Abstract
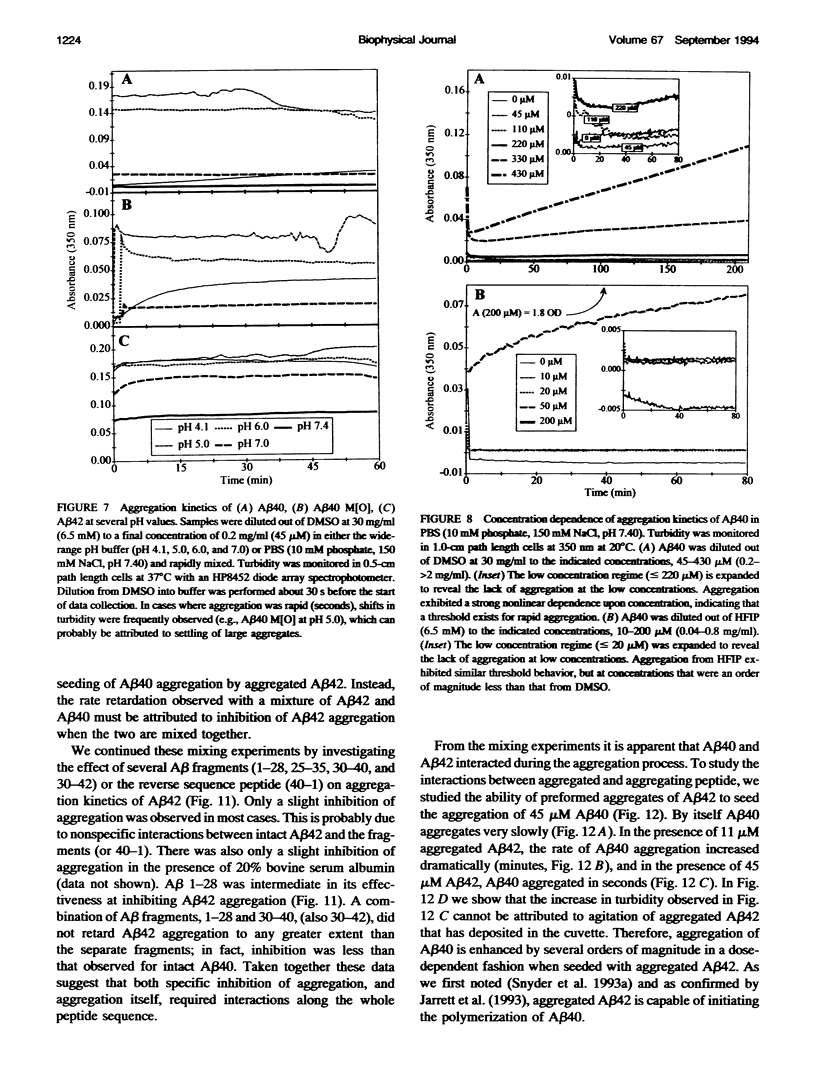
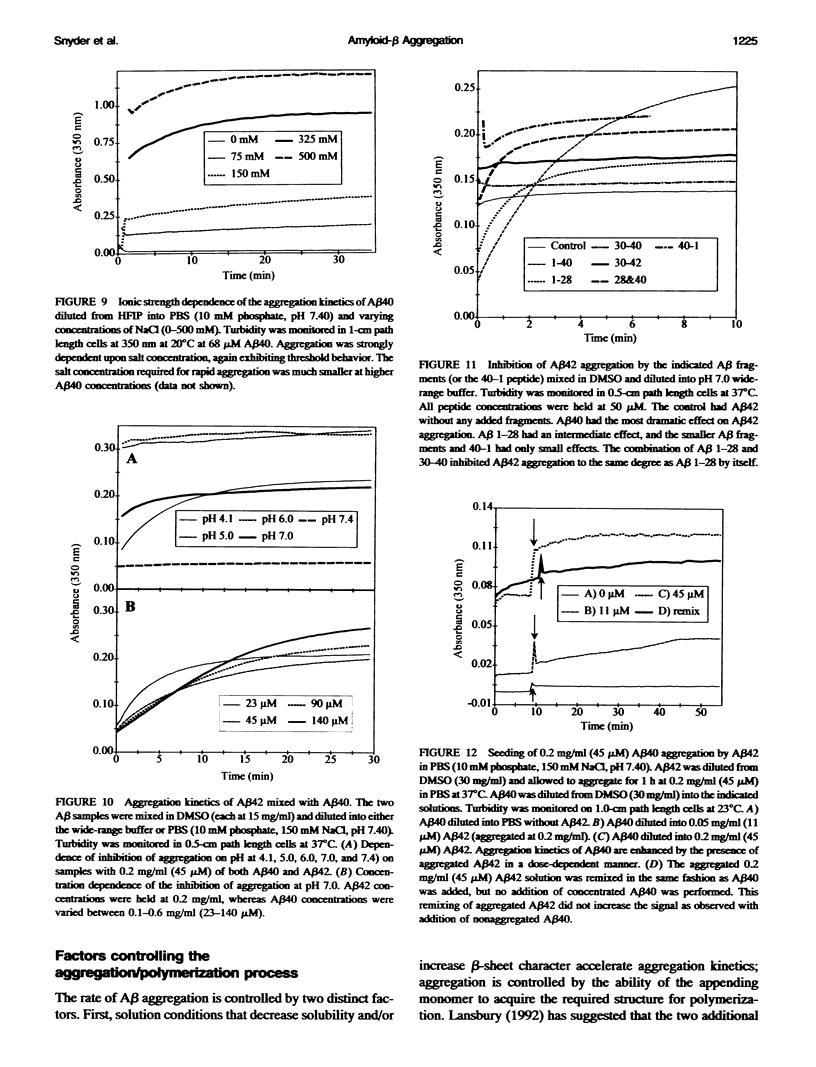
One of the clinical manifestations of Alzheimer's disease is the deposition of the 39-43 residue amyloid-beta (A beta) peptide in aggregated fibrils in senile plaques. Characterization of the aggregation behavior of A beta is one of the critical issues in understanding the role of A beta in the disease process. Using solution hydrodynamics, A beta was observed to form three types of species in phosphate-buffered saline: insoluble aggregates with sedimentation coefficients of approximately 50,000 S and molecular masses of approximately 10(9) Da, "soluble aggregates" with sedimentation coefficients of approximately 30 S and masses of approximately 10(6) Da, and monomer. When starting from monomer, the aggregation kinetics of A beta 1-40 (A beta 40) and A beta 1-42 (A beta 42), alone and in combination, reveal large differences in the tendency of these peptides to aggregate as a function of pH and other solution conditions. At pH 4.1 and 7.0-7.4, aggregation is significantly slower than at pH 5 and 6. Under all conditions, aggregation of the longer A beta 42 was more rapid than A beta 40. Oxidation of Met-35 to the sulfoxide in A beta 40 enhances the aggregation rate over that of the nonoxidized peptide. Aggregation was found to be dependent upon temperature and to be strongly dependent on peptide concentration and ionic strength, indicating that aggregation is driven by a hydrophobic effect. When A beta 40 and A beta 42 are mixed together, A beta 40 retards the aggregation of A beta 42 in a concentration-dependent manner. Shorter fragments have a decreasing ability to interfere with A beta 42 aggregation. Conversely, the rate of aggregation of A beta 40 can be significantly enhanced by seeding slow aggregating solutions with preformed aggregates of A beta 42. Taken together, the inhibition of A beta 42 aggregation by A beta 40, the seeding of A beta 40 aggregation by A beta 42 aggregates, and the chemical oxidation of A beta 40 suggest that the relative abundance and rates of production of different-length A beta and its exposure to radical damage may be factors in the accumulation of A beta in plaques in vivo.
