Abstract
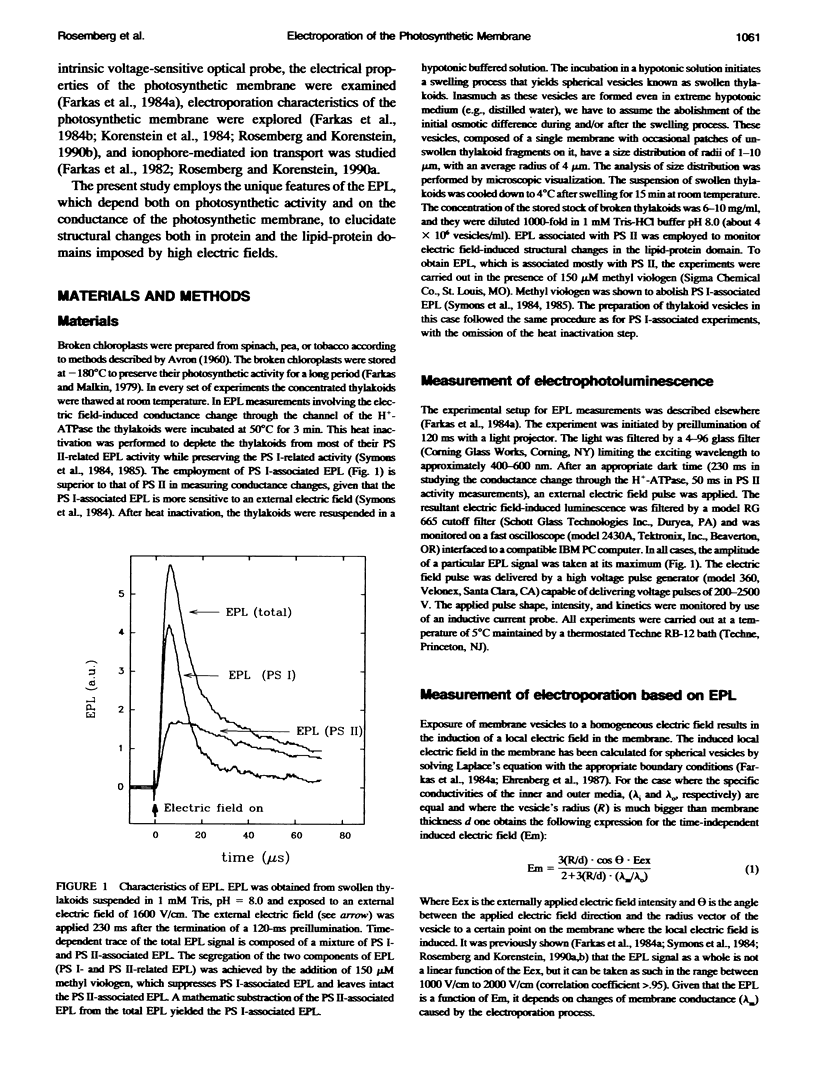
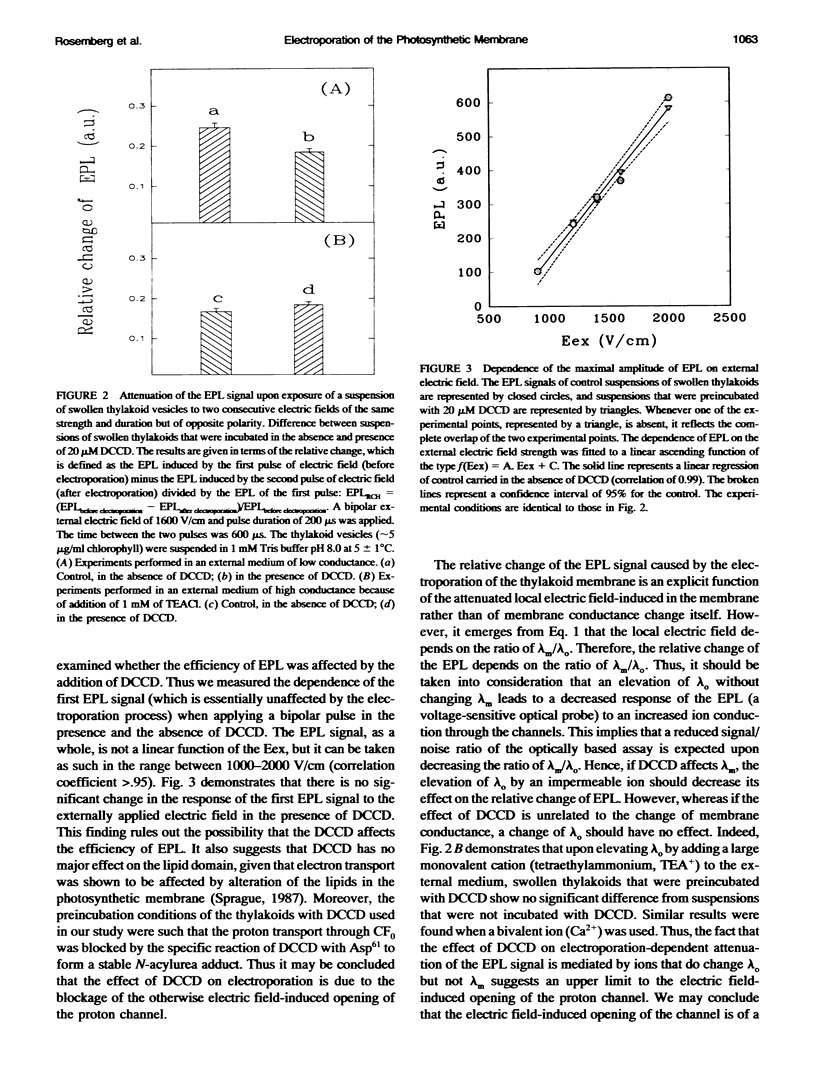
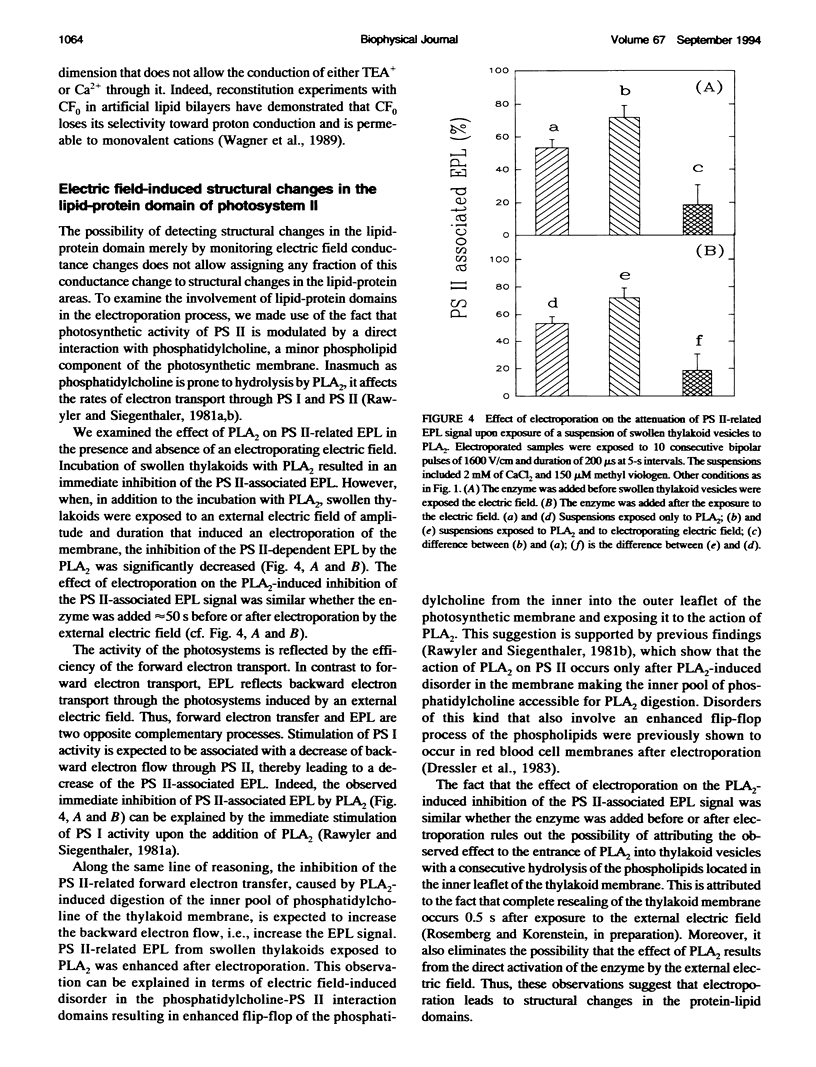
A biological membrane undergoes a reversible permeability increase through structural changes in the lipid domain when exposed to high external electric fields. The present study shows the occurrence of electric field-induced changes in the conductance of the proton channel of the H(+)-ATPase as well as electric field-induced structural changes in the lipid-protein domain of photosystem (PS) II in the photosynthetic membrane. The study was carried out by analyzing the electric field-stimulated delayed luminescence (EPL), which originates from charge recombination in the protein complexes of PS I and II of photosynthetic vesicles. We established that a small fraction of the total electric field-induced conductance change was abolished by N,N'-dicyclohexylcarbodiimide (DCCD), an inhibitor of the H(+)-ATPase. This reversible electric field-induced conductance change has characteristics of a small channel and possesses a lifetime < or = 1 ms. To detect electric field-induced changes in the lipid-protein domains of PS II, we examined the effects of phospholipase A2 (PLA2) on EPL. Higher values of EPL were observed from vesicles that were exposed in the presence of PLA2 to an electroporating electric field than to a nonelectroporating electric field. The effect of the electroporating field was a long-lived one, lasting for a period > or = 2 min. This effect was attributed to long-lived electric field-induced structural changes in the lipid-protein domains of PS II.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M. Photophosphorylation by swiss-chard chloroplasts. Biochim Biophys Acta. 1960 May 20;40:257–272. doi: 10.1016/0006-3002(60)91350-0. [DOI] [PubMed] [Google Scholar]
