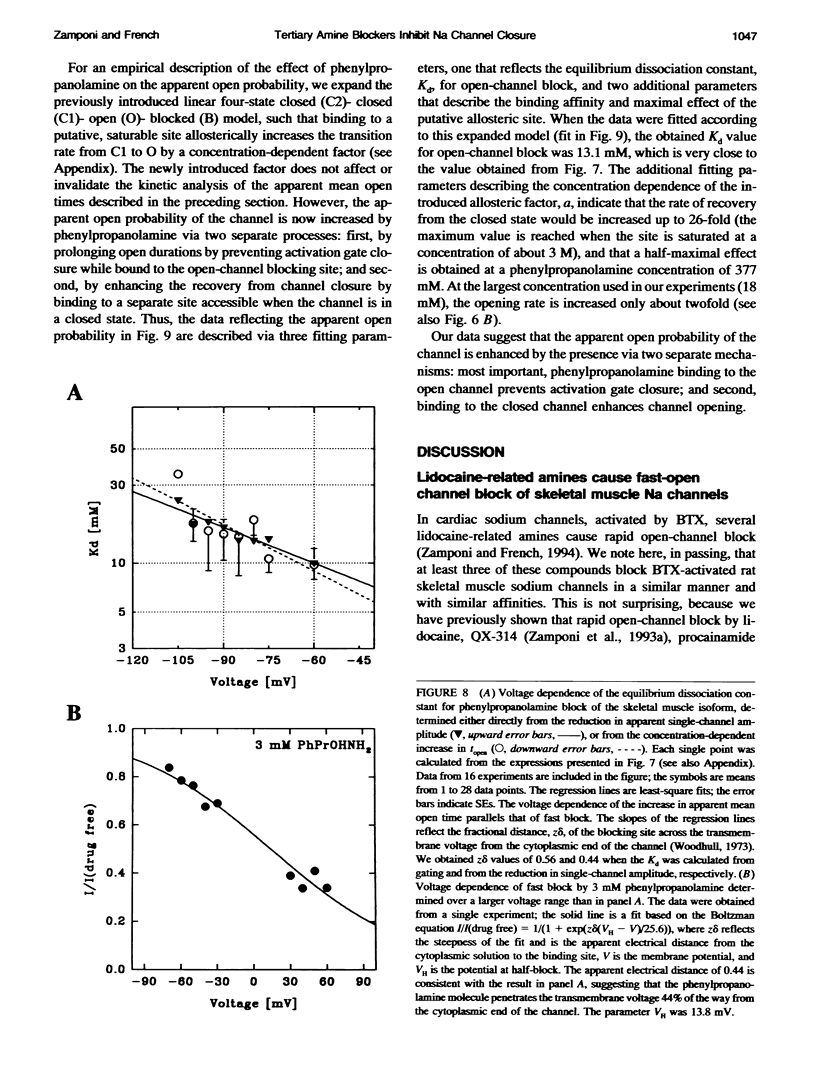
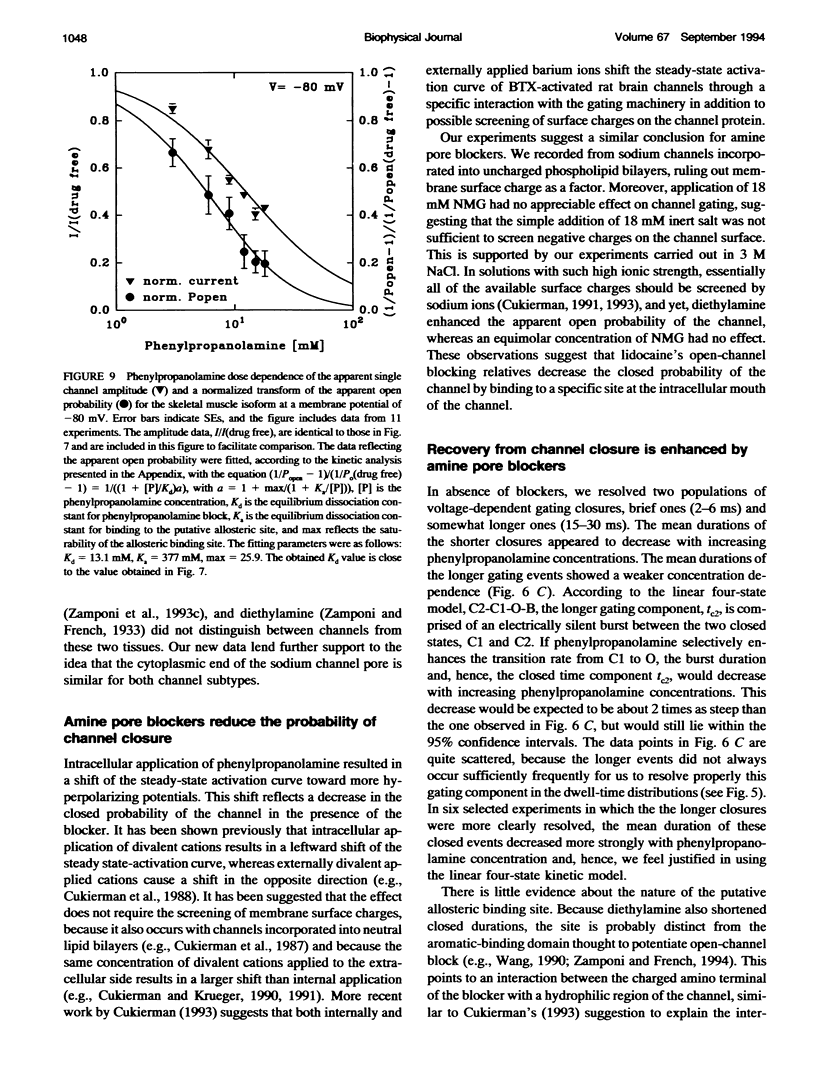
We have studied the action of several pore-blocking amines on voltage-dependent activation gating of batrachotoxin(BTX)-activated sodium channels, from bovine heart and rat skeletal muscle, incorporated into planar lipid bilayers. Although structurally simpler, the compounds studied show general structural features and channel-inhibiting actions that resemble those of lidocaine. When applied to the cytoplasmic end of the channel, these compounds cause a rapid, voltage-dependent, open-channel block seen as a reduction in apparent single-channel amplitude (companion paper). Internal application of phenylpropanolamine, phenylethylamine, phenylmethylamine, and diethylamine, as well as causing open-channel block, reduces the probability of channel closure, producing a shift of the steady-state activation curve toward more hyperpolarizing potentials. These gating effects were observed for both cardiac and skeletal muscle channels and were not evoked by addition of equimolar N-Methyl-D-Glucamine, suggesting a specific interaction of the blockers with the channel rather than a surface charge effect. Kinetic analysis of phenylpropanolamine action on skeletal muscle channels indicated that phenylpropanolamine reduced the closed probability via two separate mechanisms. First, mean closed durations were slightly abbreviated in its presence. Second, and more important, the frequency of the gating closures was reduced. This action was correlated with the degree, and the voltage dependence, of open-channel block, suggesting that the activation gate cannot close while the pore is occluded by the blocker. Such a mechanism might underlie the previously reported immobilization of gating charge associated with local anesthetic block of unmodified sodium channels.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Bezanilla F. Inactivation of the sodium channel. II. Gating current experiments. J Gen Physiol. 1977 Nov;70(5):567–590. doi: 10.1085/jgp.70.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S., Prusak-Sochaczewski E., Zamponi G., Beck-Sickinger A. G., Gordon R. D., French R. J. Action of derivatives of mu-conotoxin GIIIA on sodium channels. Single amino acid substitutions in the toxin separately affect association and dissociation rates. Biochemistry. 1992 Sep 8;31(35):8229–8238. doi: 10.1021/bi00150a016. [DOI] [PubMed] [Google Scholar]