Full text
PDF



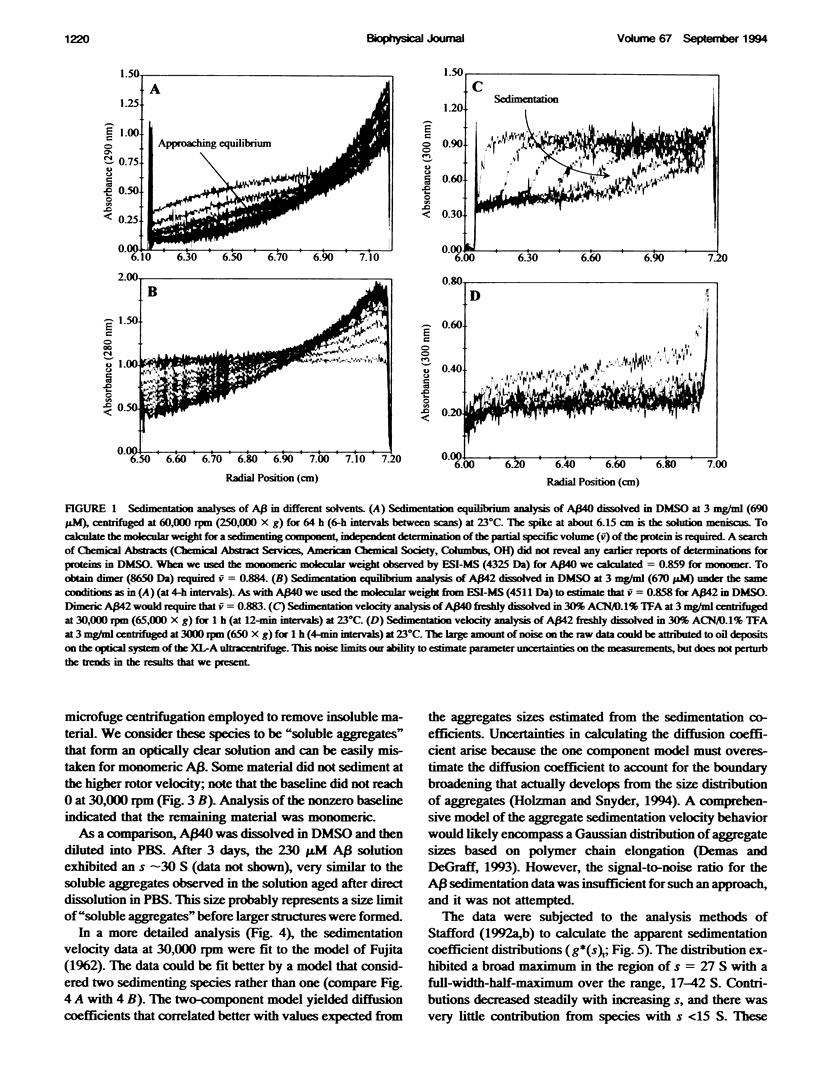
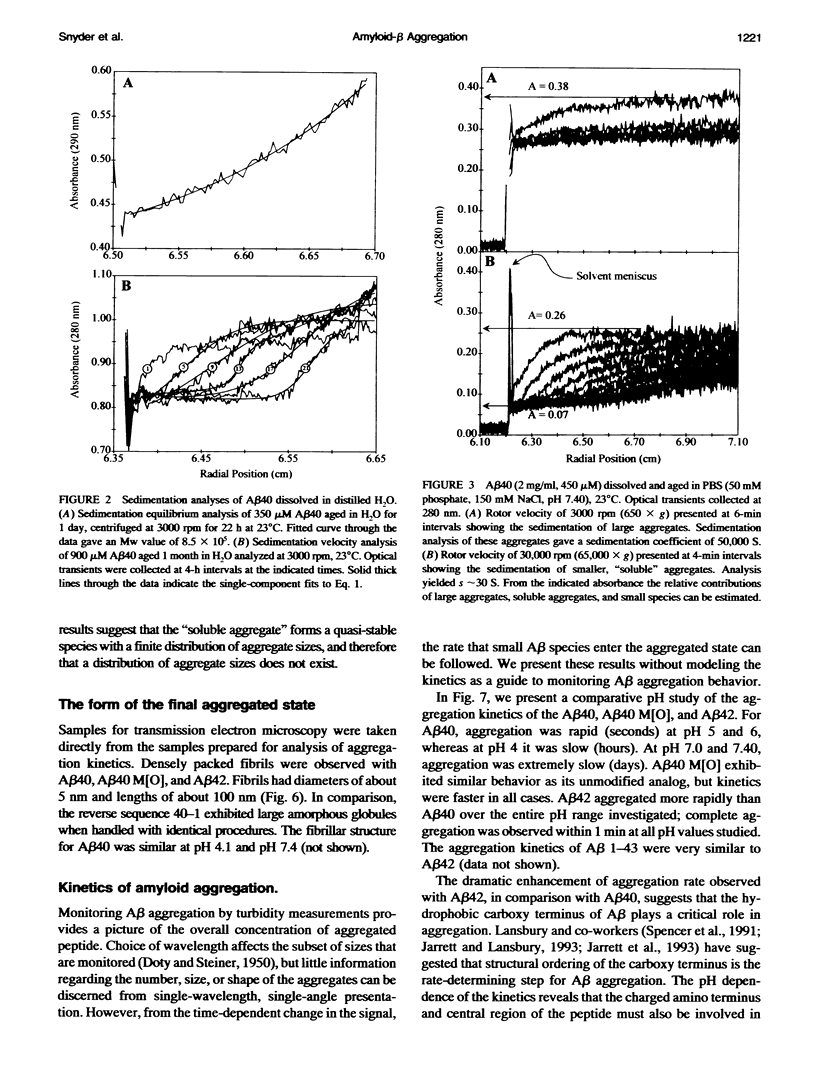
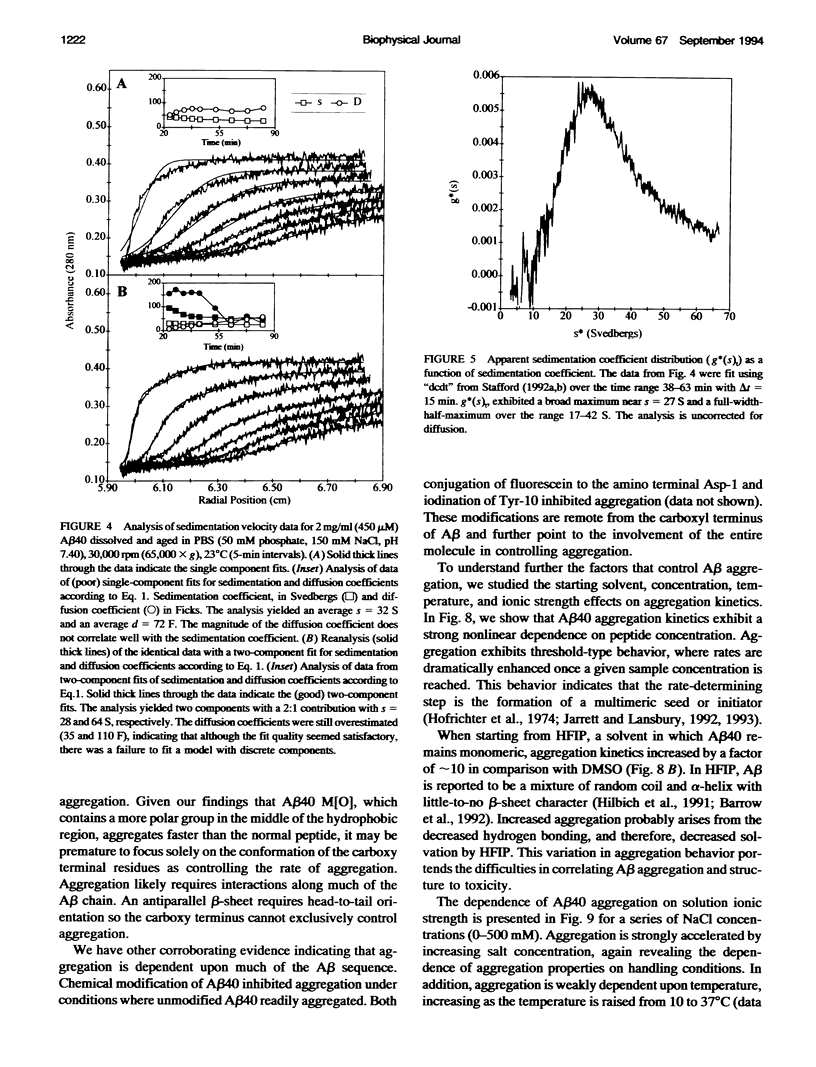




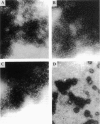
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreu J. M., Timasheff S. N. The measurement of cooperative protein self-assembly by turbidity and other techniques. Methods Enzymol. 1986;130:47–59. doi: 10.1016/0076-6879(86)30007-7. [DOI] [PubMed] [Google Scholar]
- Barrow C. J., Yasuda A., Kenny P. T., Zagorski M. G. Solution conformations and aggregational properties of synthetic amyloid beta-peptides of Alzheimer's disease. Analysis of circular dichroism spectra. J Mol Biol. 1992 Jun 20;225(4):1075–1093. doi: 10.1016/0022-2836(92)90106-t. [DOI] [PubMed] [Google Scholar]
- Behl C., Davis J., Cole G. M., Schubert D. Vitamin E protects nerve cells from amyloid beta protein toxicity. Biochem Biophys Res Commun. 1992 Jul 31;186(2):944–950. doi: 10.1016/0006-291x(92)90837-b. [DOI] [PubMed] [Google Scholar]
- Caputo C. B., Fraser P. E., Sobel I. E., Kirschner D. A. Amyloid-like properties of a synthetic peptide corresponding to the carboxy terminus of beta-amyloid protein precursor. Arch Biochem Biophys. 1992 Jan;292(1):199–205. doi: 10.1016/0003-9861(92)90068-8. [DOI] [PubMed] [Google Scholar]
- Caputo C. B., Sobel I. R., Sygowski L. A., Lampe R. A., Spreen R. C. The influence of amino acid sequence on the fibrillogenicity and amyloidogenicity of the carboxy-terminus of beta-amyloid precursor protein. Arch Biochem Biophys. 1993 Nov 1;306(2):321–330. doi: 10.1006/abbi.1993.1518. [DOI] [PubMed] [Google Scholar]
- Fraser P. E., Nguyen J. T., Inouye H., Surewicz W. K., Selkoe D. J., Podlisny M. B., Kirschner D. A. Fibril formation by primate, rodent, and Dutch-hemorrhagic analogues of Alzheimer amyloid beta-protein. Biochemistry. 1992 Nov 10;31(44):10716–10723. doi: 10.1021/bi00159a011. [DOI] [PubMed] [Google Scholar]
- Fraser P. E., Nguyen J. T., Surewicz W. K., Kirschner D. A. pH-dependent structural transitions of Alzheimer amyloid peptides. Biophys J. 1991 Nov;60(5):1190–1201. doi: 10.1016/S0006-3495(91)82154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano T., Pan J. B., Monteggia L. M., Holzman T. F., Snyder S. W., Krafft G., Ghanbari H., Kowall N. W. Similarities between beta amyloid peptides 1-40 and 40-1: effects on aggregation, toxicity in vitro, and injection in young and aged rats. Exp Neurol. 1994 Feb;125(2):175–182. doi: 10.1006/exnr.1994.1022. [DOI] [PubMed] [Google Scholar]