- Brumfeld V., Miller I. R. Effect of membrane potential on the conformation of bacteriorhodopsin reconstituted in lipid vesicles. Biophys J. 1988 Oct;54(4):747–750. doi: 10.1016/S0006-3495(88)83011-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumfeld V., Miller I. R., Korenstein R. Electric field-induced lateral mobility of photosystem I in the photosynthetic membrane: A study by electrophotoluminescence. Biophys J. 1989 Sep;56(3):607–614. doi: 10.1016/S0006-3495(89)82707-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler V., Schwister K., Haest C. W., Deuticke B. Dielectric breakdown of the erythrocyte membrane enhances transbilayer mobility of phospholipids. Biochim Biophys Acta. 1983 Jul 13;732(1):304–307. doi: 10.1016/0005-2736(83)90216-x. [DOI] [PubMed] [Google Scholar]
- Ellenson J. L., Sauer K. The electrophotoluminescence of chloroplasts. Photochem Photobiol. 1976 Feb;23(2):113–123. doi: 10.1111/j.1751-1097.1976.tb06782.x. [DOI] [PubMed] [Google Scholar]
- Farkas D. L., Korenstein R., Malkin S. Electrophotoluminescence and the electrical properties of the photosynthetic membrane. I. Initial kinetics and the charging capacitance of the membrane. Biophys J. 1984 Feb;45(2):363–373. doi: 10.1016/S0006-3495(84)84160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheriani-Gruszka N., Almog S., Biltonen R. L., Lichtenberg D. Hydrolysis of phosphatidylcholine in phosphatidylcholine-cholate mixtures by porcine pancreatic phospholipase A2. J Biol Chem. 1988 Aug 25;263(24):11808–11813. [PubMed] [Google Scholar]
- Glaser R. W., Leikin S. L., Chernomordik L. V., Pastushenko V. F., Sokirko A. I. Reversible electrical breakdown of lipid bilayers: formation and evolution of pores. Biochim Biophys Acta. 1988 May 24;940(2):275–287. doi: 10.1016/0005-2736(88)90202-7. [DOI] [PubMed] [Google Scholar]
