Abstract
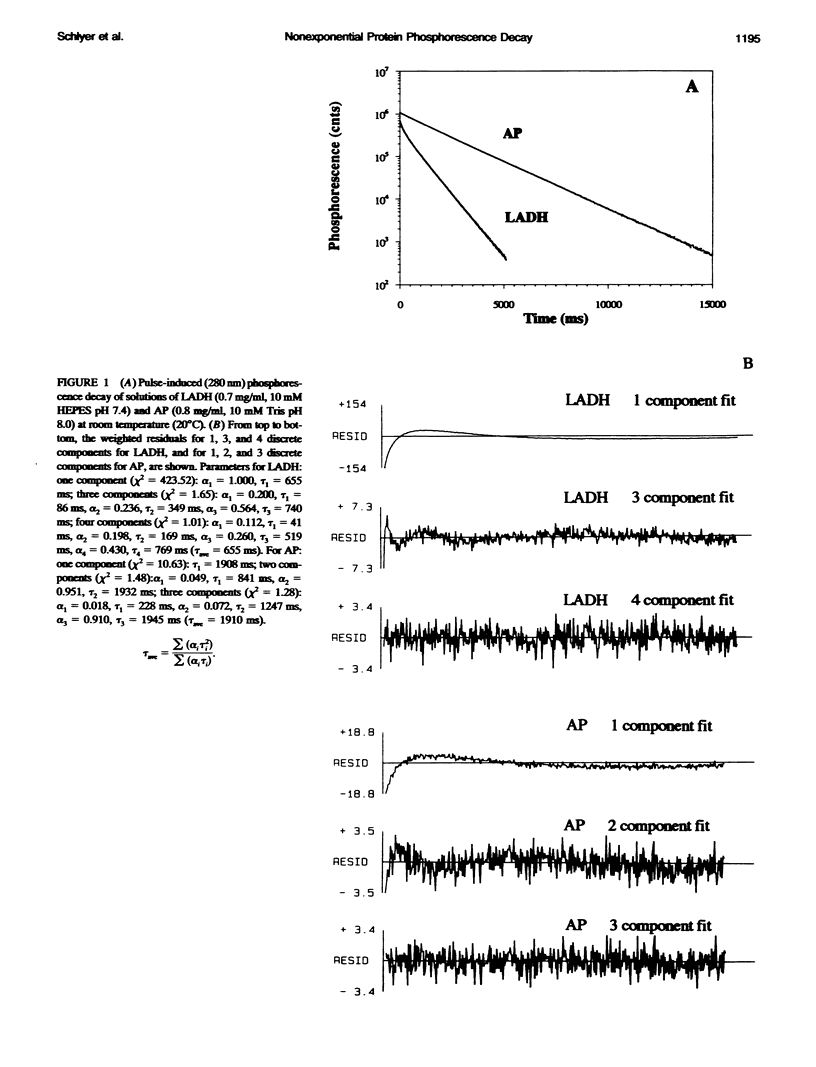
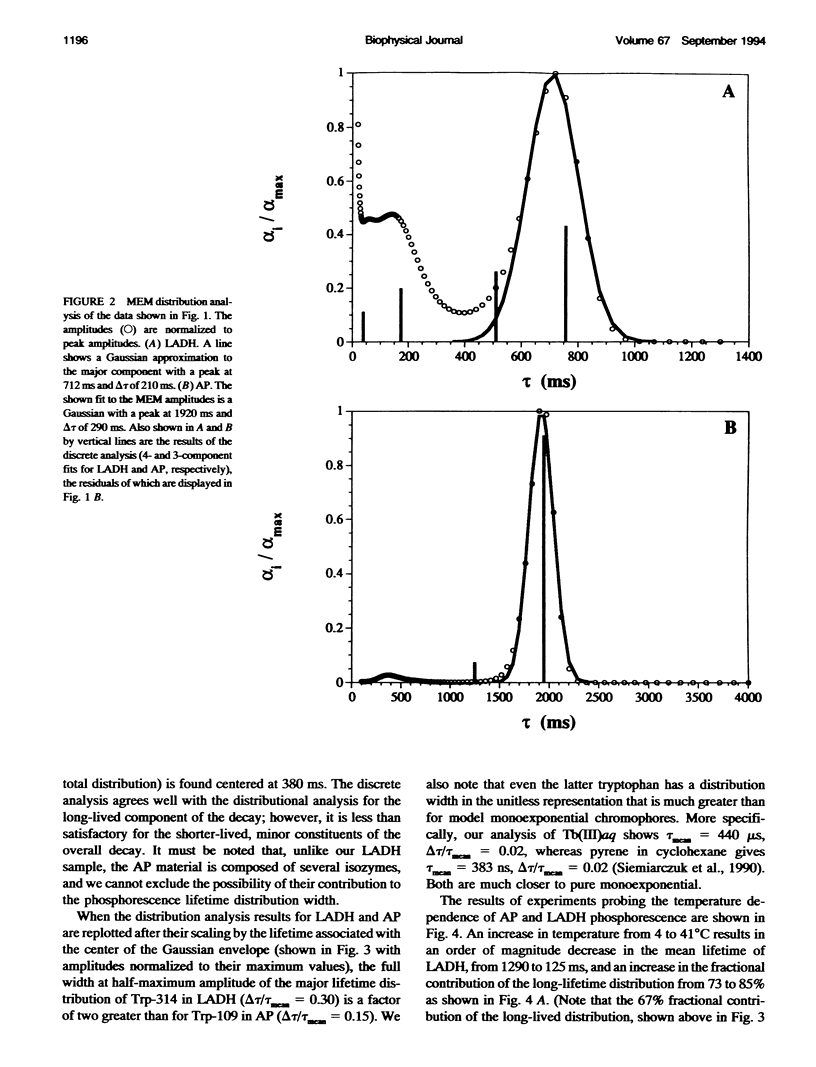
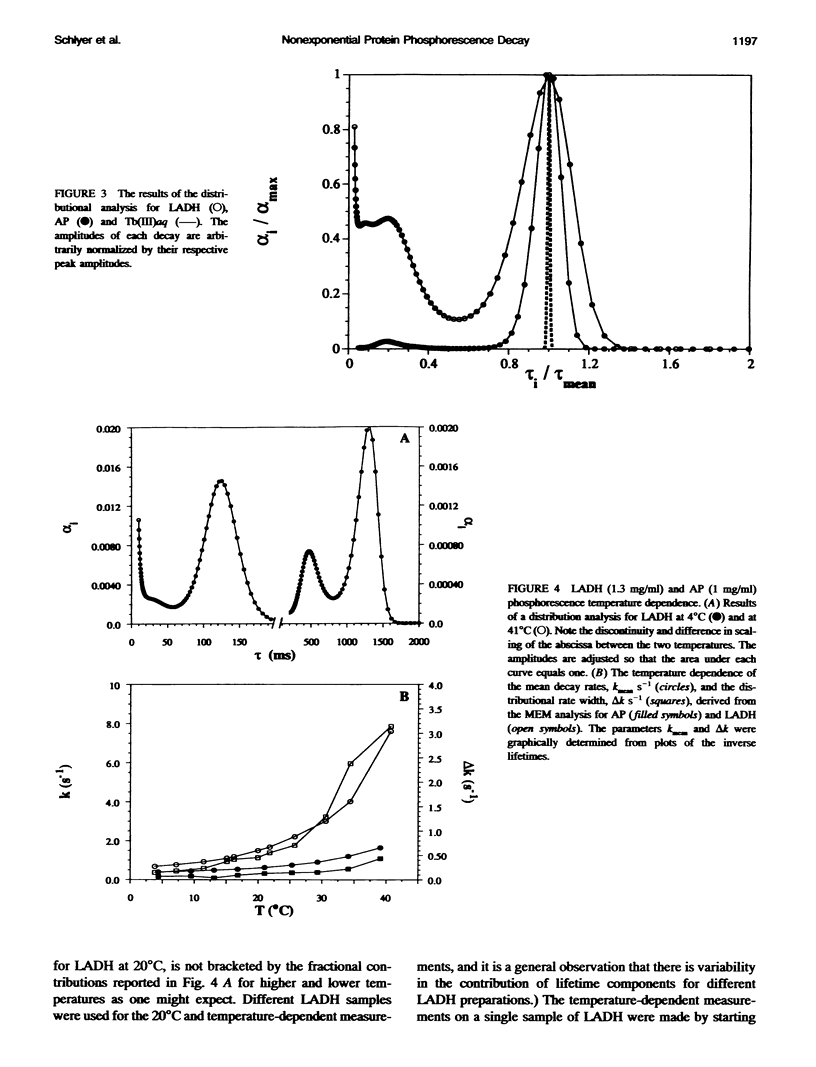
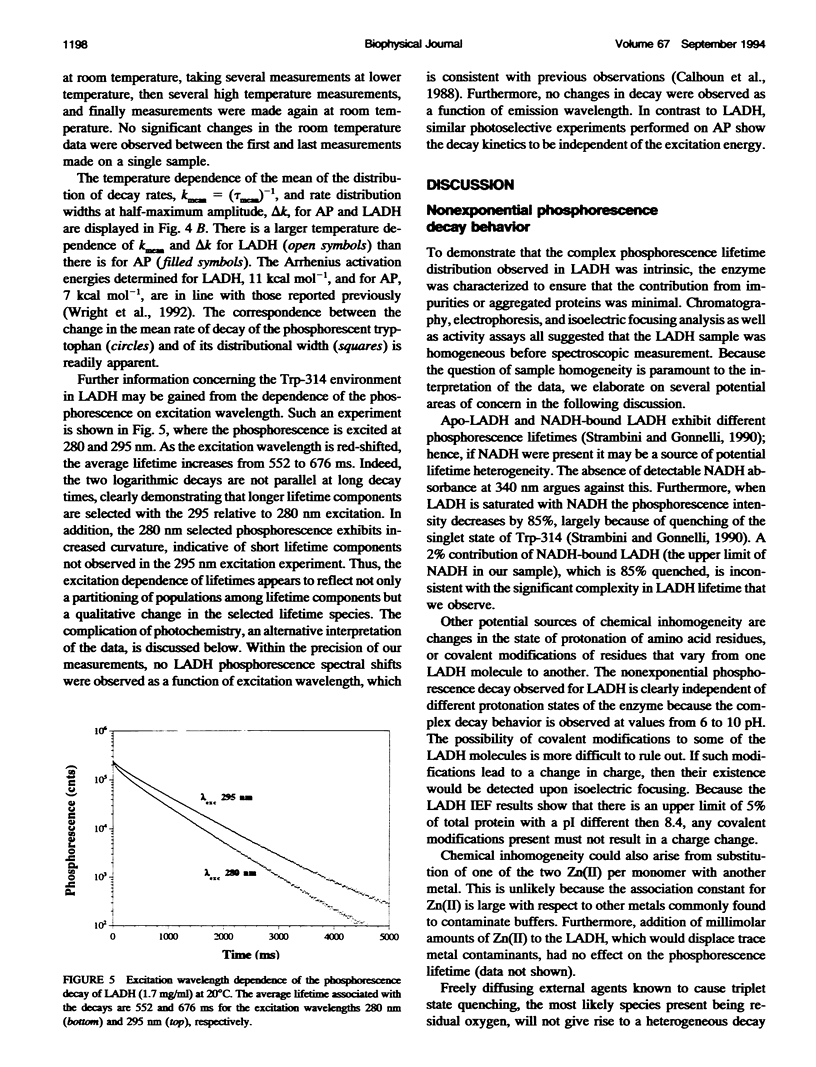
The single room temperature phosphorescent (RTP) residue of horse liver alcohol dehydrogenase (LADH). Trp-314, and of alkaline phosphatase (AP), Trp-109, show nonexponential phosphorescence decays when the data are collected to a high degree of precision. Using the maximum entropy method (MEM) for the analysis of these decays, it is shown that AP phosphorescence decay is dominated by a single Gaussian distribution, whereas for LADH the data reveal two amplitude packets. The lifetime-normalized width of the MEM distribution for both proteins is larger than that obtained for model monoexponential chromophores (e.g., terbium in water and pyrene in cyclohexane). Experiments show that the nonexponential decay is fundamental; i.e., an intrinsic property of the pure protein. Because phosphorescence reports on the state of the emitting chromophore, such nonexponential behavior could be caused by the presence of excited state reactions. However, it is also well known that the phosphorescence lifetime of a tryptophan residue is strongly dependent on the local flexibility around the indole moiety. Hence, the nonexponential phosphorescence decay may also be caused by the presence of at least two states of different local rigidity (in the vicinity of the phosphorescing tryptophan) corresponding to different ground state conformers. The observation that in the chemically homogeneous LADH sample the phosphorescence decay kinetics depends on the excitation