- Glenner G. G. Amyloid deposits and amyloidosis. The beta-fibrilloses (first of two parts). N Engl J Med. 1980 Jun 5;302(23):1283–1292. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- Goldgaber D., Lerman M. I., McBride O. W., Saffiotti U., Gajdusek D. C. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer's disease. Science. 1987 Feb 20;235(4791):877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- Gorevic P. D., Munoz P. C., Casey T. T., DiRaimondo C. R., Stone W. J., Prelli F. C., Rodrigues M. M., Poulik M. D., Frangione B. Polymerization of intact beta 2-microglobulin in tissue causes amyloidosis in patients on chronic hemodialysis. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7908–7912. doi: 10.1073/pnas.83.20.7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C., Schlossmacher M. G., Hung A. Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B. L., Lieberburg I., Koo E. H., Schenk D., Teplow D. B. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992 Sep 24;359(6393):322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- Halverson K., Fraser P. E., Kirschner D. A., Lansbury P. T., Jr Molecular determinants of amyloid deposition in Alzheimer's disease: conformational studies of synthetic beta-protein fragments. Biochemistry. 1990 Mar 20;29(11):2639–2644. doi: 10.1021/bi00463a003. [DOI] [PubMed] [Google Scholar]
- Hensley K., Carney J. M., Mattson M. P., Aksenova M., Harris M., Wu J. F., Floyd R. A., Butterfield D. A. A model for beta-amyloid aggregation and neurotoxicity based on free radical generation by the peptide: relevance to Alzheimer disease. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3270–3274. doi: 10.1073/pnas.91.8.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbich C., Kisters-Woike B., Reed J., Masters C. L., Beyreuther K. Aggregation and secondary structure of synthetic amyloid beta A4 peptides of Alzheimer's disease. J Mol Biol. 1991 Mar 5;218(1):149–163. doi: 10.1016/0022-2836(91)90881-6. [DOI] [PubMed] [Google Scholar]
- Hofrichter J., Ross P. D., Eaton W. A. Kinetics and mechanism of deoxyhemoglobin S gelation: a new approach to understanding sickle cell disease. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4864–4868. doi: 10.1073/pnas.71.12.4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye H., Fraser P. E., Kirschner D. A. Structure of beta-crystallite assemblies formed by Alzheimer beta-amyloid protein analogues: analysis by x-ray diffraction. Biophys J. 1993 Feb;64(2):502–519. doi: 10.1016/S0006-3495(93)81393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett J. T., Berger E. P., Lansbury P. T., Jr The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer's disease. Biochemistry. 