Abstract
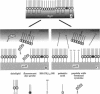
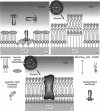
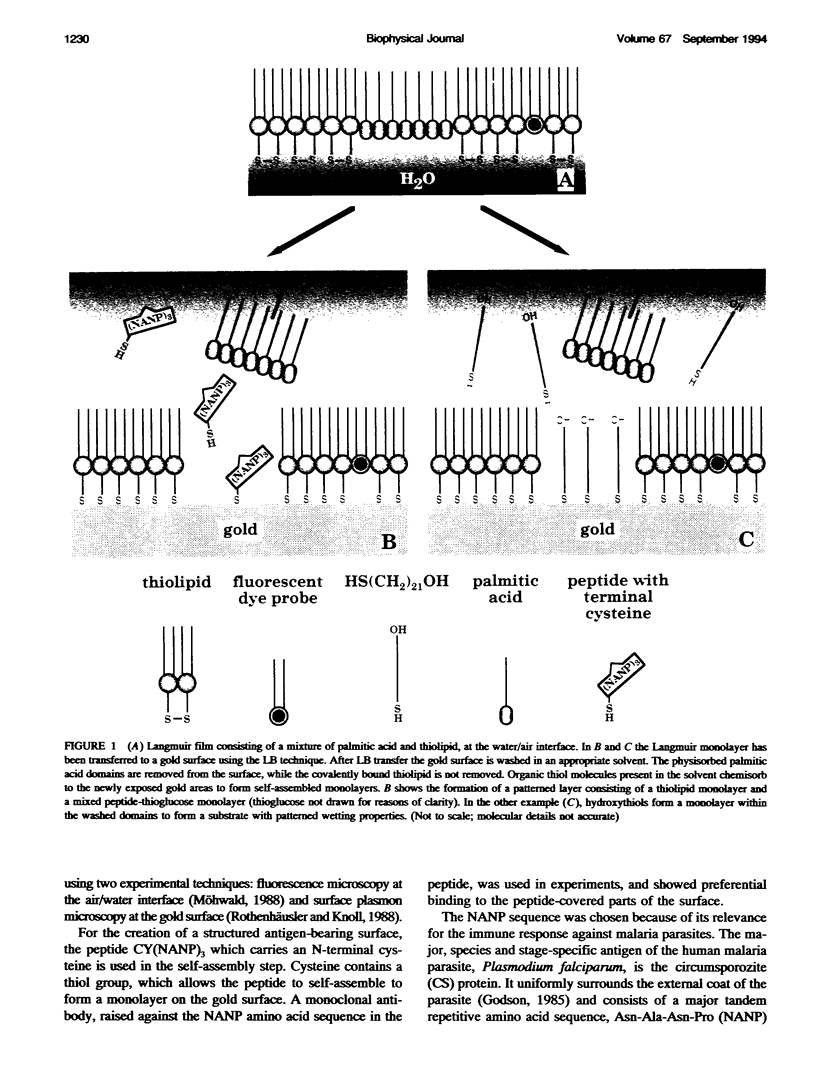
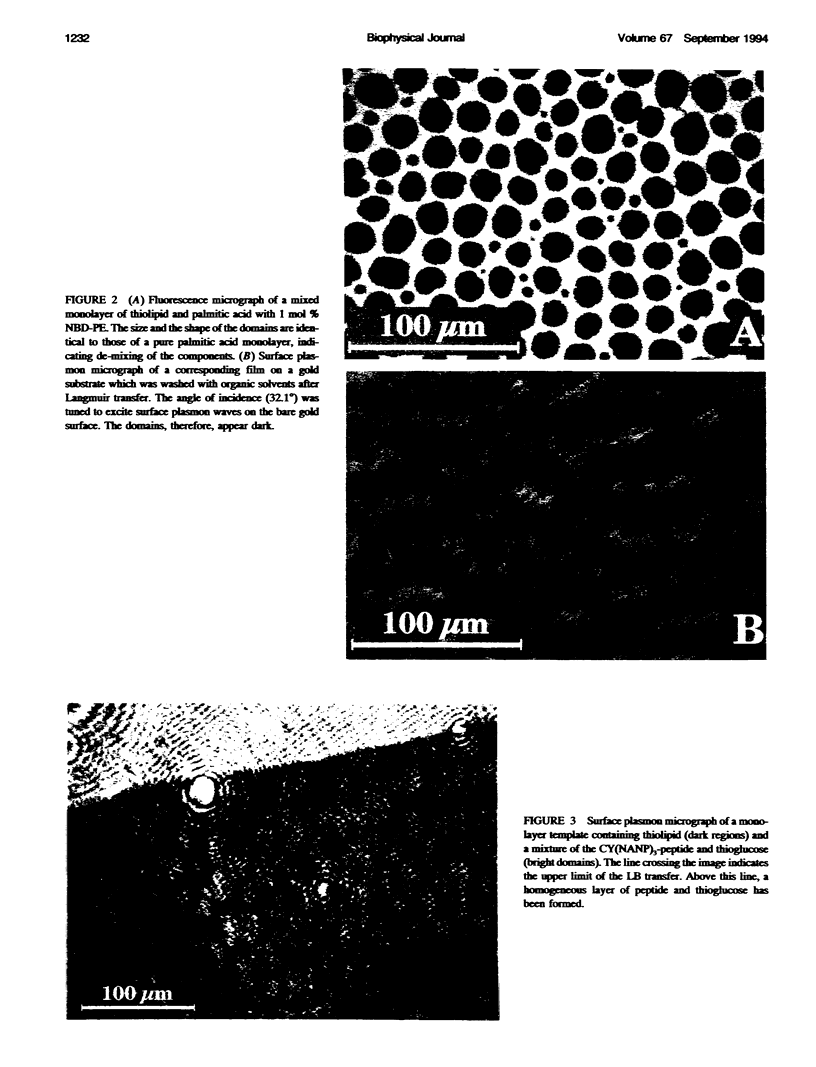
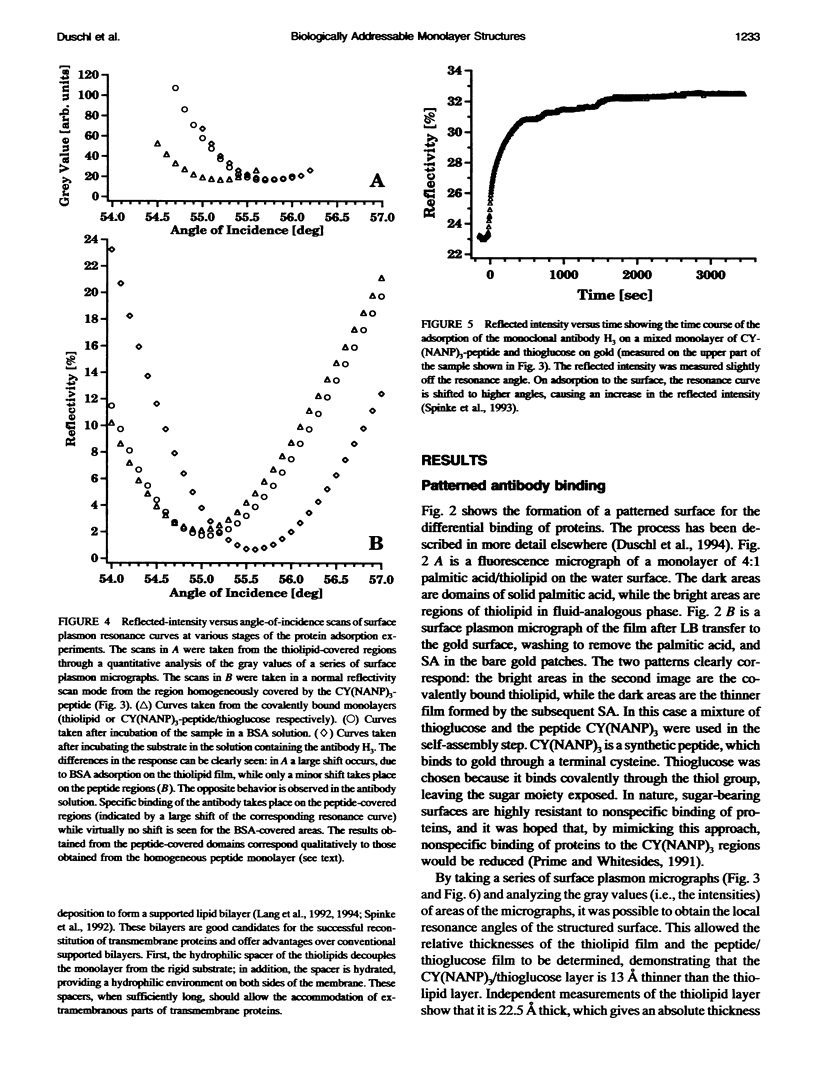
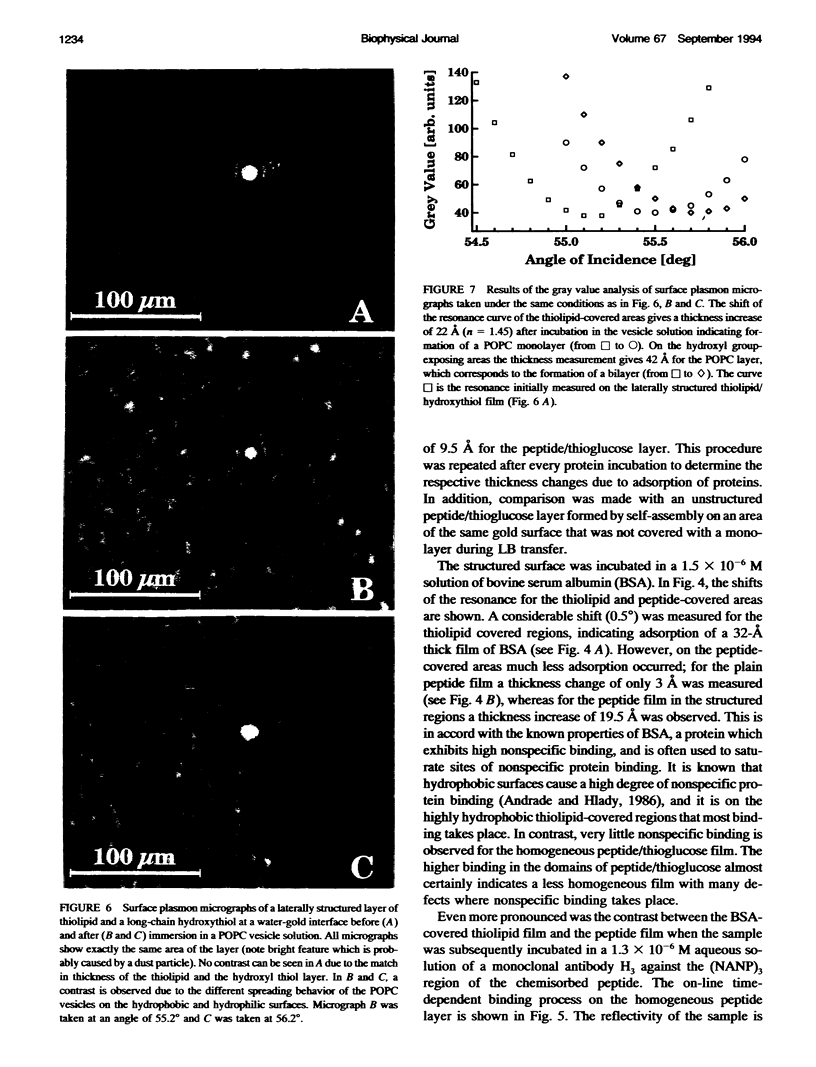
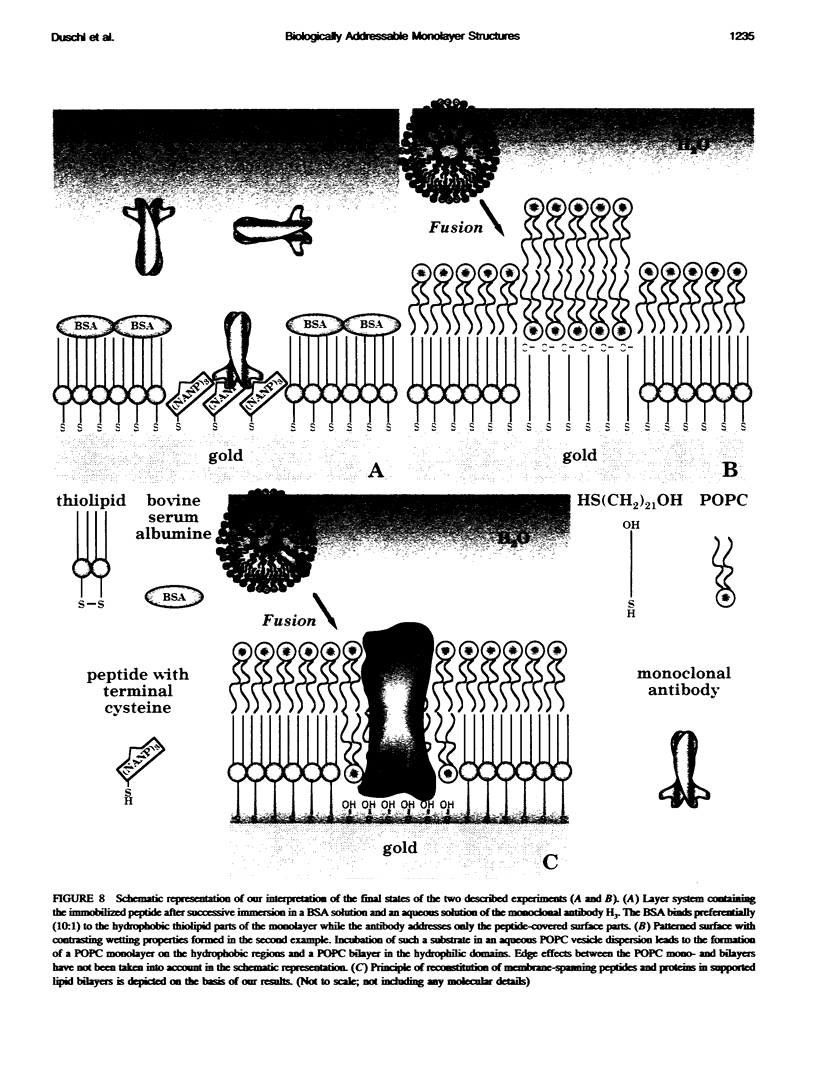
We demonstrate that the combined application of Langmuir-Blodgett and self-assembly techniques allows the fabrication of patterns with contrasting surface properties on gold substrates. The process is monitored using fluorescence microscopy and surface plasmon spectroscopy and microscopy. These structures are suitable for the investigation of biochemical processes at surfaces and in ultrathin films. Two examples of such processes are shown. In the first example, the structures are addressed through the binding of a monoclonal antibody to a peptide. This demonstrates the formation of self-assembled monolayers by cysteine-bearing peptides on gold, and the directed binding of proteins to the structured layers. A high contrast between specific and unspecific binding of proteins is observed