Abstract
Background
Glucagon like peptide-1 receptor agonist (GLP-1RA) use in individuals with high atherosclerotic cardiovascular disease (ASCVD) risk reduces major adverse cardiovascular events (MACE). However, its clinical impact, in terms of numbers needed to treat (NNT), efficacy and safety profile in reducing the risk of myocardial infarction (MI) and the individual ASCVD constituents remain unclear.
Methods
Electronic databases, Medline and Embase were reviewed for randomized trials from inception to 29 May 2025. Risk-reduction effect of GLP-1RA were pooled using pairwise meta-analysis with random-effects model. The primary outcome was MI, and secondary outcomes were the individual ASCVD constituents.
Results
109,846 patients from 25 unique studies were included. Over a follow-up duration of 3.48 ± 1.51 (1.55 to 5.47) years, GLP-1RA reduced the risk of total MI (RR: 0.86, p < 0.01), with numbers needed to benefit (NNTB) of 207 to prevent one event of MI. Higher body mass index was associated with greater MI risk reduction (β: -0.09, p = 0.03) in GLP-1RA users. GLP-1RA reduced cardiovascular mortality (RR: 0.87, p < 0.01, NNTB 170), MACE (RR: 0.87, p < 0.01, NNTB 67) and stroke (RR: 0.88, p < 0.01, NNTB 335) compared to placebo. GLP-1RA commonly resulted in gastrointestinal side-effects amongst other systems (RR: 1.55, p < 0.01, NNTH 9).
Conclusion
GLP-1RA reduced the risk of MI, stroke, cardiovascular mortality and MACE in a broad range of patients with and without T2DM and/or prior ASCVD, supporting its role in ASCVD prevention, especially in the cohort with high BMI.
Trial registration: Open Science Framework (https://doi.org/10.17605/OSF.IO/7VXN5).
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-025-02840-3.
Keywords: GLP-1 receptor agonist, GLP-1RA, Myocardial infarction, Stroke, Cardiovascular mortality, Cardiovascular disease, Numbers-needed-to-treat
Research insights
What is currently known about this topic?
Landmark cardiovascular outcome trials like LEADER and SELECT have demonstrated the cardiometabolic benefits of GLP1-RA in the reduction of MACE events in populations with T2DM as well as populations with overweight or obesity in the absence of T2DM. This was further substantiated by systematic reviews, which have described pooled overall MACE risk reductions in both the abovementioned population groups.
What is the key research question?
What is the NNT, efficacy and safety profile of GLP-1RA in reducing the risk of myocardial infarction (MI) and individual ASCVD constituents, namely non-fatal MI, unstable angina, coronary revascularization, and/or cardiovascular mortality?
What is new?
GLP-1RA use was associated with a 13% risk reduction in total MI with an NNT of 207 to prevent one event of MI over a follow-up of 3.48 ± 1.51 (range: 1.55 to 5.46) years. However, MI risk reduction was attenuated in the population with T2DM and in the secondary preventative cohort with prior ASCVD. The former is likely attributable to the competing multimorbid status in the generally higher risk population with T2DM, that can attenuate the overall beneficial effects of GLP-1RA.
Increased BMI is associated with significantly larger MI risk reduction with GLP-1RA, implying that weight reduction may further reduce residual ASCVD risk with GLP-1RA use.
In the secondary preventative cohort, GLP-1RA may be considered for its significant reduction in unstable angina and coronary revascularization risk. Relative to its clinical impact on MI reduction, measured by NNT, GLP-1RA was more effective in preventing one event of cardiovascular mortality and MACE.
How might this study influence clinical practice?
Evidence supports the role of GLP-1RA as part of therapy in overall ASCVD risk reduction, especially in the cohort with high BMI.
Introduction
Cardiovascular disease (CVD)-related mortality [1, 2] and morbidity have surged in recent decades[3], despite advancements in medical and healthcare resources [4]. The global CVD burden is expected to see a 3.6% year-on-year increase from 2025 to 2050 [5], thus emphasizing the need for effective strategies in cardiometabolic prevention within the global health conundrum [6–9]. With the success of landmark cardiovascular outcome trials on glucagon-like peptide-1 receptor agonist (GLP-1RA) and sodium-glucose cotransporter-2 inhibitors (SGLT2i) [10], this has transformed the landscape of cardiometabolic disease management [11]. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results trial (LEADER) led to a paradigm shift in the wider use of GLP-1RA from promising antihyperglycemic agents to cornerstone cardiometabolic therapies, demonstrating the significant reduction of major adverse cardiovascular events (MACE) with the use of liraglutide in the population with type 2 diabetes mellitus (T2DM) [12, 13]. The overall cardiometabolic benefit of GLP-1RA rapidly expanded beyond the T2DM population, with the Semaglutide Effects on Cardiovascular Outcomes in People with Overweight or Obesity (SELECT) trial describing MACE reduction in the population with overweight and obesity, even in the absence of T2DM [14].
In the era of precision prevention, together with updated pooled cohort equations such as the Predicting Risk of Cardiovascular Disease Events (PREVENT) calculator [15, 16] that distinguish the individual’s risk of atherosclerotic cardiovascular disease (ASCVD) and/or heart failure (HF), primary preventative strategies could be tailored in accordance to the individual’s risk profile [17–19]. Clinical practice guidelines[20, 21] have recommended the use of SGLT2i in individuals at higher risk of HF, while GLP-1RA may be prioritized in those with T2DM at higher ASCVD risk (calculated 10-year ASCVD risk ≥ 10%, Class IIa, level of evidence: B) given their neutral effect on HF hospitalization [22]. While other reviews have described the MACE risk reduction in the general population with T2DM [23, 24], non-T2DM [25], or both [26], the present study seeks to address the gap in the literature in providing a comprehensive meta-analysis on the number need to treat (NNT), efficacy and safety profile of GLP-1RA in reducing the risk of myocardial infarction (MI) and individual ASCVD constituents, namely non-fatal MI, unstable angina, coronary revascularization, and/or cardiovascular mortality.
Methods
Study design and search strategy
This systematic review and meta-analysis was conducted with reference to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) 2020. Two electronic databases, Embase and Medline, were searched for randomized controlled trials (RCTs) relating to treatment with GLP-1RA and prevention of MI from inception to 22 May 2025. Key search terms including “glucagon-like peptide 1 receptor agonist” and “myocardial infarction” were used in the search strategy and the full search strategy can be found in Supplementary Material 1. This review was prospective registered in Open Science Framework (https://doi.org/10.17605/OSF.IO/7VXN5).
Eligibility and selection criteria
References were imported into Covidence (Melbourne, Victoria, Australia) for the compilation and removal of duplicates. Abstracts were independently screened by two pairs of authors (FS, JTYH, SKSC, GS). Any discrepancies were resolved by consensus or in consultation with a senior author (ASPT, JQ, YHC, NWC). Subsequently, full text reviews were conducted to check for eligibility of studies for inclusion in this review. Only original randomized trials with published results in English or professionally translated into English were included in this study. The inclusion of each study was contingent on the use of a GLP-1RA arm and the incidence of MI both groups during the trial. Non-randomized controlled trials and observational studies, including cohort and cross-sectional studies, were excluded from this study. Reviews, study protocols, letters, commentaries, conference abstracts were also excluded. Studies in pediatric populations were also excluded in this study. References of related reviews and grey literature were screened to ensure a comprehensive review.
Data extraction and outcomes
Two pairs of authors (FS, JTYH, SKSC, GS) independently extracted data including, but not limited to: (1) study characteristics (e.g. author, title, publication year, trial name, trial registry), (2) patient demographics (e.g. age, sex, ethnicity, body mass index [BMI]), (3) baseline comorbid status (e.g. T2DM, hypertension, obesity, hyperlipidemia), (4) treatment characteristics (e.g. drug formulation, dosage, duration of intervention), (5) prognostic outcomes, (6) safety data (e.g. general, gastrointestinal, endocrine, and neurological side-effects). Any discrepancies were resolved by consensus or in consultation with a senior author (ASPT, JQ, YHC, NWC). The primary outcome of interest was the incidence of MI. Secondary outcomes were the incidence of MACE, stroke, unstable angina, coronary revascularization and cardiovascular mortality. Where available, the hazard ratios of these outcomes were also collated. MACE outcomes were collated, with most adhering to the MACE-3 definition (comprising non-fatal MI, non-fatal stroke and cardiovascular mortality). A list of MACE definitions used across the included studies are summarized in Supplementary Material 2. Cardiovascular mortality was defined as deaths resulting from any cardiovascular causes, which include, but are not limited to, cardiac arrest, MI, and arrhythmia.
Statistical methods
All statistical analysis was conducted in RStudio (version 4.4.0). Statistical significance was considered for outcomes with a p value of ≤ 0.05. To assess the cardioprotective impact of GLP-1RA on MI, secondary cardiovascular outcomes and safety profile, pairwise meta-analysis was conducted using the Paul-Mandel estimator to obtain the risk ratio (RR) and corresponding 95% confidence interval [27]. Due to the differing mechanisms of tirzepatide, a dual gastric inhibitory polypeptide (GIP) and GLP-1RA, compared with the rest of the GLP-1RA medications, the results of studies examining tirzepatide were systematically reviewed without inclusion in the meta-analysis. Statistical heterogeneity was assessed via I2, where a value of 25%, 50% and 75% indicated low, moderate and high heterogeneity respectively, and Cochran Q test, where p < 0.10 was considered significant for heterogeneity [28]. Hartung-Knapp adjustments were employed to adjust for confidence intervals by controlling for the heterogeneity arising from between-study estimations [29]. Zero total event trials for each outcome were accounted in our analysis using the continuity correction of 0.5 [30]. The random effects model was used regardless of heterogeneity scores, based on evidence of more robust estimates when compared to fixed-effect models [31–33].