1993 May 11;32(18):4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- Jarrett J. T., Lansbury P. T., Jr Amyloid fibril formation requires a chemically discriminating nucleation event: studies of an amyloidogenic sequence from the bacterial protein OsmB. Biochemistry. 1992 Dec 15;31(49):12345–12352. doi: 10.1021/bi00164a008. [DOI] [PubMed] [Google Scholar]
- Jarrett J. T., Lansbury P. T., Jr Seeding "one-dimensional crystallization" of amyloid: a pathogenic mechanism in Alzheimer's disease and scrapie? Cell. 1993 Jun 18;73(6):1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kirschner D. A., Abraham C., Selkoe D. J. X-ray diffraction from intraneuronal paired helical filaments and extraneuronal amyloid fibers in Alzheimer disease indicates cross-beta conformation. Proc Natl Acad Sci U S A. 1986 Jan;83(2):503–507. doi: 10.1073/pnas.83.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner D. A., Inouye H., Duffy L. K., Sinclair A., Lind M., Selkoe D. J. Synthetic peptide homologous to beta protein from Alzheimer disease forms amyloid-like fibrils in vitro. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6953–6957. doi: 10.1073/pnas.84.19.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaguchi N., Takahashi Y., Tokushima Y., Shiojiri S., Ito H. Novel precursor of Alzheimer's disease amyloid protein shows protease inhibitory activity. Nature. 1988 Feb 11;331(6156):530–532. doi: 10.1038/331530a0. [DOI] [PubMed] [Google Scholar]
- Knauer M. F., Soreghan B., Burdick D., Kosmoski J., Glabe C. G. Intracellular accumulation and resistance to degradation of the Alzheimer amyloid A4/beta protein. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7437–7441. doi: 10.1073/pnas.89.16.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowall N. W., Beal M. F., Busciglio J., Duffy L. K., Yankner B. A. An in vivo model for the neurodegenerative effects of beta amyloid and protection by substance P. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7247–7251. doi: 10.1073/pnas.88.16.7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladror U. S., Snyder S. W., Wang G. T., Holzman T. F., Krafft G. A. Cleavage at the amino and carboxyl termini of Alzheimer's amyloid-beta by cathepsin D. J Biol Chem. 1994 Jul 15;269(28):18422–18428. [PubMed] [Google Scholar]