wavelength further supports this latter interpretation. This dependence is caused by the wavelength-selective excitation of Trp-314 in a subensemble of LADH molecules with differing hydrophobic and rigid environments. With this interpretation, the data show that interconversion of these states occurs on a time scale long compared with the phosphorescence decay (0.1-1.0 s). Further experiments reveal that with increasing temperature the distributed phosphorescence decay rates for both AP and LADH broaden, thus indicating that either 1) the number of conformational states populated at higher temperature increases or 2) the temperature differentially affects individual conformer states. The nature of the observed heterogeneous triplet state kinetics and their relationship to aspects of protein dynamics are discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bent D. V., Hayon E. Excited state chemistry of aromatic amino acids and related peptides. III. Tryptophan. J Am Chem Soc. 1975 May 14;97(10):2612–2619. doi: 10.1021/ja00843a004. [DOI] [PubMed] [Google Scholar]
- Berger J. W., Vanderkooi J. M. Characterization of lens alpha-crystallin tryptophan microenvironments by room temperature phosphorescence spectroscopy. Biochemistry. 1989 Jun 27;28(13):5501–5508. doi: 10.1021/bi00439a027. [DOI] [PubMed] [Google Scholar]
- Calhoun D. B., Englander S. W., Wright W. W., Vanderkooi J. M. Quenching of room temperature protein phosphorescence by added small molecules. Biochemistry. 1988 Nov 1;27(22):8466–8474. doi: 10.1021/bi00422a026. [DOI] [PubMed] [Google Scholar]