To estimate the absolute risk reduction and its corresponding numbers needed to treat, data extracted for the efficacy outcomes mentioned in Sect. "Data Extraction and Outcomes" were used to calculate NNT, which yielded either numbers needed to benefit (NNTB) and number needed to harm (NNTH) based on formulas recommended by the Cochrane guidelines [34].
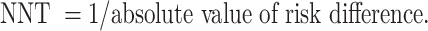 |
Subgroup analyses (duration of trial, type of medication) and sensitivity analyses (secondary prevention, T2DM only) were conducted. To assess the modulatory effect of demographic factors, such as age, sex, ethnicity, BMI and comorbid status, mixed-model meta-regression with Hartung-Knapp estimator was conducted [35]. In view of limited information (n < 3) when making head-to-head comparisons of identified outcomes with other glucose lowering agents, the results were systematically reported.
Study quality, publication bias and certainty of outcomes
Each study was independently screened by two pairs of authors (FS, JTYH, SKSC, GS). Any discrepancies were resolved by consensus or in consultation with a senior author (ASPT, JQ, YHC, NWC). Quality assessment of included articles was conducted using the Cochrane Risk of Bias 2 (ROB-2) tool [34]. RoB2 analyses the risk of bias by grading the quality of evidence through five bias domains including randomization process, deviation from intended intervention, missing outcome data, outcome measurement and reported result selection. A final grade of low risk of bias, some concerns of bias and high risk of bias was attributed to each article considering the score in each section. Publication bias was examined with Egger’s [36] regression, where ≥ 10 studies were present. Funnel plots were generated for analyses involving ≥ 10 studies for visual inspection of asymmetrical distribution of data points across the vertical treatment effect axis. Grading of Recommendations Assessment, Development and Evaluation (GRADE) scoring was also performed for each of the main outcomes analyzed. GRADE is a system of grading used to assess the overall quality and certainty of the evidence obtained from the specific outcome or intervention [37]. This helps the reader determine the level of confidence and robustness the data estimates have in each of the specific outcomes.
Results
Summary of included articles
The initial search of the literature yielded a total of 2218 articles. After the removal of 559 duplicates and automated removal by Covidence of 399 ineligible articles, 1260 articles remained for title and abstract screening. Further removal of 986 articles resulted in 274 articles being sought for retrieval and full-text review. A total of 26 articles [12, 14, 38–61], derived from 25 unique trials, published between 2013 and 2025, involving 109,846 patients were selected for inclusion in this study. The selection process is depicted in Fig. 1. All trials were randomized trials conducted in adult patients (≥ 18 years). Most studies were conducted in a multi-national setting, except for two studies conducted only in Japan. Majority of studies were also conducted in patients with T2DM, except for one study conducted in a cohort with obesity in the absence of T2DM [62]. In addition, the Evaluate Renal Function with Semaglutide Once Weekly (FLOW) trial [60] recruited participants with chronic kidney disease. The proportion of males ranged from 24 to 76%, and the mean age was 62.6 ± 9.2 years. Based on the ROB 2 tool, most studies were of low risk of bias, while only a minority was of some concern or higher. Majority of studies compared GLP-1RA to placebo [12, 14, 38–40, 44, 47, 48, 51–61], while others used insulin regiments and other anti-diabetic medications [41–43, 45–47, 49, 50] as the comparators. A summary of the study characteristics and study quality can be found in Table 1 and Supplementary Material 3 respectively.
Fig. 1.
PRISMA flowchart
Table 1.
Summary of included studies
| Study | Trial name | Trial registry number | Intervention arms | Sample size | Sex (male) | Age (years) | Risk of bias | |
|---|---|---|---|---|---|---|---|---|
| Mean | SD | |||||||
| Lincoff et al. 2023 | SELECT | NCT03574597 |
- Semaglutide - Placebo |
17,604 | 0.72 | 61.60 | 8.85 | High |
| Ruff et al. 2022 | FREEDOM CVO | NCT01455896 |
- Exenatide - Placebo |
4156 | 0.63 | 62.83 | 7.7993 | Low |
| Perkovic et al. 2024 | FLOW | NCT03819153 |
- Semaglutide - Placebo |
3533 | 0.70 | 66.60 | 9.00 | Low |
| Gerstein et al. 2019 | REWIND | NCT01394952 |
- Dulaglutide - Placebo |
9901 | 0.54 | 66.20 | 6.50 | Low |
| Pfeffer et al. 2015 | ELIXA | NCT01147250 |
- Lixisenatide + standard of care - Placebo + standard of care |
6068 | 0.69 | 60.25 | 9.66 | Low |
| Green et al. 2024 | GRADE | NCT01794143 |
- Liraglutide + metformin - Insulin Glargine + metformin - Glimepiride + metformin - Sitagliptin + metformin |
5047 | 0.64 | 57.20 | 10.00 | Some concerns |
| Del Prato et al. 2022 | SURPASS-4 | NCT03730662 |
- Tirzepatide (± metformin, sulfonylurea, SGLT2 inhibitor) - Insulin Glargine (± metformin, sulfonylurea, SGLT2 inhibitor) |
1995 | 0.62 | 63.60 | 8.60 | Low |
| Pei et al. 2021 | DUAL II China | NCT03175120 |
- IDegLira (Degludec, Liraglutide) + basal insulin + metformin ± oral antidiabetic drugs (OADs) - Insulin degludec + basal insulin + metformin ± oral antidiabetic drugs (OADs) |
453 | 0.60 | 54.70 | 9.90 | Low |
| Frias et al. 2019 |
- Dulaglutide + metformin - Placebo + metformin |
317 | 0.50 | 56.80 | 9.74 | Low | ||
| Philis-Tsimikas et al. 2019 | DUALTM IX | NCT02773368 |
-Insulin Degludec/Liraglutide (IDegLira) + SGLT2 inhibitor -Insulin Glargine + SGLT2 inhibitor |
420 | 0.59 | 56.65 | 10.30 | Low |
| Seino et al. 2018 | NCT02254291 |
-Semaglutide -Sitagliptin |
308 | 0.76 | 58.30 | 10.70 | Low | |
| Jabbour et al. 2018 | DURATION-8 | NCT02229396 |
-Exenatide/Dapagliflozin + metformin -Exenatide/Placebo + metformin -Dapagliflozin/Placebo + metformin |
685 | 0.48 | 54.17 | 9.53 | Low |
| Le Roux et al. 2017 | SCALE | NCT01272219 |
-Liraglutide + reduced-calorie diet and increased physical activity -Placebo + reduced-calorie diet and increased physical activity |
2254 | 0.24 | 47.43 | 11.73 | High |
| Araki et al. 2015 | NCT01584232 |
-Dulaglutide + sulphonylureas (± biguanides) -Insulin Glargine + sulphonylureas (± biguanides) |
361 | 0.71 | 56.80 | 10.90 | Low | |
| Gough et al. 2014 | DUAL I | NCT01336023 |
-Insulin Degludec/Liraglutide + metformin (± pioglitazone) -Liraglutide + metformin (± pioglitazone) -Insulin Degludec + metformin (± pioglitazone) |
1663 | 0.51 | 55.03 | 9.92 | Low |
| Pan et al. 2014 | GetGoal-M-Asia | NCT01169779 |
-Lixisenatide + metformin (± sulfonylurea) -Placebo + metformin (± sulfonylurea) |
390 | 0.49 | 54.80 | 10.39 | Low |
| Pinget et al. 2013 | GETGOAL-P | NCT00763815 |
-Lixisenatide + pioglitazone (± metformin) -Placebo + pioglitazone (± metformin) |
484 | 0.52 | 55.77 | 9.50 | Low |
| Riddle et al. 2013 | GetGoal-Duo1 | NCT00975286 |
-Insulin Glargine + metformin (± TZDs) + lixisenatide -Insulin Glargine + metformin (± TZDs) + placebo |
446 | 0.50 | 56.00 | 10.00 | Low |
| Gerstein et al. 2023 | AMPLITUDE O | NCT03496298 |
- Efpeglenatide - Placebo |
4076 | 0.67 | 64.50 | 8.20 | Low |
| Gerstein et al. 2021 | AMPLITUDE O | NCT03496298 |
- Efpeglenatide - Placebo |
4076 | 0.67 | 64.50 | 8.20 | Low |
| Holman et al. 2017 | EXSCEL | NCT01144338 |
- Exenatide + usual care - Placebo + usual care |
14,752 | 0.62 | 62.00 | 8.90 | Low |
| Hernandez et al. 2018 | HARMONY | NCT02465515 |
- Albiglutide + standard care - Placebo + standard care |
9463 | 0.69 | 64.15 | 8.70 | Low |
| Marso et al. 2016 | LEADER | NCT01179048 |
- Liraglutide + standard of care - Placebo + standard of care |
9340 | 0.64 | 64.30 | 7.20 | Low |
| Husain et al. 2019 | PIONEER 6 | NCT02692716 |
- Semaglutide - Placebo |
3183 | 0.68 | 66.00 | 7.00 | Low |
| Marso et al. 2016 | SUSTAIN 6 | NCT01720446 |
- Semaglutide + standard-care regimen - Placebo + standard-care regimen |
3297 | 0.61 | 64.60 | 7.40 | Low |
| McGuire et al. 2025 | SOUL | NCT03914326 |
- Oral semaglutide - Placebo" |
9650 | 0.71 | 66.10 | 7.55 | Low |
Number needed to treat
When compared to placebo, the NNT in the overall study is based on studies with a mean follow-up time in years of 3.48 ± 1.51 (range: 1.55 to 5.46). The current results show that number needed to benefit (NNTB) was 207 (95%CI: NNTB 129 to NNTB 620, Follow-up time: 3.48 ± 1.51 years) for total MI, NNTB 67 (95%CI: NNTB 46 to NNTB 135, Follow-up time: 3.48 ± 1.51 years) for MACE, NNTB 170 (95%CI: NNTB 120 to NNTB 306, Follow-up time: 3.48 ± 1.51 years) for cardiovascular mortality, and NNTB 93 (95%CI: NNTB 52 to NNTB 1717, Follow-up time: 3.45 ± 1.21 years) for coronary revascularization. These findings are summarized in Table 2.