- Lansbury P. T., Jr In pursuit of the molecular structure of amyloid plaque: new technology provides unexpected and critical information. Biochemistry. 1992 Aug 4;31(30):6865–6870. doi: 10.1021/bi00145a001. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otvos L., Jr, Szendrei G. I., Lee V. M., Mantsch H. H. Human and rodent Alzheimer beta-amyloid peptides acquire distinct conformations in membrane-mimicking solvents. Eur J Biochem. 1993 Jan 15;211(1-2):249–257. doi: 10.1111/j.1432-1033.1993.tb19893.x. [DOI] [PubMed] [Google Scholar]
- Pike C. J., Burdick D., Walencewicz A. J., Glabe C. G., Cotman C. W. Neurodegeneration induced by beta-amyloid peptides in vitro: the role of peptide assembly state. J Neurosci. 1993 Apr;13(4):1676–1687. doi: 10.1523/JNEUROSCI.13-04-01676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike C. J., Walencewicz A. J., Glabe C. G., Cotman C. W. In vitro aging of beta-amyloid protein causes peptide aggregation and neurotoxicity. Brain Res. 1991 Nov 1;563(1-2):311–314. doi: 10.1016/0006-8993(91)91553-d. [DOI] [PubMed] [Google Scholar]
- Ponte P., Gonzalez-DeWhitt P., Schilling J., Miller J., Hsu D., Greenberg B., Davis K., Wallace W., Lieberburg I., Fuller F. A new A4 amyloid mRNA contains a domain homologous to serine proteinase inhibitors. Nature. 1988 Feb 11;331(6156):525–527. doi: 10.1038/331525a0. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Physiological production of the beta-amyloid protein and the mechanism of Alzheimer's disease. Trends Neurosci. 1993 Oct;16(10):403–409. doi: 10.1016/0166-2236(93)90008-a. [DOI] [PubMed] [Google Scholar]
- Seubert P., Vigo-Pelfrey C., Esch F., Lee M., Dovey H., Davis D., Sinha S., Schlossmacher M., Whaley J., Swindlehurst C. Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature. 1992 Sep 24;359(6393):325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- Shen C. L., Scott G. L., Merchant F., Murphy R. M. Light scattering analysis of fibril growth from the amino-terminal fragment beta(1-28) of beta-amyloid peptide. Biophys J. 1993 Dec;65(6):2383–2395. doi: 10.1016/S0006-3495(93)81312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji M., Golde T. E., Ghiso J., Cheung T. T., Estus S., Shaffer L. M., Cai X. D., McKay D. M., Tintner R., Frangione B. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992 Oct 2;258(5079):126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- Simmons L. K., May P. C., Tomaselli K. J., Rydel R. E., Fuson K. S., Brigham E. F., Wright S., Lieberburg I., Becker G. W., Brems D. N. Secondary structure of amyloid beta peptide correlates with neurotoxic activity in vitro. Mol Pharmacol. 1994 Mar;45(3):373–379. [PubMed] [Google Scholar]