by the patterned presentation of antigens. Such films possess considerable potential for the design of multichannel sensor devices. In the second example, a structured phospholipid layer is produced by controlled self-assembly from vesicle solution. The structures created--areas of phospholipid bilayer, surrounded by a matrix of phospholipid monolayer--allow formation of a supported bilayer which is robust and strongly bound to the gold support, with small areas of free-standing bilayer which very closely resemble a phospholipid cell membrane.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betzig E., Trautman J. K. Near-field optics: microscopy, spectroscopy, and surface modification beyond the diffraction limit. Science. 1992 Jul 10;257(5067):189–195. doi: 10.1126/science.257.5067.189. [DOI] [PubMed] [Google Scholar]
- Del Giudice G., Tougne C., Renia L., Ponnudurai T., Corradin G., Pessi A., Verdini A. S., Louis J. A., Mazier D., Lambert P. H. Characterization of murine monoclonal antibodies against a repetitive synthetic peptide from the circumsporozoite protein of the human malaria parasite, Plasmodium falciparum. Mol Immunol. 1991 Sep;28(9):1003–1009. doi: 10.1016/0161-5890(91)90186-n. [DOI] [PubMed] [Google Scholar]
- Kalb E., Frey S., Tamm L. K. Formation of supported planar bilayers by fusion of vesicles to supported phospholipid monolayers. Biochim Biophys Acta. 1992 Jan 31;1103(2):307–316. doi: 10.1016/0005-2736(92)90101-q. [DOI] [PubMed] [Google Scholar]
- Müller W., Ringsdorf H., Rump E., Wildburg G., Zhang X., Angermaier L., Knoll W., Liley M., Spinke J. Attempts to mimic docking processes of the immune system: recognition-induced formation of protein multilayers. Science. 1993 Dec 10;262(5140):1706–1708. doi: 10.1126/science.8259513. [DOI] [PubMed] [Google Scholar]
- Nussenzweig V., Nussenzweig R. S. Rationale for the development of an engineered sporozoite malaria vaccine. Adv Immunol. 1989;45:283–334. doi: 10.1016/s0065-2776(08)60695-1. [DOI] [PubMed] [Google Scholar]
- Prime K. L., Whitesides G. M. Self-assembled organic monolayers: model systems for studying adsorption of proteins at surfaces. Science. 1991 May 24;252(5009):1164–1167. doi: 10.1126/science.252.5009.1164. [DOI] [PubMed] [Google Scholar]
- Radmacher M., Tillamnn R. W., Fritz M., Gaub H. E. From molecules to cells: imaging soft samples with the atomic force microscope. Science. 1992 Sep 25;257(5078):1900–1905. doi: 10.1126/science.1411505. [DOI] [PubMed] [Google Scholar]
- Spinke J., Yang J., Wolf H., Liley M., Ringsdorf H., Knoll W. Polymer-supported bilayer on a solid substrate. Biophys J. 1992 Dec;63(6):1667–1671. doi: 10.1016/S0006-3495(92)81742-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts T. H., Gaub H. E., McConnell H. M. T-cell-mediated association of peptide antigen and major histocompatibility complex protein detected by energy transfer in an evanescent wave-field. Nature. 1986 Mar 13;320(6058):179–181. doi: 10.1038/320179a0. [DOI] [PubMed] [Google Scholar]
- Whitesides G. M., Mathias J. P., Seto C. T. Molecular self-assembly and nanochemistry: a chemical strategy for the synthesis of nanostructures. Science. 1991 Nov 29;254(5036):1312–1319. doi: 10.1126/science.1962191. [DOI] [PubMed] [Google Scholar]