Abstract
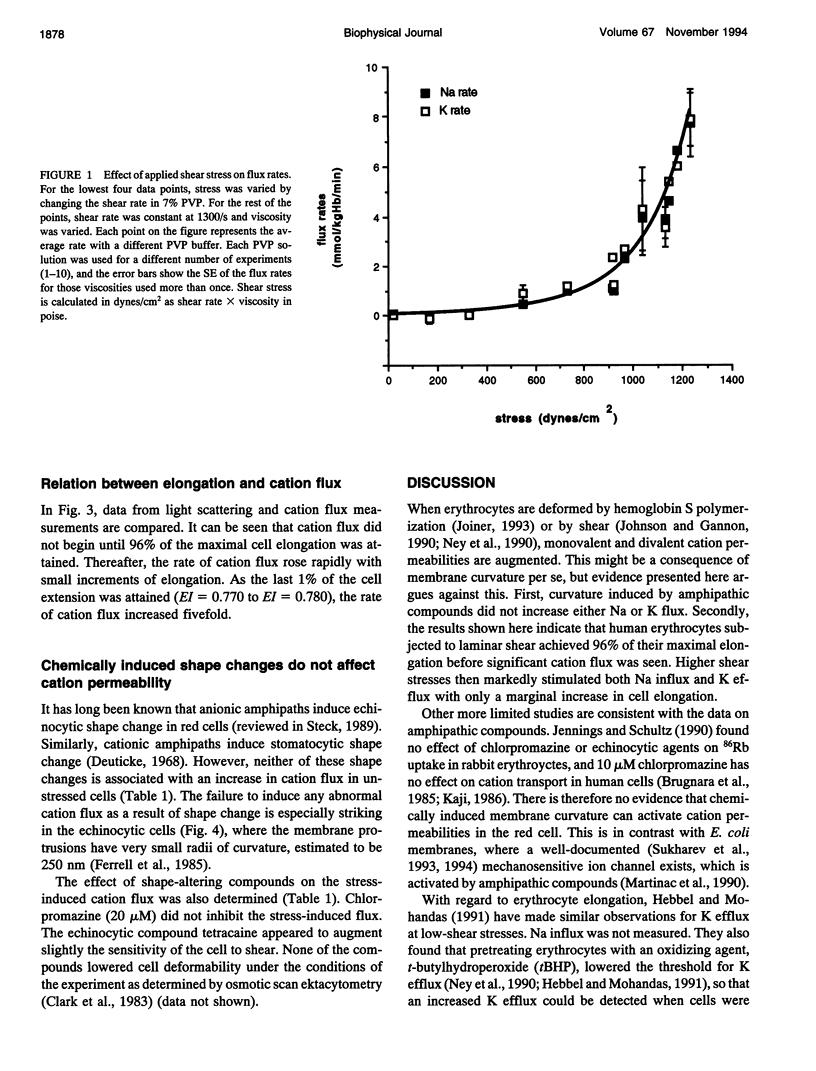
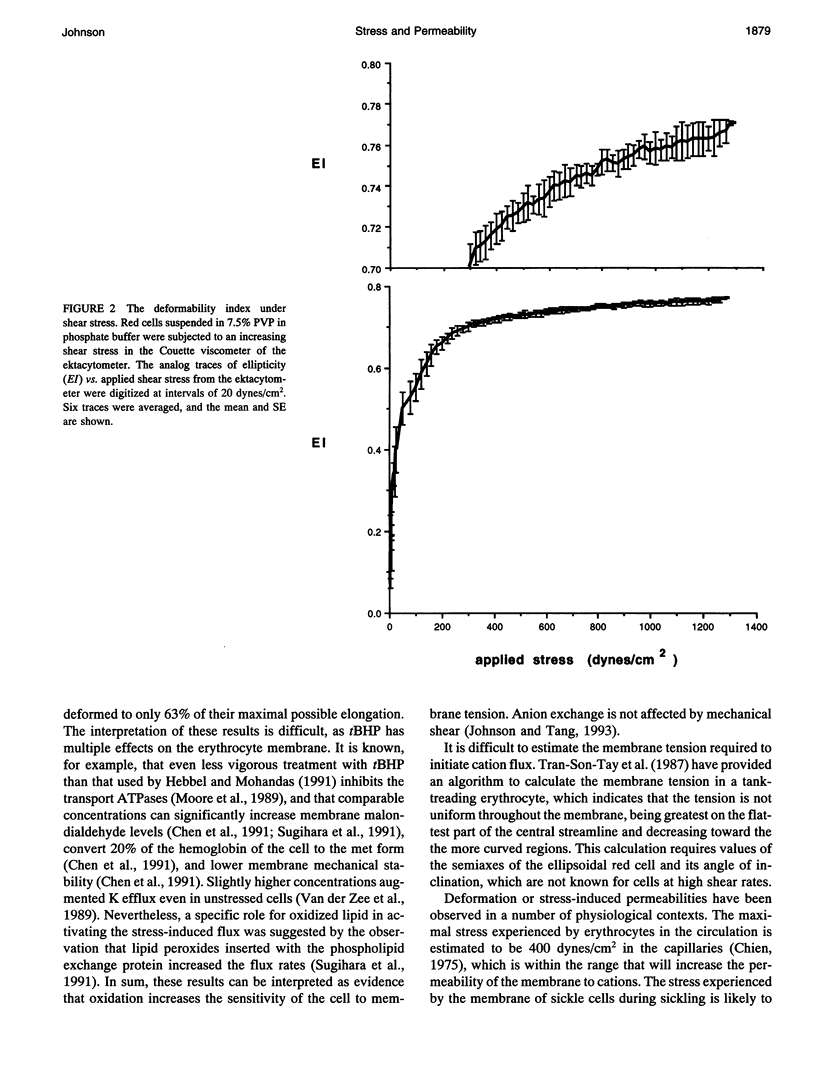
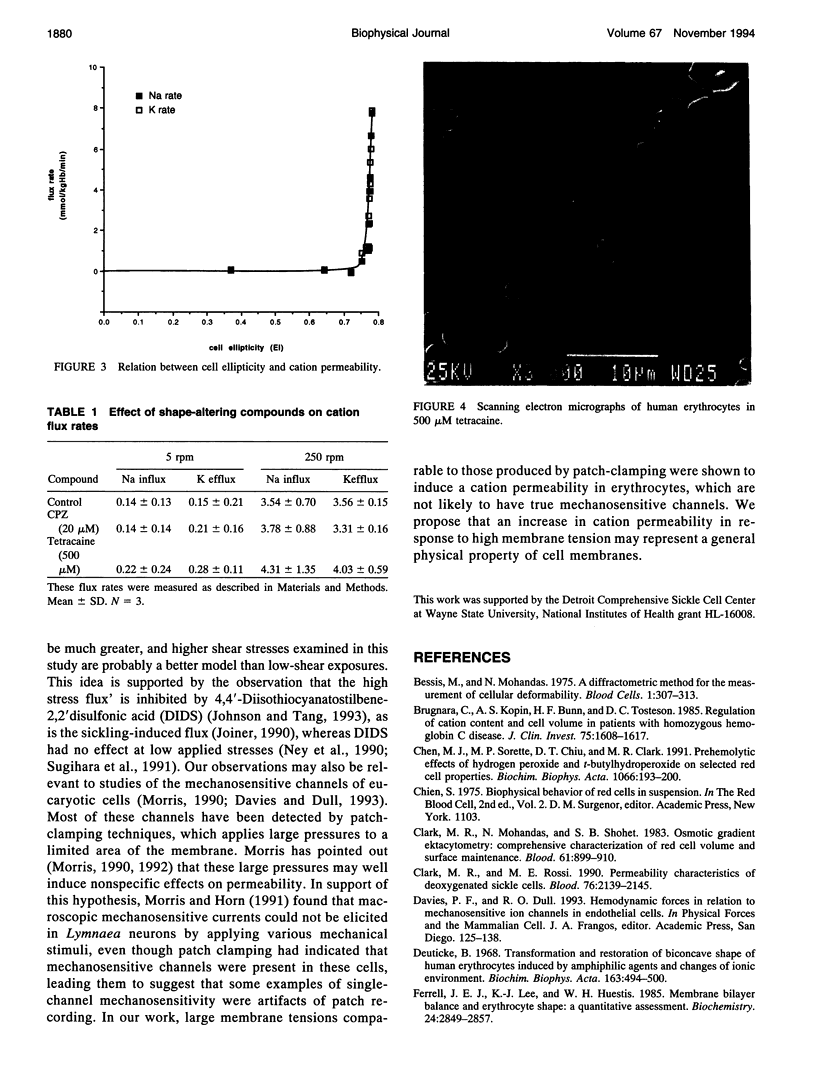
The human red cell is known to increase its cation permeability when deformed by mechanical forces. Light-scattering measurements were used to quantitate the cell deformation, as ellipticity under shear. Permeability to sodium and potassium was not proportional to the cell deformation. An ellipticity of 0.75 was required to increase the permeability of the membrane to cations, and flux thereafter increased rapidly as the limits of cell extension were reached. Induction of membrane curvature by chemical agents also did not increase cation permeability. These results indicate that membrane deformation per se does not increase permeability, and that membrane tension is the effector for increased cation permeability. This may be relevant to some cation permeabilities observed by patch clamping.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brugnara C., Kopin A. S., Bunn H. F., Tosteson D. C. Regulation of cation content and cell volume in hemoglobin erythrocytes from patients with homozygous hemoglobin C disease. J Clin Invest. 1985 May;75(5):1608–1617. doi: 10.1172/JCI111867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. J., Sorette M. P., Chiu D. T., Clark M. R. Prehemolytic effects of hydrogen peroxide and t-butylhydroperoxide on selected red cell properties. Biochim Biophys Acta. 1991 Jul 22;1066(2):193–200. doi: 10.1016/0005-2736(91)90186-c. [DOI] [PubMed] [Google Scholar]
- Clark M. R., Mohandas N., Shohet S. B. Osmotic gradient ektacytometry: comprehensive characterization of red cell volume and surface maintenance. Blood. 1983 May;61(5):899–910. [PubMed] [Google Scholar]