- Cahalan M. D. Local anesthetic block of sodium channels in normal and pronase-treated squid giant axons. Biophys J. 1978 Aug;23(2):285–311. doi: 10.1016/S0006-3495(78)85449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K. L., Aldrich R. W., Yellen G. Tetraethylammonium blockade distinguishes two inactivation mechanisms in voltage-activated K+ channels. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5092–5095. doi: 10.1073/pnas.88.12.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Ogden D. C. Activation of ion channels in the frog end-plate by high concentrations of acetylcholine. J Physiol. 1988 Jan;395:131–159. doi: 10.1113/jphysiol.1988.sp016912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman S., Zinkand W. C., French R. J., Krueger B. K. Effects of membrane surface charge and calcium on the gating of rat brain sodium channels in planar bilayers. J Gen Physiol. 1988 Oct;92(4):431–447. doi: 10.1085/jgp.92.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus O. B., Blatz A. L., Magleby K. L. Sampling, log binning, fitting, and plotting durations of open and shut intervals from single channels and the effects of noise. Pflugers Arch. 1987 Nov;410(4-5):530–553. doi: 10.1007/BF00586537. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J., Sine S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987 Dec;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto D., Yeh J. Z. Kinetics of 9-aminoacridine block of single Na channels. J Gen Physiol. 1984 Sep;84(3):361–377. doi: 10.1085/jgp.84.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. Z., Tanguy J. Na channel activation gate modulates slow recovery from use-dependent block by local anesthetics in squid giant axons. Biophys J. 1985 May;47(5):685–694. doi: 10.1016/S0006-3495(85)83965-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi G. W., Doyle D. D., French R. J. State-dependent block underlies the tissue specificity of lidocaine action on batrachotoxin-activated cardiac sodium channels. Biophys J. 1993 Jul;65(1):91–100. doi: 10.1016/S0006-3495(93)81043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi G. W., French R. J. Dissecting lidocaine action: diethylamide and phenol mimic separate modes of lidocaine block of sodium channels from heart and skeletal muscle. Biophys J. 1993 Dec;65(6):2335–2347. doi: 10.1016/S0006-3495(93)81292-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi G. W., French R. J. Transcainide causes two modes of open-channel block with different voltage sensitivities in batrachotoxin-activated sodium channels. Biophys J. 1994 Sep;67(3):1028–1039. doi: 10.1016/S0006-3495(94)80568-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi G. W., Sui X., Codding P. W., French R. J. Dual actions of procainamide on batrachotoxin-activated sodium channels: open channel block and prevention of inactivation. Biophys J. 1993 Dec;65(6):2324–2334. doi: 10.1016/S0006-3495(93)81291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


