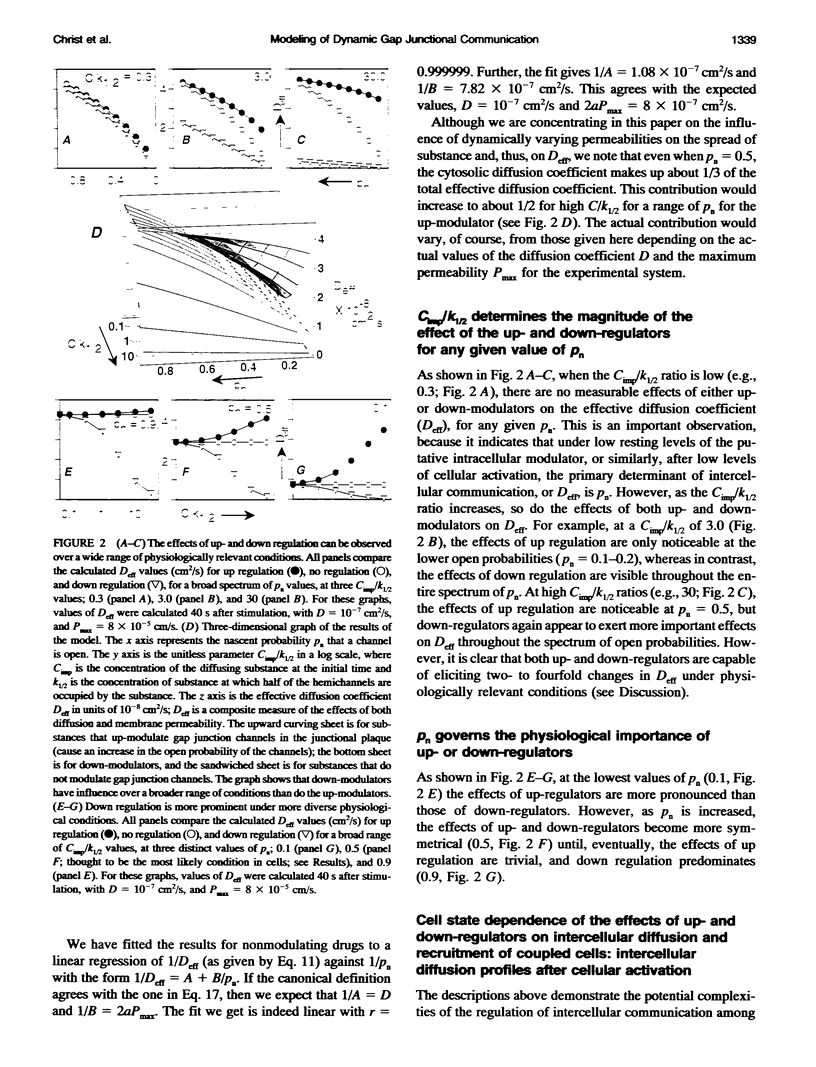
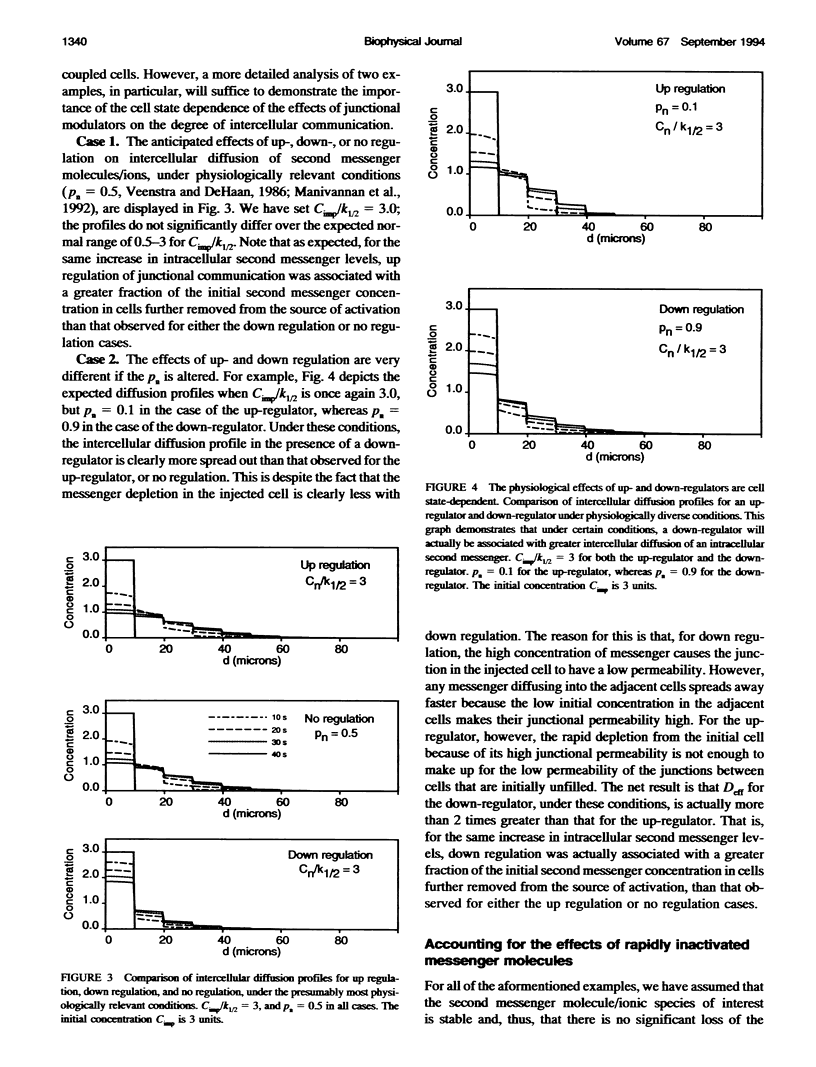
Abstract
Gap junctions are aqueous intercellular channels formed by a diverse class of membrane-spanning proteins, known as connexins. These aqueous pores provide partial cytoplasmic continuity between cells in most tissues, and are freely permeable to a host of physiologically relevant second messenger molecules/ionic species (e.g., Ca2+, IP3, cAMP, cGMP). Despite the fact that these second messenger molecules/ionic species have been shown to alter junctional patency, there is no clear basis for understanding how dynamic and transient changes in the intracellular concentration of second messenger molecules might modulate the extent of intercellular communication among coupled cells. Thus, we have modified the tissue monolayer model of Ramanan and Brink (1990) to account for both the up-regulatory and down-regulatory effects on junctions by second messenger molecules that diffuse through gap junctions. We have chosen the vascular wall as our morphological correlate because of its anisotropy and large investment of gap junctions. The model allows us to illustrate the putative behavior of gap junctions under a variety of physiologically relevant conditions. The modeling studies demonstrated that transient alterations in intracellular second messenger concentrations are capable of producing 50-125% changes in the number of cells recruited into a functional syncytial unit, after activation of a single cell. Moreover, the model conditions required to demonstrate such physiologically relevant changes in intercellular diffusion among coupled cells are commonly observed in intact tissues and cultured cells.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azadzoi K. M., Kim N., Brown M. L., Goldstein I., Cohen R. A., Saenz de Tejada I. Endothelium-derived nitric oxide and cyclooxygenase products modulate corpus cavernosum smooth muscle tone. J Urol. 1992 Jan;147(1):220–225. doi: 10.1016/s0022-5347(17)37201-4. [DOI] [PubMed] [Google Scholar]
- Brink P. R., Ramanan S. V. A model for the diffusion of fluorescent probes in the septate giant axon of earthworm. Axoplasmic diffusion and junctional membrane permeability. Biophys J. 1985 Aug;48(2):299–309. doi: 10.1016/S0006-3495(85)83783-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett A. L., Lowenstein C. J., Bredt D. S., Chang T. S., Snyder S. H. Nitric oxide: a physiologic mediator of penile erection. Science. 1992 Jul 17;257(5068):401–403. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- Christ G. J., Brink P. R., Zhao W., Moss J., Gondré C. M., Roy C., Spray D. C. Gap junctions modulate tissue contractility and alpha 1 adrenergic agonist efficacy in isolated rat aorta. J Pharmacol Exp Ther. 1993 Aug;266(2):1054–1065. [PubMed] [Google Scholar]