- Clark M. R., Rossi M. E. Permeability characteristics of deoxygenated sickle cells. Blood. 1990 Nov 15;76(10):2139–2145. [PubMed] [Google Scholar]
- Deuticke B. Transformation and restoration of biconcave shape of human erythrocytes induced by amphiphilic agents and changes of ionic environment. Biochim Biophys Acta. 1968 Dec 10;163(4):494–500. doi: 10.1016/0005-2736(68)90078-3. [DOI] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Lee K. J., Huestis W. H. Membrane bilayer balance and erythrocyte shape: a quantitative assessment. Biochemistry. 1985 Jun 4;24(12):2849–2857. doi: 10.1021/bi00333a006. [DOI] [PubMed] [Google Scholar]
- Groner W., Mohandas N., Bessis M. New optical technique for measuring erythrocyte deformability with the ektacytometer. Clin Chem. 1980 Sep;26(10):1435–1442. [PubMed] [Google Scholar]
- Hebbel R. P., Mohandas N. Reversible deformation-dependent erythrocyte cation leak. Extreme sensitivity conferred by minimal peroxidation. Biophys J. 1991 Sep;60(3):712–715. doi: 10.1016/S0006-3495(91)82100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings M. L., Schulz R. K. Swelling-activated KCl cotransport in rabbit red cells: flux is determined mainly by cell volume rather than shape. Am J Physiol. 1990 Dec;259(6 Pt 1):C960–C967. doi: 10.1152/ajpcell.1990.259.6.C960. [DOI] [PubMed] [Google Scholar]
- Johnson R. M., Gannon S. A. Erythrocyte cation permeability induced by mechanical stress: a model for sickle cell cation loss. Am J Physiol. 1990 Nov;259(5 Pt 1):C746–C751. doi: 10.1152/ajpcell.1990.259.5.C746. [DOI] [PubMed] [Google Scholar]
- Johnson R. M., Tang K. DIDS inhibition of deformation-induced cation flux in human erythrocytes. Biochim Biophys Acta. 1993 May 14;1148(1):7–14. doi: 10.1016/0005-2736(93)90154-r. [DOI] [PubMed] [Google Scholar]