- Calhoun D. B., Vanderkooi J. M., Woodrow G. V., 3rd, Englander S. W. Penetration of dioxygen into proteins studied by quenching of phosphorescence and fluorescence. Biochemistry. 1983 Mar 29;22(7):1526–1532. doi: 10.1021/bi00276a002. [DOI] [PubMed] [Google Scholar]
- Cioni P., Puntoni A., Strambini G. B. Tryptophan phosphorescence as a monitor of the solution structure of phosphoglycerate kinase from yeast. Biophys Chem. 1993 Feb;46(1):47–55. doi: 10.1016/0301-4622(93)87006-i. [DOI] [PubMed] [Google Scholar]
- Coleman P. L., Weiner H. Interaction of chloride ion with horse liver alcohol dehydrogenase-reduced nicotinamide adenine dinucleotide complexes. Biochemistry. 1973 Apr 24;12(9):1702–1705. doi: 10.1021/bi00733a006. [DOI] [PubMed] [Google Scholar]
- Ehrig T., Muhoberac B. B., Hurley T. D., Bosron W. F. Tryptophan fluorescence quenching by alkaline pH and ternary complex formation in human beta 1 beta 1 and horse EE alcohol dehydrogenases. FEBS Lett. 1992 Apr 6;300(3):283–285. doi: 10.1016/0014-5793(92)80864-d. [DOI] [PubMed] [Google Scholar]
- Englander S. W., Calhoun D. B., Englander J. J. Biochemistry without oxygen. Anal Biochem. 1987 Mar;161(2):300–306. doi: 10.1016/0003-2697(87)90454-4. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Parak F., Young R. D. Conformational substates in proteins. Annu Rev Biophys Biophys Chem. 1988;17:451–479. doi: 10.1146/annurev.bb.17.060188.002315. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Sligar S. G., Wolynes P. G. The energy landscapes and motions of proteins. Science. 1991 Dec 13;254(5038):1598–1603. doi: 10.1126/science.1749933. [DOI] [PubMed] [Google Scholar]