- Christ G. J., Moreno A. P., Melman A., Spray D. C. Gap junction-mediated intercellular diffusion of Ca2+ in cultured human corporal smooth muscle cells. Am J Physiol. 1992 Aug;263(2 Pt 1):C373–C383. doi: 10.1152/ajpcell.1992.263.2.C373. [DOI] [PubMed] [Google Scholar]
- Cole W. C., Garfield R. E., Kirkaldy J. S. Gap junctions and direct intercellular communication between rat uterine smooth muscle cells. Am J Physiol. 1985 Jul;249(1 Pt 1):C20–C31. doi: 10.1152/ajpcell.1985.249.1.C20. [DOI] [PubMed] [Google Scholar]
- Cornell-Bell A. H., Finkbeiner S. M., Cooper M. S., Smith S. J. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990 Jan 26;247(4941):470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- De Mello W. C. Increase in junctional conductance caused by isoproterenol in heart cell pairs is suppressed by cAMP-dependent protein-kinase inhibitor. Biochem Biophys Res Commun. 1988 Jul 29;154(2):509–514. doi: 10.1016/0006-291x(88)90169-6. [DOI] [PubMed] [Google Scholar]
- Eghbali B., Kessler J. A., Reid L. M., Roy C., Spray D. C. Involvement of gap junctions in tumorigenesis: transfection of tumor cells with connexin 32 cDNA retards growth in vivo. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10701–10705. doi: 10.1073/pnas.88.23.10701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Edwards F. R. Sympathetic neuroeffector transmission in arteries and arterioles. Physiol Rev. 1989 Apr;69(2):546–604. doi: 10.1152/physrev.1989.69.2.546. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Biological actions and properties of endothelium-derived nitric oxide formed and released from artery and vein. Circ Res. 1989 Jul;65(1):1–21. doi: 10.1161/01.res.65.1.1. [DOI] [PubMed] [Google Scholar]
- Kanno Y. Modulation of cell communication and carcinogenesis. Jpn J Physiol. 1985;35(5):693–707. doi: 10.2170/jjphysiol.35.693. [DOI] [PubMed] [Google Scholar]
- Larson D. M., Haudenschild C. C., Beyer E. C. Gap junction messenger RNA expression by vascular wall cells. Circ Res. 1990 Apr;66(4):1074–1080. doi: 10.1161/01.res.66.4.1074. [DOI] [PubMed] [Google Scholar]
- Manivannan K., Ramanan S. V., Mathias R. T., Brink P. R. Multichannel recordings from membranes which contain gap junctions. Biophys J. 1992 Jan;61(1):216–227. doi: 10.1016/S0006-3495(92)81828-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkoff R., Rundus V. R., Parker S. B., Beyer E. C., Hertzberg E. L. Connexin expression in the developing avian cardiovascular system. Circ Res. 1993 Jul;73(1):71–78. doi: 10.1161/01.res.73.1.71. [DOI] [PubMed] [Google Scholar]
- Moore L. K., Beyer E. C., Burt J. M. Characterization of gap junction channels in A7r5 vascular smooth muscle cells. Am J Physiol. 1991 May;260(5 Pt 1):C975–C981. doi: 10.1152/ajpcell.1991.260.5.C975. [DOI] [PubMed] [Google Scholar]