- Gopher A., Blatt Y., Schönfeld M., Okamura M. Y., Feher G., Montal M. The effect of an applied electric field on the charge recombination kinetics in reaction centers reconstituted in planar lipid bilayers. Biophys J. 1985 Aug;48(2):311–320. doi: 10.1016/S0006-3495(85)83784-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamamoto T., Ohno K., Kagawa Y. Net adenosine triphosphate synthesis driven by an external electric field in rat liver mitochondria. J Biochem. 1982 May;91(5):1759–1766. doi: 10.1093/oxfordjournals.jbchem.a133868. [DOI] [PubMed] [Google Scholar]
- Liu D. S., Astumian R. D., Tsong T. Y. Activation of Na+ and K+ pumping modes of (Na,K)-ATPase by an oscillating electric field. J Biol Chem. 1990 May 5;265(13):7260–7267. [PubMed] [Google Scholar]
- Rawyler A., Siegenthaler P. A. Transmembrane distribution of phospholipids and their involvement in electron transport, as revealed by phospholipids A2 treatment of spinach thylakoids. Biochim Biophys Acta. 1981 Apr 13;635(2):348–358. doi: 10.1016/0005-2728(81)90033-5. [DOI] [PubMed] [Google Scholar]
- Rephaeli A., Richards D. E., Karlish S. J. Electrical potential accelerates the E1P(Na)----E2P conformational transition of (Na,K)-ATPase in reconstituted vesicles. J Biol Chem. 1986 Sep 25;261(27):12437–12440. [PubMed] [Google Scholar]
- Rosemberg Y., Korenstein R. A novel method for measuring membrane conductance changes by a voltage-sensitive optical probe. FEBS Lett. 1990 Apr 9;263(1):155–158. doi: 10.1016/0014-5793(90)80727-z. [DOI] [PubMed] [Google Scholar]
- Rosemberg Y., Rozen P., Malkin S., Korenstein R. Spatial and temporal electroselection patterns in electric field stimulation of polarized luminescence from photosynthetic membrane vesicles. Biophys J. 1992 Jun;61(6):1585–1594. doi: 10.1016/S0006-3495(92)81962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpersu E. H., Tsong T. Y. Activation of electrogenic Rb+ transport of (Na,K)-ATPase by an electric field. J Biol Chem. 1984 Jun 10;259(11):7155–7162. [PubMed] [Google Scholar]