- Gabellieri E., Strambini G. B., Gualtieri P. Tryptophan phosphorescence and the conformation of liver alcohol dehydrogenase in solution and in the crystalline state. Biophys Chem. 1988 May;30(1):61–67. doi: 10.1016/0301-4622(88)85004-x. [DOI] [PubMed] [Google Scholar]
- Ghiron C., Bazin M., Santus R. Determination of the dioxygen quenching constant for protein and model indole triplets. Biochim Biophys Acta. 1988 Nov 23;957(2):207–216. doi: 10.1016/0167-4838(88)90274-9. [DOI] [PubMed] [Google Scholar]
- Gonnelli M., Strambini G. B. The rate of equine liver alcohol dehydrogenase denaturation by urea. Dependence on temperature and denaturant concentration. Biophys Chem. 1986 Jul;24(2):161–167. doi: 10.1016/0301-4622(86)80009-6. [DOI] [PubMed] [Google Scholar]
- Gregory R. B., Lumry R. Hydrogen-exchange evidence for distinct structural classes in globular proteins. Biopolymers. 1985 Feb;24(2):301–326. doi: 10.1002/bip.360240203. [DOI] [PubMed] [Google Scholar]
- Johansson J., Vallee B. L., Jörnvall H. Closely related isozymes of alcohol dehydrogenase. Carboxymethylation: gamma 1 gamma 1 differs widely from both beta 1 beta 1 and its equine equivalence EE. FEBS Lett. 1991 Feb 11;279(1):119–122. doi: 10.1016/0014-5793(91)80265-5. [DOI] [PubMed] [Google Scholar]
- Koloczek H., Vanderkooi J. M. Domain structural flexibility in rhodanese examined by quenching of a phosphorescent probe. Biochim Biophys Acta. 1987 Nov 26;916(2):236–244. doi: 10.1016/0167-4838(87)90114-2. [DOI] [PubMed] [Google Scholar]