- Moreno A. P., Campos de Carvalho A. C., Christ G., Melman A., Spray D. C. Gap junctions between human corpus cavernosum smooth muscle cells: gating properties and unitary conductance. Am J Physiol. 1993 Jan;264(1 Pt 1):C80–C92. doi: 10.1152/ajpcell.1993.264.1.C80. [DOI] [PubMed] [Google Scholar]
- Moreno A. P., Fishman G. I., Spray D. C. Phosphorylation shifts unitary conductance and modifies voltage dependent kinetics of human connexin43 gap junction channels. Biophys J. 1992 Apr;62(1):51–53. doi: 10.1016/S0006-3495(92)81775-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Ramanan S. V., Brink P. R. Exact solution of a model of diffusion in an infinite chain or monolayer of cells coupled by gap junctions. Biophys J. 1990 Sep;58(3):631–639. doi: 10.1016/S0006-3495(90)82406-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal S. S., Duling B. R. Flow control among microvessels coordinated by intercellular conduction. Science. 1986 Nov 14;234(4778):868–870. doi: 10.1126/science.3775368. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Burt J. M. Structure-activity relations of the cardiac gap junction channel. Am J Physiol. 1990 Feb;258(2 Pt 1):C195–C205. doi: 10.1152/ajpcell.1990.258.2.C195. [DOI] [PubMed] [Google Scholar]
- Sáez J. C., Connor J. A., Spray D. C., Bennett M. V. Hepatocyte gap junctions are permeable to the second messenger, inositol 1,4,5-trisphosphate, and to calcium ions. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2708–2712. doi: 10.1073/pnas.86.8.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takens-Kwak B. R., Jongsma H. J. Cardiac gap junctions: three distinct single channel conductances and their modulation by phosphorylating treatments. Pflugers Arch. 1992 Nov;422(2):198–200. doi: 10.1007/BF00370421. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Weingart R. Inotropic effect of cyclic AMP in calf ventricular muscle studied by a cut end method. J Physiol. 1976 Aug;260(1):117–141. doi: 10.1113/jphysiol.1976.sp011507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra R. D., DeHaan R. L. Measurement of single channel currents from cardiac gap junctions. Science. 1986 Aug 29;233(4767):972–974. doi: 10.1126/science.2426781. [DOI] [PubMed] [Google Scholar]
- WEIDMANN S. Shortening of the cardiac action potential due to a brief injection of KCl following the onset of activity. J Physiol. 1956 Apr 27;132(1):157–163. doi: 10.1113/jphysiol.1956.sp005510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H., Hollstein M., Mesnil M., Martel N., Aguelon A. M. Selective lack of intercellular communication between transformed and nontransformed cells as a common property of chemical and oncogene transformation of BALB/c 3T3 cells. Cancer Res. 1987 Nov 1;47(21):5658–5664. [PubMed] [Google Scholar]