Table 2.
Numbers needed to treat for total MI (overall)
| Outcome | Number of studies | Numbers needed to treat (95% confidence interval) | Follow-up time (Years)a |
|---|---|---|---|
| Total MI | 15 | NNTB 207 (NNTB 129 to NNTB 620) | 3.48 ± 1.51 (Range: 1.55 to 5.47) |
| Fatal MI | 6 | NNTB 1744 (NNTB 401 to ∞ to NNTH 312) | 4.15 ± 1.70 (Range: 3.27 to 5.47) |
| Non-Fatal MI | 13 | NNTB 208 (NNTB 123 to NNTB 931) | 4.06 ± 1.18 (Range: 3.32 to 5.47) |
| MACE | 12 | NNTB 67 (NNTB 46 to NNTB 135) | 3.48 ± 1.51 (Range: 1.55 to 5.47) |
| Stroke | 11 | NNTB 335 (NNTB 224 to NNTB 714) | 3.48 ± 1.51 (Range: 1.55 to 5.47) |
| Coronary Revascularization | 6 | NNTB 93 (NNTB 52 to NNTB 1717) | 3.45 ± 1.21 (Range: 3.27 to 3.96) |
| Cardiovascular Mortality | 13 | NNTB 170 (NNTB 120 to NNTB 306) | 3.48 ± 1.51 (Range: 1.55 to 5.47) |
| Heart Failure | 10 | NNTB 551 (NNTB 119 to ∞ to NNTH 153) | 4.10 ± 1.52 (Range: 3.27 to 5.47) |
| Unstable Angina | 8 | NNTB 2919 (NNTB 659 to ∞ NNTH 1031) | 3.03 ± 1.18 (Range: 3.32 to 5.47) |
When the numbers needed to benefit crosses infinity, it means that for the current outcome, no clear benefit for the outcome can be seen statistically
Legend: NNTB, Numbers needed to treat; NNTH, Numbers needed to harm; MI, Myocardial Infarction; MACE, Major Adverse Cardiovascular Events; ∞, Infinity
a Follow-up Time is presented in Mean ± Standard deviation (Range) unless stated otherwise
Subgroup analysis based on GLP-1RA type showed that NNTB for albiglutide was 51, followed by semaglutide (NNTB 82), whilst the NNT for efpeglenatide, liraglutide, dulaglutide and exenatide was insignificant (Supplementary Material 4).
Efficacy
Comparison to placebo
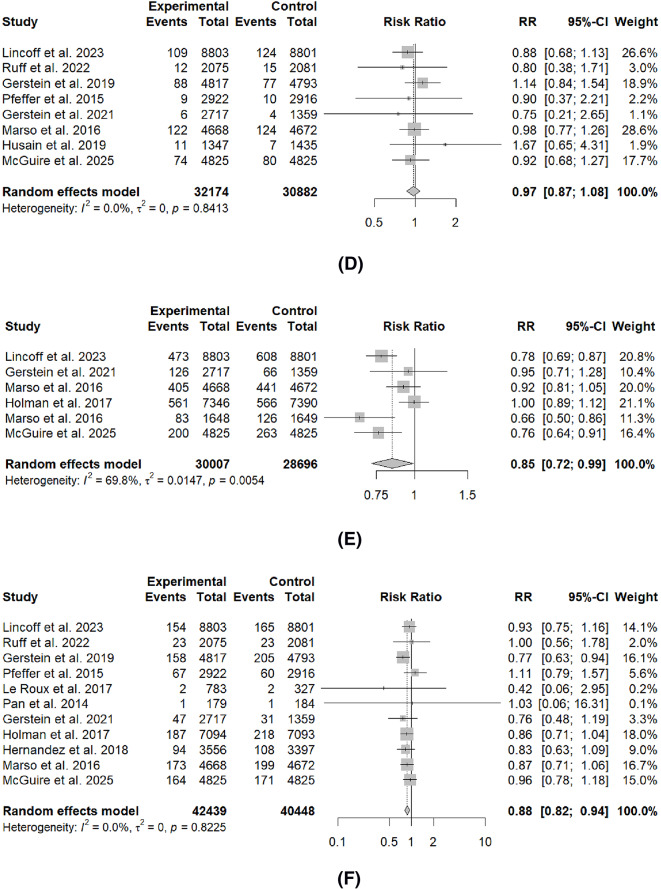
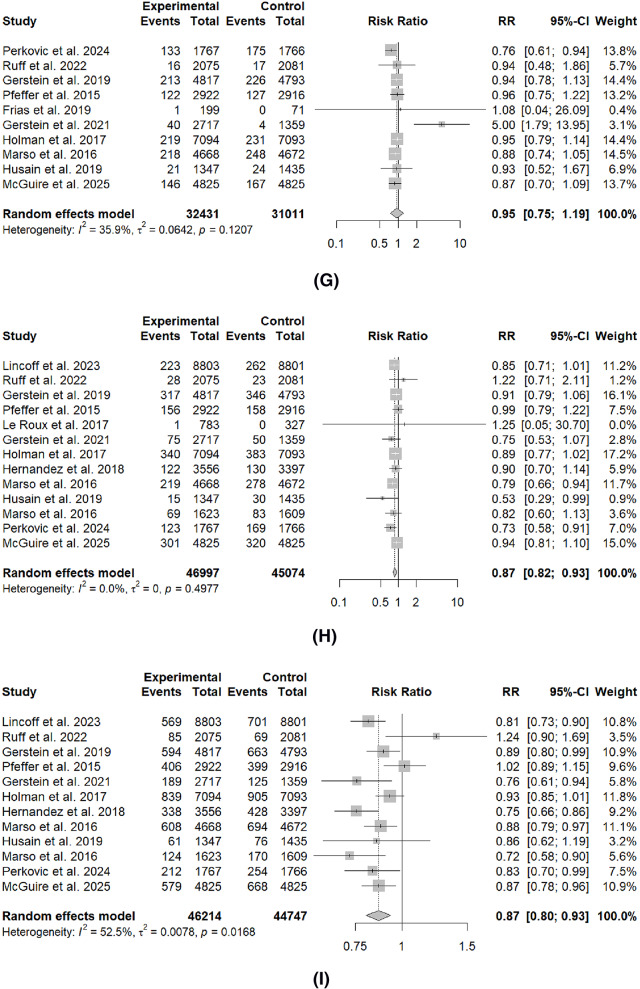
Table 3; Fig. 2 summarize the comparison of GLP-1RA and placebo on the effect of cardiovascular outcomes. Across the main outcomes, GLP-1RA significantly reduced the rates of most outcomes, including total MI (RR: 0.86, 95%I 0.78 to 0.95, p < 0.01), non-fatal MI (RR: 0.87, 95%CI: 0.79 to 0.97, p = 0.02), MACE (RR: 0.87, 95%CI: 0.80 to 0.93, p < 0.01), stroke (RR: 0.88, 95%CI: 0.82 to 0.94, p < 0.01), rates of coronary revascularization (RR: 0.85, 95CI: 0.72 to 0.99, p = 0.04) and cardiovascular mortality (RR: 0.87, 95%CI: 0.82 to 0.93, p < 0.01). There were no significant differences for fatal MI, HF and unstable angina.
Table 3.
Effect on cardiovascular outcomes compared to placebo and insulin regimen
| Outcomes | Number of Studies | Risk Ratio (95% Confidence Interval) | I2 | Cochran Q | p-value |
|---|---|---|---|---|---|
| Comparison to Placebo | |||||
| Total MI | 15 | 0.86 (0.78 to 0.95) | 47.10% | 0.02 | < 0.01* |
| Fatal MI | 6 | 0.87 (0.44 to 1.72) | 32.80% | 0.19 | 0.62 |
| Non-fatal MI | 13 | 0.87 (0.79 to 0.97) | 38.30% | 0.08 | 0.02* |
| MACE | 12 | 0.87 (0.80 to 0.93) | 52.50% | 0.02 | < 0.01* |
| Stroke | 11 | 0.88 (0.82 to 0.94) | 00.00% | 0.82 | < 0.01* |
| Coronary Revascularization | 6 | 0.85 (0.72 to 0.99) | 72.00% | < 0.01 | 0.04* |
| Cardiovascular Mortality | 13 | 0.87 (0.82 to 0.93) | 00.00% | 0.50 | < 0.01* |
| Heart Failure | 10 | 0.95 (0.75 to 1.19) | 35.90% | 0.12 | 0.60 |
| Unstable Angina | 8 | 0.97 (0.87 to 1.08) | 00.00% | 0.84 | 0.54 |
Fig. 2.
Forest plots of cardiovascular outcome prevention compared to placebo
Comparison to insulin and glucose-lowering agents
Table 3 summarizes the comparison between the impact of GLP-1RA to other glucose-lowering agents on cardiovascular outcomes. GLP-1RA significantly reduces the rates of MACE (RR: 0.73, 95%CI: 0.69 to 0.79, p = 0.01), rates of coronary revascularization (RR: 0.61, 95%CI: 0.40 to 0.94, p = 0.02) and cardiovascular mortality RR: 0.43, 95%CI: 0.34 to 0.54, p < 0.01). However, no statistical differences were observed for other outcomes (MI, non-fatal MI, stroke, heart failure, unstable angina). Comparison with other glucose-lowering agents, comprising sulfonylurea, dipeptidyl peptidase 4 inhibitors (DPP4-I) and SGLT2i are summarized in Supplementary Material 5.