- Johnson R. M., Tang K. Induction of a Ca(2+)-activated K+ channel in human erythrocytes by mechanical stress. Biochim Biophys Acta. 1992 Jun 30;1107(2):314–318. doi: 10.1016/0005-2736(92)90418-l. [DOI] [PubMed] [Google Scholar]
- Joiner C. H. Cation transport and volume regulation in sickle red blood cells. Am J Physiol. 1993 Feb;264(2 Pt 1):C251–C270. doi: 10.1152/ajpcell.1993.264.2.C251. [DOI] [PubMed] [Google Scholar]
- Joiner C. H. Deoxygenation-induced cation fluxes in sickle cells: II. Inhibition by stilbene disulfonates. Blood. 1990 Jul 1;76(1):212–220. [PubMed] [Google Scholar]
- Joiner C. H., Morris C. L., Cooper E. S. Deoxygenation-induced cation fluxes in sickle cells. III. Cation selectivity and response to pH and membrane potential. Am J Physiol. 1993 Mar;264(3 Pt 1):C734–C744. doi: 10.1152/ajpcell.1993.264.3.C734. [DOI] [PubMed] [Google Scholar]
- Kaji D. Volume-sensitive K transport in human erythrocytes. J Gen Physiol. 1986 Dec;88(6):719–738. doi: 10.1085/jgp.88.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverett L. B., Hellums J. D., Alfrey C. P., Lynch E. C. Red blood cell damage by shear stress. Biophys J. 1972 Mar;12(3):257–273. doi: 10.1016/S0006-3495(72)86085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinac B., Adler J., Kung C. Mechanosensitive ion channels of E. coli activated by amphipaths. Nature. 1990 Nov 15;348(6298):261–263. doi: 10.1038/348261a0. [DOI] [PubMed] [Google Scholar]
- Mohandas N., Clark M. R., Jacobs M. S., Shohet S. B. Analysis of factors regulating erythrocyte deformability. J Clin Invest. 1980 Sep;66(3):563–573. doi: 10.1172/JCI109888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas N., Rossi M. E., Clark M. R. Association between morphologic distortion of sickle cells and deoxygenation-induced cation permeability increase. Blood. 1986 Aug;68(2):450–454. [PubMed] [Google Scholar]