- Li Z., Galley W. C. Evidence for ligand-induced conformational changes in proteins from phosphorescence spectroscopy. Biophys J. 1989 Aug;56(2):353–360. doi: 10.1016/S0006-3495(89)82681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey A. K., Brochon J. C. Analyzing the distribution of decay constants in pulse-fluorimetry using the maximum entropy method. Biophys J. 1987 Nov;52(5):693–706. doi: 10.1016/S0006-3495(87)83264-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp S., Vanderkooi J. M. Tryptophan phosphorescence at room temperature as a tool to study protein structure and dynamics. Photochem Photobiol. 1989 Jun;49(6):775–784. doi: 10.1111/j.1751-1097.1989.tb05576.x. [DOI] [PubMed] [Google Scholar]
- Saviotti M. L., Galley W. C. Room temperature phosphorescence and the dynamic aspects of protein structure. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4154–4158. doi: 10.1073/pnas.71.10.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strambini G. B., Cioni P., Peracchi A., Mozzarelli A. Conformational changes and subunit communication in tryptophan synthase: effect of substrates and substrate analogs. Biochemistry. 1992 Aug 25;31(33):7535–7542. doi: 10.1021/bi00148a014. [DOI] [PubMed] [Google Scholar]
- Strambini G. B. Singular oxygen effects on the room-temperature phosphorescence of alcohol dehydrogenase from horse liver. Biophys J. 1983 Jul;43(1):127–130. doi: 10.1016/S0006-3495(83)84331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderkooi J. M., Englander S. W., Papp S., Wright W. W., Owen C. S. Long-range electron exchange measured in proteins by quenching of tryptophan phosphorescence. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5099–5103. doi: 10.1073/pnas.87.13.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]