Sensitivity analysis based on prior ASCVD
Sensitivity analysis was conducted on the population with prior ASCVD events and summarized in Supplementary Material 6. In the secondary prevention population, the use of GLP-1RA led to significant reduction in unstable angina events (RR: 0.88, 95%CI: 0.82 to 0.95, p = 0.03) and coronary revascularization (RR: 0.78, 95%CI: 0.69 to 0.87, p < 0.01) compared to placebo. However, the rates of total MI and non-fatal MI were not significantly reduced in the prior ASCVD population (Total MI RR: 0.82, 95%CI: 0.49 to 1.36, p = 0.23;non-fatal MI RR: 0.87, 95%CI: 0.09 to 7.96, p = 0.56).
Sensitivity analysis based on T2DM
The effects of GLP-1RA in the T2DM population were largely congruent with the overall population, except for its effects on the rates of coronary revascularization. While the levels of risk reduction were similar (Overall RR: 0.85 vs. T2DM RR: 0.86), this effect did not achieve statistical significance within this analysis. The results of this sensitivity analysis is summarized in Supplementary Material 6.
Meta-regression on modulators of GLP-1RA effectiveness
Sufficient studies (n ≥ 10) were available for the conductance of meta-regression on the following baseline, biochemical and demographic factors. Higher BMI was independently associated with reductions in total MI incidence (β: -0.09, 95%CI: -0.18 to -0.01, p = 0.03) with GLP-1RA use. On the other hand, the Asian ethnicity was associated with increased risk of total MI incidence with GLP-1RA use (β: 3.49, 95%CI: 0.95 to 6.03, p = 0.01). Age, sex and hemoglobin A1c (HbA1c) levels was not associated with total MI risk reduction. These findings are summarized in Table 4.
Table 4.
Meta-regression of demographic factors on myocardial infarction compared to placebo
| Demographic Factor | Number of Studies | Sample size | β coefficient (95% Confidence Interval) | p-value |
|---|---|---|---|---|
| Age | 15 | 84,374 | -0.02 (-0.06 to 0.03) | 0.37 |
| Male | 15 | 84,374 | -1.24 (-2.69 to 0.20) | 0.09 |
| Caucasian | 14 | 83,969 | 0.21 (-1.51 to 1.93) | 0.79 |
| African American | 11 | 64,727 | 0.30 (-4.10 to 9.91) | 0.37 |
| Asian | 11 | 56,882 | 3.49 (0.95 to 6.03) | 0.01* |
| Bodymass Index | 14 | 74,724 | -0.09 (-0.18 to -0.01) | 0.03* |
| HbA1C | 13 | 70,568 | 0.02 (-0.09 to 0.12) | 0.76 |
Bolded p value with asterix (*) are of p ≤ 0.05, denotingstatistical significance
Legend: HbA1C, hemoglobin A1C;
Safety analysis
Amongst the 16 safety domains (Supplementary Material 7), most of the side-effects were related to the gastrointestinal system. Patients taking GLP-1RA were at elevated risk of nausea (NNTH 8, RR: 3.56, 95%CI: 2.43 to 5.21, p < 0.01), vomiting (NNTH 9, RR: 3.99, 95%CI: 2.44 to 6.54, p < 0.01), diarrhea (NNTH 20, RR: 1.85, 95%CI: 1.33 to 2.59, p < 0.01), and constipation (NNTH 21, RR: 2.04, 95%CI: 1.46 to 2.83, p < 0.01). Overall, patients were 55% more likely to develop gastrointestinal side-effects (NNTH 8, RR: 1.55, 95%CI: 1.22 to 1.97, p < 0.01) compared to placebo. Other side effects included, but are not limited to, minor (NNTH 36, RR: 2.17, 95%CI: 1.38 to 3.43, p = 0.03), symptomatic (NNTH 22, RR: 1.78, 95%CI: 1.15 to 2.74, p = 0.03) hypoglycemic events, dizziness (NNTH 60, RR: 1.39, 95%CI: 1.03 to 1.87, p = 0.04) (Table 5).
Table 5.
Safety analysis (Numbers needed to Harm)
| Outcome | Number of Studies | Numbers Needed to Harm (95% Confidence Interval) |
|---|---|---|
| Gastrointestinal | ||
| Gastrointestinal side effects | 8 | NNTH 8 (NNTH 5 to NNTH 21) |
| Nausea | 12 | NNTH 8 (NNTH 5 to NNTH 14) |
| Vomiting | 9 | NNTH 12 (NNTH 7 to NNTH 25) |
| Diarrhea | 12 | NNTH 20 (NNTH 11 to NNTH 53) |
| Constipation | 4 | NNTH 21 (NNTH 12 to NNTH 46) |
| Endocrinology | ||
| Minor hypoglycaemic events | 2 | NNTH 36 (NNTH 18 to NNTH 113) |
| Symptomatic hypoglycaemic events | 3 | NNTH 22 (NNTH 10 to NNTH 113) |
| Severe symptomatic hypoglycaemic events | 7 | NNTH 2307 (NNTH 82 to ∞ to NNTB 125) |
| Neurology | ||
| Dizziness | 4 | NNTH 60 (NNTH 27 to NNTH 848) |
| Headache | 6 | NNTH 152 (NNTH 45 to ∞ to NNTB 162) |
| Hepatobiliary | ||
| Overall pancreatitis | s5 | NNTH 1743 (NNTH 368 to ∞ to NNTB 1612) |
| Acute Pancreatitis | 5 | NNTH 2550 (NNTH 523 to ∞ to NNTB 436) |
| Cholelithiasis | 2 | NNTH 459 (NNTH 132 ∞ to NNTB 670) |
| Rheumatology | ||
| Severe allergic reactions | 3 | NNTH 425 (NNTH 42 to ∞ to NNTB 105) |
| Hypersensitivity syndrome or symptoms | 2 | NNTB 638 (NNTB 589 to NNTB 695) |
When the numbers needed to harm crosses infinity, it means that for the current outcome, no clear harm for the outcome can be seen statistically
Legend: NNTH, Numbers needed to harm; TEAE, Treatment-emergent adverse events; ∞, Infinity;
Publication bias and certainty of outcomes
From the visual assessment of funnel plots, there was no significant publication bias in the analysis of cardiovascular outcomes. The funnel plots are shown in Supplementary Material 8. Egger’s regression performed also did not reveal any statistically significant publication bias, with p < 0.05 as the threshold for significance. The GRADE methodology was employed and the assessment noted moderate to high level of certainty in all the main outcomes Supplementary Material 9). Nonetheless, attenuation of the certainty of outcomes for total MI, fatal MI, MACE and coronary revascularization was attributed to the domain of inconsistency and imprecision.
Discussion
Current clinical practice guidelines [63, 64] recommend the use of GLP-1RA in patients with T2DM at high risk of ASCVD. While these guidelines are informed by the surmounting evidence of MACE reduction in the primary preventative cohort at risk of ASCVD [26], the present study adds to the current evidence by providing an in-depth assessment of clinical impact, in terms of the NNT, efficacy and safety of GLP-1RA on MI risk and the individual constituents of ASCVD risk reduction. There were several principal findings from the comprehensive evaluation of 25 trials enrolling over 109,846 participants. The therapeutic effect of GLP-1RA was observed with 14% risk reduction in total MI, although these effects should be interpreted in the context of the NNT of 207 to prevent one event of MI over a follow-up of 3.48 ± 1.51 years. However, the significant MI risk reduction was slightly attenuated in the population with T2DM (Overall RR: 0.86 vs. T2DM RR: 0.89) and in the secondary preventative cohort with prior ASCVD (Overall p < 0.01 vs. Prior ASCVD p = 0.23). Importantly, the meta-regression analysis revealed greater effectiveness in MI reduction in individuals with high BMI from early initiation of GLP-1RA. It is also notable that significant reduction in unstable angina and unplanned revascularization with the use of GLP-1RA was observed in the secondary preventative cohort, potentially translating to clinical impact in individuals with prior ASCVD events. The benefits in ASCVD risk reduction with GLP-1RA are evidenced by 14% risk reduction in cardiovascular mortality with an NNTB of 207 to prevent one event, 13% MACE reduction with an NNT of 67 to prevent one event and 12% risk reduction in stroke with an NNT of 335 to prevent one event, along with their favourable safety profile of 6% increased overall adverse events.
The ASCVD risk reduction of GLP-1RA is largely attributed to the 13% reduction of three-point MACE, which comprised of cardiovascular death, non-fatal MI and non-fatal stroke. However, little is known about the weight of each individual component’s contribution to the overall composite risk reduction. Prior evidence suggested that, while GLP-1RA exhibits beneficial effects on MACE, the reduction of MI may not be the primary driver to the improvement of overall cardiovascular outcomes. These studies described an insignificant trend towards the risk reduction of fatal and non-fatal MI, as well as unstable angina [65–68]. Our study challenges current observations by demonstrating significant risk reduction of MI, with the use of GLP-1RA. Several mechanistic pathways have been hypothesized to underpin these clinical observations, which involves the influence of GLP-1RA on inflammatory, oxidative stress, and angiogenesis pathways in the atherosclerotic process [69–72].
GLP-1RA has comparable efficacy in MI risk reduction compared to current guideline-guided medical therapy in ASCVD prevention. The NNT for MI risk reduction over a course of five years has been reported to be 361 for aspirin [73], 104 for statins [74], and 100 for anti-hypertensive medications [75], compared to the NNT of 207 for GLP-1RA. In terms of the individual MACE components, the present study informs clinicians that the impact of GLP-1RA, based on the NNT, is predominantly in the reduction of coronary revascularization risk, followed by total MI, then cardiovascular mortality, non-fatal MI and stroke. In addition, studies have shown that use of GLP-1RA has significant reduction in MI, CV mortality, and/or MACE in patients with a history of MI [12, 76], with our data demonstrating reduction in unstable angina and coronary revascularization risk in the secondary preventative cohort. The next important step will be to examine the cost effectiveness of GLP-1RA medications in patients following MI, and the role in further ASCVD risk reduction. Future studies are needed to compare the clinical impact of GLP-1RA, in terms of NNT, with other therapies in the pipeline that target the various pathways in the ASCVD process, such as bempedoic acid which has been associated with reduced risk of MI and coronary revascularization due to the improvement in lipid and inflammatory profiles [77]. However, the comparison of NNT across various drug classes must be nuanced given the NNT values are specific to individual trial design, study population baseline characteristics, and the time of outcome measurement.