- Serpersu E. H., Tsong T. Y. Stimulation of a ouabain-sensitive Rb+ uptake in human erthrocytes with an external electric field. J Membr Biol. 1983;74(3):191–201. doi: 10.1007/BF02332123. [DOI] [PubMed] [Google Scholar]
- Sigrist-Nelson K., Sigrist H., Azzi A. Characterization of the dicyclohexylcarbodiimide-binding protein isolated from chloroplast membranes. Eur J Biochem. 1978 Dec 1;92(1):9–14. doi: 10.1111/j.1432-1033.1978.tb12717.x. [DOI] [PubMed] [Google Scholar]
- Sprague S. G. Structural and functional consequences of galactolipids on thylakoid membrane organization. J Bioenerg Biomembr. 1987 Dec;19(6):691–703. doi: 10.1007/BF00762303. [DOI] [PubMed] [Google Scholar]
- Sugar I. P., Förster W., Neumann E. Model of cell electrofusion. Membrane electroporation, pore coalescence and percolation. Biophys Chem. 1987 May 9;26(2-3):321–335. doi: 10.1016/0301-4622(87)80033-9. [DOI] [PubMed] [Google Scholar]
- Sugar I. P., Neumann E. Stochastic model for electric field-induced membrane pores. Electroporation. Biophys Chem. 1984 May;19(3):211–225. doi: 10.1016/0301-4622(84)87003-9. [DOI] [PubMed] [Google Scholar]
- Teissie J., Tsong T. Y. Evidence of voltage-induced channel opening in Na/K ATPase of human erythrocyte membrane. J Membr Biol. 1980 Jul 15;55(2):133–140. doi: 10.1007/BF01871155. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y., Astumian R. D. Electroconformational coupling and membrane protein function. Prog Biophys Mol Biol. 1987;50(1):1–45. doi: 10.1016/0079-6107(87)90002-2. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y. Voltage modulation of membrane permeability and energy utilization in cells. Biosci Rep. 1983 Jun;3(6):487–505. doi: 10.1007/BF01120693. [DOI] [PubMed] [Google Scholar]
- Wagner R., Apley E. C., Hanke W. Single channel H+ currents through reconstituted chloroplast ATP synthase CF0-CF1. EMBO J. 1989 Oct;8(10):2827–2834. doi: 10.1002/j.1460-2075.1989.tb08429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