- Spencer R. G., Halverson K. J., Auger M., McDermott A. E., Griffin R. G., Lansbury P. T., Jr An unusual peptide conformation may precipitate amyloid formation in Alzheimer's disease: application of solid-state NMR to the determination of protein secondary structure. Biochemistry. 1991 Oct 29;30(43):10382–10387. doi: 10.1021/bi00107a004. [DOI] [PubMed] [Google Scholar]
- Stafford W. F., 3rd Boundary analysis in sedimentation transport experiments: a procedure for obtaining sedimentation coefficient distributions using the time derivative of the concentration profile. Anal Biochem. 1992 Jun;203(2):295–301. doi: 10.1016/0003-2697(92)90316-y. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Cheung T. T., Cai X. D., Odaka A., Otvos L., Jr, Eckman C., Golde T. E., Younkin S. G. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science. 1994 May 27;264(5163):1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., McClatchey A. I., Lamperti E. D., Villa-Komaroff L., Gusella J. F., Neve R. L. Protease inhibitor domain encoded by an amyloid protein precursor mRNA associated with Alzheimer's disease. Nature. 1988 Feb 11;331(6156):528–530. doi: 10.1038/331528a0. [DOI] [PubMed] [Google Scholar]
- Tomski S. J., Murphy R. M. Kinetics of aggregation of synthetic beta-amyloid peptide. Arch Biochem Biophys. 1992 May 1;294(2):630–638. doi: 10.1016/0003-9861(92)90735-f. [DOI] [PubMed] [Google Scholar]
- Turnell W. G., Finch J. T. Binding of the dye congo red to the amyloid protein pig insulin reveals a novel homology amongst amyloid-forming peptide sequences. J Mol Biol. 1992 Oct 20;227(4):1205–1223. doi: 10.1016/0022-2836(92)90532-o. [DOI] [PubMed] [Google Scholar]
- Waite J., Cole G. M., Frautschy S. A., Connor D. J., Thal L. J. Solvent effects on beta protein toxicity in vivo. Neurobiol Aging. 1992 Sep-Oct;13(5):595–599. doi: 10.1016/0197-4580(92)90062-3. [DOI] [PubMed] [Google Scholar]
- Yankner B. A., Duffy L. K., Kirschner D. A. Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides. Science. 1990 Oct 12;250(4978):279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- Zagorski M. G., Barrow C. J. NMR studies of amyloid beta-peptides: proton assignments, secondary structure, and mechanism of an alpha-helix----beta-sheet conversion for a homologous, 28-residue, N-terminal fragment. Biochemistry. 1992 Jun 23;31(24):5621–5631. doi: 10.1021/bi00139a028. [DOI] [PubMed] [Google Scholar]