The possibility that specific GLP-1RAs may differentially impact on MI risk cannot be dismissed. Four GLP-1RAs demonstrated similar degree of protection against total MI, with albiglutide associated with the lowest NNTB for total MI, followed by semaglutide, efpeglenatide, and liraglutide. While lixisenatide was found to be the only GLP-1RA subtype to increase the risk of MI, it is important to consider that the ELIXA trial, which studied lixisenatide in patients with T2DM, had the highest risk population as it included participants with recent acute coronary events in the past 180 days [40]. Moreover, the lack of cardiovascular benefits observed with lixisenatide may be contributed to its relatively short half-life [78]. Interestingly, our analysis revealed that the unfavorable impact of lixisenatide on MI rates was reversed upon the removal of the ELIXA trial [40] in the sensitivity analysis (NNTB: 569, 95%CI: NNTB 252 to ∞ to NNTH 10). In addition, our study revealed that exenatide demonstrated the least favorable NNTB profile in terms of MI risk reduction. This was partly contributed by the relatively higher discontinuation rate observed in the EXSCEL trial [55], which studied once-weekly exenatide on cardiovascular outcomes in T2DM, given that the study did not have a run-in period to optimize medication adherence. Together with the EXSCEL trial’s shorter follow-up duration, this could have attenuated the significance in the study outcome. It will be important to consider the specific GLP1-RAs that have shown clear benefit in the reduction of MI risk when integrating them into contemporary primary and secondary ASCVD prevention guidelines.
Specific patient factors can modify the beneficial effect of GLP-1RA in MI risk reduction [79, 80]. The presence of obesity is intricately associated with the risk of ischemic heart diseases [81], associated with a dose–response relationship[82]. Our findings are suggestive that an increased BMI is associated with significantly larger MI risk reduction with GLP-1RA. With weight reduction, GLP-1RA can improve dysregulated lipid profile and insulin resistance of individuals with obesity, thus reducing residual ASCVD risk [83–85]. In addition, GLP-1RA did not result in improvements in total MI and non-fatal MI compared to placebo, in our sensitivity analysis of the T2DM as well as the secondary prevention populations. This is likely attributable to the competing multimorbid status in the generally higher risk population with T2DM, that can attenuate the overall beneficial effects of GLP-1RA [86–88]. Some studies suggest that the beneficial effects of GLP-1RAs can be dampened by the deleterious effects of long-standing T2DM resulting in the reduced capacity to secrete insulin and diminished incretin effects, resulting in reduced glucose-lowering efficacy of GLP-1RA [89, 90]. While others have reported on the significant MACE reduction with GLP-1RA therapy in the secondary prevention cohort, but not in the primary prevention setting [91], our findings extend the current notion that the beneficial impact on the individual MACE constituents, particularly MI risk reduction, may be attenuated in the higher risk secondary preventative population with established CVD, often characterized by advanced age and multimorbidity status [90]. Taken together, this emphasizes the importance in timely initiation of GLP-1RA, targeting the primary preventive population with overweight/obesity, prior to the manifestation of T2DM and development of ASCVD [92–95].
In the context of heart failure, GLP-1RA were associated with a directionally favorable but statistically non-significant effect. This finding diverges from the results reported by Sattar et al. 2021 [96], wherein GLP-1RA therapy was linked to a statistically significant reduction in heart failure risk. A critical distinction lies in the substantially larger sample size of the present analysis—encompassing over 9,000 additional participants—which may confer greater statistical power and reliability. Furthermore, the current findings are concordant with those of Villaschi et a. 2024 [97], suggesting that earlier signals of cardioprotective benefit may have led to pre-mature conclusions. These inconsistencies underscore the necessity for additional high-powered methodologically rigorous trials to more definitively delineate the effect of GLP-1RA therapy on heart failure outcomes, thereby informing future revisions of clinical practice guidelines.
Moreover, emerging evidence has shown that the SGLT2i and GLP-1RA combination therapy was associated with fewer cardiovascular events in the population with T2DM and MI, compared to either drug used alone [98]. Both these classes can synergistically improve the plethora of metabolic risk factors and reduce peri-infarct tissue inflammation as well as infarct size molecular, thus improving myocardial remodeling post-infarct [99, 100]. This could further position GLP-1RA as part of a comprehensive cardiometabolic strategy, optimizing its potential synergistic cardiovascular benefit with SGLT2i.
GLP-1RA demonstrated mixed evidence on the reduction of MI, coronary revascularization, HF and/or cardiovascular mortality when compared to other glucose-lowering agents [101–104]. Several studies reported larger risk reduction in coronary revascularization by 44% when compared to sulfonylurea[41] and DPP-4I[46]. Our study postulates improved MI rates, albeit statistically non-significant, with the use of GLP-1RA compared to other glucose-lowering agents. Larger head-to-head analysis comparing GLP-1RA and other glucose lowering agents are needed to evaluate the effects on atherosclerosis and plaque regression [95, 105, 106].
Limitations
This study has its limitations. First, in composite outcomes such as MACE, differences in definition introduce heterogeneity in the analysis as seen in Supplementary Material 2. Thus, caution should be taken in the interpretation of the NNT and RRs derived from the MACE analysis. Second, most studies included participants with T2DM, except the SELECT trial. Therefore, generalizing the MI reduction effect on populations without T2DM must be done with caution. Further analyses are warranted to examine the cardioprotective effects of GLP-1RA in this subgroup without T2DM. Third, the included studies consisted mostly of mixed prior and non-prior ASCVD patients, preventing a direct comparison of the utility of GLP-1RA between primary and secondary prevention cohorts. Future study designs with homogeneous inclusion criteria are warranted to delineate primary and secondary prevention cohorts to facilitate more informative interpretation of the effects and safety of GLP-1RA. Fourth, despite the mounting evidence of the clinical impact of GLP-1RA on MACE reduction, the present study extends the current literature on the clinical impact and efficacy of GLP-1RA on the MI risk reduction and its individual ASCVD components. However, comparisons with novel glucose-lowering medications (e.g. SGLT2i) and other newer GLP-1RA medications such as the dual GIP and GLP-1RA medications (tirzepatide and mazdutide) on their effectiveness in ASCVD risk reduction remain lacking. Thus, future studies are warranted to examine a head-to-head or indirect comparison through network meta-analyses to compare the clinical utility in ASCVD risk reduction across these emerging pharmacotherapies. Fifth, other studies have reported high one-year discontinuation rates (64.8% in non-T2DM and 46.5% in T2DM cohorts) and low 1-year re-initiation rates of GLP-1RA (36.3% in non-T2DM and 47.3% in T2DM cohorts), largely contributed by moderate or severe incident gastrointestinal adverse events and lower income status [107–111]. As such, suboptimal medication adherence and access can underestimate long-term ASCVD risk reduction correlates associated with GLP-1RA therapy. Sixth, the current estimates for NNT show large uncertainty, as seen with wide confidence intervals in some of the NNT results. This is largely owing to trial variability, incorporation of trials with smaller scale trials reporting on MI rates [44, 47, 48, 51, 52, 59], the heterogeneity of the study population, and incorporation of zero total event trials in the current analysis [47, 48, 51, 52, 59]. Though this has been shown to lead to larger variability, numerous simulation papers have shown that this leads to a more conservative estimate and allows for the most generalizable estimate of treatment effect. Nevertheless, larger-scale research with harmonized study endpoints of ASCVD risk should be done to better clarify the overall treatment effect of GLP-1RA in cardiovascular outcomes.
Conclusions
In this meta-analysis of 100,196 patients, across the full spectrum of those with and without prior ASCVD and/or T2DM, GLP-1RA demonstrated significant reduction of MI risk by 12% with NNT of 248 to prevent one event of MI. This benefit was more pronounced in those with higher BMI. In the secondary preventative cohort, GLP-1RA may be considered for its significant reduction in unstable angina and coronary revascularization risk. Relative to its clinical impact on MI reduction, measured by NNT, GLP-1RA was more effective in preventing 1 event of cardiovascular mortality and MACE. Together with its favorable safety profile, the evidence supports the role of GLP-1RA as part of therapy in overall ASCVD risk reduction, especially in the cohort with high BMI.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- GLP1-RA
Glucagon like peptide-1 receptor agonist
- ASCVD
Atherosclerotic cardiovascular disease
- MACE
Major adverse cardiovascular events
- NNT
Numbers Needed to Treat
- MI
Myocardial Infarction
- CVD
Cardiovascular Disease
- SGLT2i
Sodium-glucose cotransporter-2 inhibitors
- LEADER
Liraglutide effect and action in diabetes: evaluation of cardiovascular outcome results
- T2DM
Type 2 Diabetes mellitus
- SELECT
Semaglutide effects on cardiovascular outcomes in people with overweight or obesity
- PREVENT
Predicting risk of cardiovascular disease events
- HF
Heart Failure
- PRISMA
Preferred reporting items for systematic review and meta-analyses
- RCT
Randomized controlled trial
- BMI
Body mass index
- RR
Risk ratio
- NNTB
Numbers needed to benefit
- NNTH
Number needed to harm
- ROB-2
Cochrane risk of bias 2 (ROB-2) tool
- FLOW
Evaluate renal function with semaglutide once weekly
Author contributions
All authors have made substantial contributions to all of the following: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be submitted. No writing assistance was obtained in the preparation of the manuscript. The manuscript, including related data, figures and tables has not been previously published, and the manuscript is not under consideration elsewhere.Conceptualization and Design: MAM, YHC, NWCAcquisition of Data: ASPT, JTYH, SKSC, JQ, GS, FS, KECAnalysis and Interpretation of Data: ASPT, VVA, BC, YHC, NWSCWriting– original draft: ASPT, JTYH, SKSC, JQ, GS, FS, KEC, VVA, BC, AM, SAT, MM, GKD, CWLR, MYC, MAM, YHC, NWSCWriting– review & editing: ASPT, JTYH, SKSC, JQ, GS, FS, KEC, VVA, BC, AM, SAT, MM, GKD, CWLR, MYC, MAM, YHC, NWSC.
Funding
This research was supported by the National Medical Research Council Transition Award (TA24jul-0008), the CSDU Clinician-Scientist Grant and the CArdiovascular DiseasE National Collaborative Enterprise (CADENCE) Singapore National Clinical Translational Program.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki. The study was exempt from IRB review as no confidential information was involved.
Consent for publication
Not applicable.
All other authors of the manuscript do not have a conflict of interest to declare.
Authorship statement
All authors approve the final version of the manuscript, including the authorship list and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Presentations
No part of this manuscript or its contents have been presented in any capacity outside of this manuscript as of the time of submission.
Registration and protocol
This review was registered on the Open Science Framework (OSF) registries prior to conduction (10.17605/OSF.IO/7VXN5).
Competing interests
Dr Nicholas Chew is supported by the NUHS Seed Fund (NUHSRO/2022/058/RO5+6/Seed-Mar/03), National University of Singapore Yong Loo Lin School of Medicine’s Academic Fellowship Scheme, and NUHS Clinician Scientist Program (NCSP2.0/2024/NUHS/NCWS).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ansel Shao Pin Tang, Jovan Teng Yuan Hsu, Sheena Kar Shuan Chong: Co-first authorship
Mamas Andreas Mamas, Yip Han Chin, Nicholas WS. Chew: Co-last authorship
References
- 1.Federation WH. Deaths from cardiovascular disease surged 60% globally over the last 30 years: Report: World Heart Federation; [updated 23/05/2023; cited 2024 15/10/2024]. Available from: https://world-heart-federation.org/news/deaths-from-cardiovascular-disease-surged-60-globally-over-the-last-30-years-report/
- 2.Woodruff RC, Tong X, Khan SS, Shah NS, Jackson SL, Loustalot F, et al. Trends in cardiovascular disease mortality rates and excess deaths, 2010–2022. Am J Prev Med. 2024;66(4):582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaduganathan M, Mensah George A, Turco Justine V, Fuster V, Roth GA. The global burden of cardiovascular diseases and risk. J Am Coll Cardiol. 2022;80(25):2361–71. [DOI] [PubMed] [Google Scholar]
- 4.Nebuwa C, Omoike OJ, Fagbenro A, Uwumiro F, Erhus E, Okpujie V, et al. Rising cardiovascular mortality despite increased resource utilization: insights from the nationwide inpatient sample database. Cureus. 2024;16(4): e57856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chong B, Jayabaskaran J, Jauhari SM, Chan SP, Goh R, Kueh MTW, et al. Global burden of cardiovascular diseases: projections from 2025 to 2050. Eur J Prevent Cardiol. 2024. 10.1093/eurjpc/zwae281. [DOI] [PubMed] [Google Scholar]
- 6.Chew NW, Figtree GA, Kong G, Vernon S, Muthiah M, Ng CH, et al. Hepatic steatosis and advanced fibrosis are independent predictors of mortality in acute myocardial infarction without standard modifiable risk factors. Diabetes Obes Metab. 2022;24(12):2454–8. [DOI] [PubMed] [Google Scholar]
- 7.Lee EC, Anand VV, Razavi AC, Alebna PL, Muthiah MD, Siddiqui MS, et al. The global epidemic of metabolic fatty liver disease. Curr Cardiol Rep. 2024;26(4):199–210. [DOI] [PubMed] [Google Scholar]
- 8.Ng CH, Chan KE, Chin YH, Zeng RW, Tsai PC, Lim WH, et al. The effect of diabetes and prediabetes on the prevalence, complications and mortality in nonalcoholic fatty liver disease. Clin Mol Hepatol. 2022;28(3):565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yong JN, Ng CH, Lee CW-M, Chan YY, Tang ASP, Teng M, et al. Non-alcoholic fatty liver disease association with structural heart, systolic and diastolic dysfunction: a meta-analysis. Hepatol Int. 2022;16(2):269–81. [DOI] [PubMed] [Google Scholar]
- 10.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Furtado RHM, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139(17):2022–31. [DOI] [PubMed] [Google Scholar]
- 11.Chew NWS, Mehta A, Goh RSJ, Zhang A, Chen Y, Chong B, et al. Cardiovascular-liver-metabolic health: recommendations in screening, diagnosis, and management of metabolic dysfunction-associated steatotic liver disease in cardiovascular disease via modified Delphi approach. Circulation. 2025;151(1):98–119. [DOI] [PubMed] [Google Scholar]
- 12.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kueh MT, Chew NW, Al-Ozairi E, le Roux CW. The emergence of obesity in type 1 diabetes. Int J Obes. 2024;48(3):289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lincoff AM, Brown-Frandsen K, Colhoun HM, Deanfield J, Emerson SS, Esbjerg S, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023;389(24):2221–32. [DOI] [PubMed] [Google Scholar]
- 15.Heaton J, Alshami A, Imburgio S, Upadhyaya V, Saybolt M, Apolito R, et al. Comparison of pooled cohort equation and PREVENT™ risk calculator for statin treatment allocation. Atherosclerosis. 2024;399: 118626. [DOI] [PubMed] [Google Scholar]
- 16.Larkin H. What to know about PREVENT, the AHA’s new cardiovascular disease risk calculator. JAMA. 2024;331(4):277–9. [DOI] [PubMed] [Google Scholar]
- 17.Chong B, Jayabaskaran J, Ruban J, Goh R, Chin YH, Kong G, et al. Epicardial adipose tissue assessed by computed tomography and echocardiography are associated with adverse cardiovascular outcomes: a systematic review and meta-analysis. Circ Cardiovasc Imaging. 2023;16(5):e015159. [DOI] [PubMed] [Google Scholar]
- 18.Chew NW, Ng CH, Chan KE, Chee D, Syn N, Tamaki N, et al. FIB-4 predicts MACE and cardiovascular mortality in patients with nonalcoholic fatty liver disease. Can J Cardiol. 2022;38(11):1779–80. [DOI] [PubMed] [Google Scholar]
- 19.Chew NW, Chong B, Kuo SM, Jayabaskaran J, Cai M, Zheng H, et al. Trends and predictions of metabolic risk factors for acute myocardial infarction: findings from a multiethnic nationwide cohort. The Lancet Regional Health-Western Pacific. 2023;37:100803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Virani SS, Newby LK, Arnold SV, Bittner V, Brewer LC, Demeter SH, et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA guideline for the management of patients with chronic coronary disease: a report of the American heart association/American college of cardiology joint committee on clinical practice guidelines. Circulation. 2023;148(9):e9–119. [DOI] [PubMed] [Google Scholar]
- 21.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–726. [DOI] [PubMed] [Google Scholar]
- 22.Marx N, Federici M, Schütt K, Müller-Wieland D, Ajjan RA, Antunes MJ, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023;44(39):4043–140. [DOI] [PubMed] [Google Scholar]
- 23.Giugliano D, Scappaticcio L, Longo M, Caruso P, Maiorino MI, Bellastella G, et al. GLP-1 receptor agonists and cardiorenal outcomes in type 2 diabetes: an updated meta-analysis of eight CVOTs. Cardiovasc Diabetol. 2021;20:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bethel MA, Patel RA, Merrill P, Lokhnygina Y, Buse JB, Mentz RJ, et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol. 2018;6(2):105–13. [DOI] [PubMed] [Google Scholar]
- 25.Leite AR, Angélico-Gonçalves A, Vasques-Nóvoa F, Borges-Canha M, Leite-Moreira A, Neves JS, et al. Effect of glucagon-like peptide-1 receptor agonists on cardiovascular events in overweight or obese adults without diabetes: a meta-analysis of placebo-controlled randomized trials. Diabetes Obes Metab. 2022;24(8):1676–80. [DOI] [PubMed] [Google Scholar]
- 26.Rivera FB, Cruz LLA, Magalong JV, Ruyeras JMMJ, Aparece JP, Bantayan NRB, et al. Cardiovascular and renal outcomes of glucagon-like peptide 1 receptor agonists among patients with and without type 2 diabetes mellitus: a meta-analysis of randomized placebo-controlled trials. Am J Prevent Cardiol. 2024;18: 100679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paule RC, Mandel J. Consensus values and weighting factors. J Res Natl Bur Stand. 1982;87(5):377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrer M, Cuijpers P, Furukawa T, Ebert D. Doing meta-analysis with R: A hands-on guide. Boca Raton: Chapman and Hall/CRC; 2021. [Google Scholar]
- 30.Weber F, Knapp G, Ickstadt K, Kundt G, Glass Ä. Zero-cell corrections in random-effects meta-analyses. Res Synth Methods. 2020;11(6):913–9. [DOI] [PubMed] [Google Scholar]
- 31.Deeks JJ, Higgins JP, Altman DG, Group CSM. Analysing data and undertaking meta‐analyses. Cochrane handbook for systematic reviews of interventions. 2019;241–84.
- 32.Tufanaru C, Munn Z, Stephenson M, Aromataris E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. JBI Evidence Implementation. 2015;13(3):196–207. [DOI] [PubMed] [Google Scholar]
- 33.Bell A, Fairbrother M, Jones K. Fixed and random effects models: making an informed choice. Qual Quant. 2019;53:1051–74. [Google Scholar]
- 34.Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Assessing risk of bias in a randomized trial. In: Cochrane handbook for systematic reviews of interventions 2019; pp 205–28.
- 35.IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruff CT, Baron M, Im K, O’Donoghue ML, Fiedorek FT, Sabatine MS. Subcutaneous infusion of exenatide and cardiovascular outcomes in type 2 diabetes: a non-inferiority randomized controlled trial. Nat Med. 2022;28(1):89–95. [DOI] [PubMed] [Google Scholar]
- 39.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. The Lancet. 2019;394(10193):121–30. [DOI] [PubMed] [Google Scholar]
- 40.Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–57. [DOI] [PubMed] [Google Scholar]
- 41.Green JB, Everett BM, Ghosh A, Younes N, Krause-Steinrauf H, Barzilay J, et al. Cardiovascular outcomes in GRADE (Glycemia reduction approaches in type 2 diabetes: a comparative effectiveness study). Circulation. 2024;149(13):993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Del Prato S, Kahn SE, Pavo I, Weerakkody GJ, Yang Z, Doupis J, et al. Efficacy and safety of tirzepatide versus insulin glargine in patients with type 2 diabetes and increased cardiovascular risk (SURPASS-4). Diabetologie und Stoffwechsel. 2022;17(01):008. [Google Scholar]
- 43.Pei Y, Agner BR, Luo B, Dong X, Li D, Liu J, et al. DUAL II China: Superior HbA1c reductions and weight loss with insulin degludec/liraglutide (IDegLira) versus insulin degludec in a randomized trial of Chinese people with type 2 diabetes inadequately controlled on basal insulin. Diabetes Obes Metab. 2021;23(12):2687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frias JP, Wynne AG, Matyjaszek-Matuszek B, Bartaskova D, Cox DA, Woodward B, et al. Efficacy and safety of an expanded dulaglutide dose range: a phase 2, placebo-controlled trial in patients with type 2 diabetes using metformin. Diabetes Obes Metab. 2019;21(9):2048–57. [DOI] [PubMed] [Google Scholar]
- 45.Philis-Tsimikas A, Billings LK, Busch R, Portillo CM, Sahay R, Halladin N, et al. Superior efficacy of insulin degludec/liraglutide versus insulin glargine U100 as add-on to sodium-glucose co-transporter-2 inhibitor therapy: a randomized clinical trial in people with uncontrolled type 2 diabetes. Diabetes Obes Metab. 2019;21(6):1399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seino Y, Terauchi Y, Osonoi T, Yabe D, Abe N, Nishida T, et al. Safety and efficacy of semaglutide once weekly vs sitagliptin once daily, both as monotherapy in Japanese people with type 2 diabetes. Diabetes Obes Metab. 2018;20(2):378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jabbour SA, Frias JP, Hardy E, Ahmed A, Wang H, Öhman P, et al. Safety and efficacy of exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy: 52-week results of the DURATION-8 randomized controlled trial. Diabetes Care. 2018;41(10):2136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Le Roux CW, Astrup A, Fujioka K, Greenway F, Lau DC, Van Gaal L, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. The Lancet. 2017;389(10077):1399–409. [DOI] [PubMed] [Google Scholar]
- 49.Araki E, Inagaki N, Tanizawa Y, Oura T, Takeuchi M, Imaoka T. Efficacy and safety of once-weekly dulaglutide in combination with sulphonylurea and/or biguanide compared with once-daily insulin glargine in Japanese patients with type 2 diabetes: a randomized, open-label, phase III, non-inferiority study. Diabetes Obes Metab. 2015;17(10):994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gough SC, Bode B, Woo V, Rodbard HW, Linjawi S, Poulsen P, et al. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2(11):885–93. [DOI] [PubMed] [Google Scholar]
- 51.Yu Pan C, Han P, Liu X, Yan S, Feng P, Zhou Z, et al. Lixisenatide treatment improves glycaemic control in Asian patients with type 2 diabetes mellitus inadequately controlled on metformin with or without sulfonylurea: a randomized, double-blind, placebo-controlled, 24-week trial (GetGoal-M-Asia). Diabetes Metab Res Rev. 2014;30(8):726–35. [DOI] [PubMed] [Google Scholar]
- 52.Riddle MC, Forst T, Aronson R, Sauque-Reyna L, Souhami E, Silvestre L, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: a 24-week, randomized, placebo-controlled study (GetGoal-Duo 1). Diabetes Care. 2013;36(9):2497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gerstein HC, Li Z, Ramasundarahettige C, Baek S, Branch KR, Del Prato S, et al. Exploring the relationship between efpeglenatide dose and cardiovascular outcomes in type 2 diabetes: insights from the AMPLITUDE-O trial. Circulation. 2023;147(13):1004–13. [DOI] [PubMed] [Google Scholar]
- 54.Gerstein HC, Sattar N, Rosenstock J, Ramasundarahettige C, Pratley R, Lopes RD, et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N Engl J Med. 2021;385(10):896–907. [DOI] [PubMed] [Google Scholar]
- 55.Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hernandez AF, Green JB, Janmohamed S, D’Agostino RB, Granger CB, Jones NP, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. The Lancet. 2018;392(10157):1519–29. [DOI] [PubMed] [Google Scholar]
- 57.Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841–51. [DOI] [PubMed] [Google Scholar]
- 58.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–44. [DOI] [PubMed] [Google Scholar]
- 59.Pinget M, Goldenberg R, Niemoeller E, Muehlen-Bartmer I, Guo H, Aronson R. Efficacy and safety of lixisenatide once daily versus placebo in type 2 diabetes insufficiently controlled on pioglitazone (GetGoal-P). Diabetes Obes Metab. 2013;15(11):1000–7. [DOI] [PubMed] [Google Scholar]
- 60.Perkovic V, Tuttle KR, Rossing P, Mahaffey KW, Mann JF, Bakris G, et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N Engl J Med. 2024;391(2):109–21. [DOI] [PubMed] [Google Scholar]
- 61.McGuire DK, Marx N, Mulvagh SL, Deanfield JE, Inzucchi SE, Pop-Busui R, et al. Oral Semaglutide and cardiovascular outcomes in high-risk type 2 diabetes. N Engl J Med. 2025;392(20):2001–12. [DOI] [PubMed] [Google Scholar]
- 62.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 63.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. 2019;140(11):e596–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: The Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Eur Heart J. 2019;41(2):255–323. [DOI] [PubMed] [Google Scholar]
- 65.Kong G, Chin YH, Chong B, Goh RSJ, Lim OZH, Ng CH, et al. Higher mortality in acute coronary syndrome patients without standard modifiable risk factors: results from a global meta-analysis of 1,285,722 patients. Int J Cardiol. 2023;371:432–40. [DOI] [PubMed] [Google Scholar]
- 66.Kong G, Chew NW, Ng CH, Chin YH, Lim OZ, Ambhore A, et al. Prognostic outcomes in acute myocardial infarction patients without standard modifiable risk factors: a multiethnic study of 8,680 Asian patients. Front Cardiovasc Med. 2022;9: 869168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Figtree GA, Vernon ST, Harmer JA, Gray MP, Arnott C, Bachour E, et al. Clinical pathway for coronary atherosclerosis in patients without conventional modifiable risk factors: JACC State-of-the-Art Review. J Am Coll Cardiol. 2023;82(13):1343–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kong G, Chew NW, Ng CH, Chin YH, Zeng R, Foo R, et al. Long-term outcomes in acute coronary syndrome patients without standard modifiable risk factors: a multi-ethnic retrospective cohort study of 5400 Asian patients. J Thromb Thrombolysis. 2022;54(4):569–78. [DOI] [PubMed] [Google Scholar]
- 69.Park B, Bakbak E, Teoh H, Krishnaraj A, Dennis F, Quan A, et al. GLP-1 receptor agonists and atherosclerosis protection: the vascular endothelium takes center stage. Am J Physiol Heart Circ Physiol. 2024;326(5):H1159–76. [DOI] [PubMed] [Google Scholar]
- 70.Tan B, Pan X-H, Chew HSJ, Goh RSJ, Lin C, Anand VV, et al. Efficacy and safety of tirzepatide for treatment of overweight or obesity. A systematic review and meta-analysis. Int J Obes. 2023;47(8):677–85. [DOI] [PubMed] [Google Scholar]
- 71.Chew NW, Ng CH, Muthiah MD, Sanyal AJ. Comprehensive review and updates on holistic approach towards non-alcoholic fatty liver disease management with cardiovascular disease. Curr Atheroscler Rep. 2022;24(7):515–32. [DOI] [PubMed] [Google Scholar]
- 72.Chew NW, Ng CH, Truong E, Noureddin M, Kowdley KV, editors. Nonalcoholic steatohepatitis drug development pipeline: an update. Seminars in Liver Disease; 2022: Thieme Medical Publishers, Inc. [DOI] [PubMed]
- 73.Zheng SL, Roddick AJ. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta-analysis. JAMA. 2019;321(3):277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.David Newman M. Statin Drugs given for 5 years for heart disease prevention (without known heart disease) 2015 [updated January 10, 2015. Available from: https://thennt.com/nnt/statins-for-heart-disease-prevention-without-prior-heart-disease-2/.
- 75.James McCormack MP-RbRR, MD and Barbara Roberts, MD. Blood Pressure Medicines for Five Years to Prevent Death, Heart Attacks, and Strokes 2014 [updated July 21, 2014. Available from: https://thennt.com/nnt/anti-hypertensives-to-prevent-death-heart-attacks-and-strokes/.
- 76.Trevisan M, Fu EL, Szummer K, Norhammar A, Lundman P, Wanner C, et al. Glucagon-like peptide-1 receptor agonists and the risk of cardiovascular events in diabetes patients surviving an acute myocardial infarction. Eur Heart J Cardiovasc Pharmacother. 2021;7(2):104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.De Filippo O, D’Ascenzo F, Iannaccone M, Bertaina M, Leone A, Borzillo I, et al. Safety and efficacy of bempedoic acid: a systematic review and meta-analysis of randomised controlled trials. Cardiovasc Diabetol. 2023;22(1):324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sheahan KH, Wahlberg EA, Gilbert MP. An overview of GLP-1 agonists and recent cardiovascular outcomes trials. Postgrad Med J. 2020;96(1133):156–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chin YH, Lim O, Lin C, Chan YY, Kong G, Ng CH, et al. Meta-analysis of the placebo and nocebo effects associated with placebo treatment in randomized trials of lipid-lowering therapies. Eur Heart J Quality Care Clin Outcomes. 2023;9(5):511–9. [DOI] [PubMed] [Google Scholar]
- 80.Yaow CYL, Chong B, Chin YH, Kueh MTW, Ng CH, Chan KE, et al. Higher risk of adverse cardiovascular outcomes in females with type 2 diabetes Mellitus: an Umbrella review of systematic reviews. Eur J Prev Cardiol. 2023;30(12):1227–35. [DOI] [PubMed] [Google Scholar]
- 81.Bogers RP, Bemelmans WJ, Hoogenveen RT, Boshuizen HC, Woodward M, Knekt P, et al. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: a meta-analysis of 21 cohort studies including more than 300 000 persons. Arch Intern Med. 2007;167(16):1720–8. [DOI] [PubMed] [Google Scholar]
- 82.Adams B, Jacocks L, Guo H. Higher BMI is linked to an increased risk of heart attacks in European adults: a Mendelian randomisation study. BMC Cardiovasc Disord. 2020;20:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dar S, Siddiqi AK, Alabduladhem TO, Rashid AM, Sarfraz S, Maniya T, et al. Effects of novel glucose-lowering drugs on the lipid parameters: a systematic review and meta-analysis. Ann Med Surg. 2022;77: 103633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fu CE, Ng CH, Yong JN, Chan KE, Xiao J, Nah B, et al. A meta-analysis on associated risk of mortality in nonalcoholic fatty liver disease. Endocr Pract. 2023;29(1):33–9. [DOI] [PubMed] [Google Scholar]
- 85.Chan KE, Ng CH, Fu CE, Quek J, Kong G, Goh YJ, et al. The spectrum and impact of metabolic dysfunction in MAFLD: a longitudinal cohort analysis of 32683 overweight and obese individuals. Clin Gastroenterol Hepatol. 2023;21(10):2560-9.e15. [DOI] [PubMed] [Google Scholar]
- 86.Kong G, Zhang A, Chong B, Lim J, Kannan S, Han Chin Y, et al. Long-term prognosis of patients with coexisting obesity and malnutrition after acute myocardial infarction: a cohort study. Circ Cardiovasc Quality Outcomes. 2023;16(4):e009340. [DOI] [PubMed] [Google Scholar]
- 87.Kong G, Chin YH, Lim J, Ng CH, Kannan S, Chong B, et al. A two-decade population-based study on the effect of hypertension in the general population with obesity in the United States. Obesity. 2023;31(3):832–40. [DOI] [PubMed] [Google Scholar]
- 88.Xiao J, Ng CH, Chan KE, Fu C, Tay P, Yong JN, et al. Hepatic, extra-hepatic outcomes and causes of mortality in NAFLD–an umbrella overview of systematic review of meta-analysis. J Clin Exp Hepatol. 2023;13(4):656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fernando K, Bain SC, Holmes P, Jones PN, Patel DC. Glucagon-like peptide 1 receptor agonist usage in type 2 diabetes in primary care for the UK and beyond: a narrative review. Diabetes Therapy. 2021;12(9):2267–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marx N, Husain M, Lehrke M, Verma S, Sattar N. GLP-1 receptor agonists for the reduction of atherosclerotic cardiovascular risk in patients with type 2 diabetes. Circulation. 2022;146(24):1882–94. [DOI] [PubMed] [Google Scholar]
- 91.Wright AK, Carr MJ, Kontopantelis E, Leelarathna L, Thabit H, Emsley R, et al. Primary prevention of cardiovascular and heart failure events with SGLT2 inhibitors, GLP-1 receptor agonists, and their combination in type 2 diabetes. Diabetes Care. 2022;45(4):909–18. [DOI] [PubMed] [Google Scholar]
- 92.Chew NW, Kong G, Venisha S, Chin YH, Ng CH, Muthiah M, et al. Long-term prognosis of acute myocardial infarction associated with metabolic health and obesity status. Endocr Pract. 2022;28(8):802–10. [DOI] [PubMed] [Google Scholar]
- 93.Lin C, Loke WH, Ng BH, Chin YH, Chong B, Goh RSJ, et al. Mortality, cardiovascular, and medication outcomes in patients with myocardial infarction and underweight in a meta-analysis of 6.3 million patients. Am J Cardiol. 2023;196:1–10. [DOI] [PubMed] [Google Scholar]
- 94.Chew NW, Pan XH, Chong B, Chandramouli C, Muthiah M, Lam CS. Type 2 diabetes mellitus and cardiometabolic outcomes in metabolic dysfunction-associated steatotic liver disease population. Diabetes Res Clin Pract. 2024;211: 111652. [DOI] [PubMed] [Google Scholar]
- 95.Lai AR, Warrier M, Ng EZ, Lin C, Chin YH, Kong G, et al. Cardiovascular outcomes in acute coronary syndrome and malnutrition: a meta-analysis of nutritional assessment tools. JACC Adv. 2023;2(8):100635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sattar N, Lee MMY, Kristensen SL, Branch KRH, Del Prato S, Khurmi NS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021;9(10):653–62. [DOI] [PubMed] [Google Scholar]
- 97.Villaschi A, Ferrante G, Cannata F, Pini D, Pagnesi M, Corrada E, et al. GLP-1-ra and heart failure-related outcomes in patients with and without history of heart failure: an updated systematic review and meta-analysis. Clin Res Cardiol. 2024;113(6):898–909. [DOI] [PubMed] [Google Scholar]
- 98.Marfella R, Prattichizzo F, Sardu C, Rambaldi PF, Fumagalli C, Marfella LV, et al. GLP-1 receptor agonists-SGLT-2 inhibitors combination therapy and cardiovascular events after acute myocardial infarction: an observational study in patients with type 2 diabetes. Cardiovasc Diabetol. 2024;23(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Marfella R, Rizzo MR, Siniscalchi M, Paolisso P, Barbieri M, Sardu C, et al. Peri-procedural tight glycemic control during early percutaneous coronary intervention up-regulates endothelial progenitor cell level and differentiation during acute ST-elevation myocardial infarction: effects on myocardial salvage. Int J Cardiol. 2013;168(4):3954–62. [DOI] [PubMed] [Google Scholar]
- 100.Paolisso P, Bergamaschi L, Santulli G, Gallinoro E, Cesaro A, Gragnano F, et al. Infarct size, inflammatory burden, and admission hyperglycemia in diabetic patients with acute myocardial infarction treated with SGLT2-inhibitors: a multicenter international registry. Cardiovasc Diabetol. 2022;21(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Muthiah M, Ng CH, Chan KE, Fu CE, Lim WH, Tan DJH, et al. Type 2 diabetes mellitus in metabolic-associated fatty liver disease versus type 2 diabetes mellitus non-alcoholic fatty liver disease: a longitudinal cohort analysis. Ann Hepatol. 2023;28(1):100762. [DOI] [PubMed] [Google Scholar]
- 102.Chin YH, Ng CH, Chew NW, Kong G, Lim WH, Tan DJH, et al. The placebo response rate and nocebo events in obesity pharmacological trials. A systematic review and meta-analysis. EClinicalMedicine. 2022;54. [DOI] [PMC free article] [PubMed]
- 103.Llewellyn DC, Logan Ellis H, Aylwin SJ, Oštarijaš E, Green S, Sheridan W, et al. The efficacy of GLP-1RAs for the management of postprandial hypoglycemia following bariatric surgery: a systematic review. Obesity. 2023;31(1):20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yeong T, Mai AS, Lim OZ, Ng CH, Chin YH, Tay P, et al. Can glucose-lowering medications improve outcomes in non-diabetic heart failure patients? A Bayesian network meta-analysis. ESC Heart Failure. 2022;9(2):1338–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chew NW, Ng C-H, Kong G, Lee K-S, Tan DJ, Lim OZ-H, et al. Meta-analysis of percutaneous coronary intervention versus coronary artery bypass grafting for left main narrowing. Am J Cardiol. 2022;173:39–47. [DOI] [PubMed] [Google Scholar]
- 106.Alebna PL, Han CY, Ambrosio M, Kong G, Cyrus JW, Harley K, et al. Association of Lipoprotein (a) with major adverse cardiovascular events across hs-CRP: a systematic review and meta-analysis. JACC Adv. 2024;3(12_Part_1):101409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rodriguez PJ, Zhang V, Gratzl S, Do D, Goodwin Cartwright B, Baker C, et al. Discontinuation and reinitiation of dual-labeled glp-1 receptor agonists among US adults with overweight or obesity. JAMA Netw Open. 2025;8(1): e2457349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Durden E, Liang M, Fowler R, Panton UH, Mocevic E. The effect of early response to GLP-1 RA therapy on long-term adherence and persistence among type 2 diabetes patients in the United States. J Manag Care Spec Pharm. 2019;25(6):669–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Weiss T, Carr RD, Pal S, Yang L, Sawhney B, Boggs R, et al. Real-world adherence and discontinuation of glucagon-like peptide-1 receptor agonists therapy in type 2 diabetes mellitus patients in the United States. Patient Prefer Adherence. 2020;14:2337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gasoyan H, Pfoh ER, Schulte R, Le P, Rothberg MB. Early- and later-stage persistence with antiobesity medications: a retrospective cohort study. Obesity (Silver Spring). 2024;32(3):486–93. [DOI] [PubMed] [Google Scholar]
- 111.Do D, Lee T, Peasah SK, Good CB, Inneh A, Patel U. GLP-1 receptor agonist discontinuation among patients with obesity and/or type 2 diabetes. JAMA Netw Open. 2024;7(5):e2413172-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.