- Moore R. B., Brummitt M. L., Mankad V. N. Hydroperoxides selectively inhibit human erythrocyte membrane enzymes. Arch Biochem Biophys. 1989 Sep;273(2):527–534. doi: 10.1016/0003-9861(89)90512-2. [DOI] [PubMed] [Google Scholar]
- Morris C. E. Are stretch-sensitive channels in molluscan cells and elsewhere physiological mechanotransducers? Experientia. 1992 Sep 15;48(9):852–858. doi: 10.1007/BF02118418. [DOI] [PubMed] [Google Scholar]
- Morris C. E., Horn R. Failure to elicit neuronal macroscopic mechanosensitive currents anticipated by single-channel studies. Science. 1991 Mar 8;251(4998):1246–1249. doi: 10.1126/science.1706535. [DOI] [PubMed] [Google Scholar]
- Morris C. E. Mechanosensitive ion channels. J Membr Biol. 1990 Feb;113(2):93–107. doi: 10.1007/BF01872883. [DOI] [PubMed] [Google Scholar]
- Ney P. A., Christopher M. M., Hebbel R. P. Synergistic effects of oxidation and deformation on erythrocyte monovalent cation leak. Blood. 1990 Mar 1;75(5):1192–1198. [PubMed] [Google Scholar]
- Pfafferott C., Nash G. B., Meiselman H. J. Red blood cell deformation in shear flow. Effects of internal and external phase viscosity and of in vivo aging. Biophys J. 1985 May;47(5):695–704. doi: 10.1016/S0006-3495(85)83966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoda M. D., Giraud F., Craescu C. T., Beuzard Y. Compartmentalization of Ca2+ in sickle cells. Cell Calcium. 1985 Oct;6(5):397–411. doi: 10.1016/0143-4160(85)90017-x. [DOI] [PubMed] [Google Scholar]
- Sugihara T., Rawicz W., Evans E. A., Hebbel R. P. Lipid hydroperoxides permit deformation-dependent leak of monovalent cation from erythrocytes. Blood. 1991 Jun 15;77(12):2757–2763. [PubMed] [Google Scholar]
- Sukharev S. I., Blount P., Martinac B., Blattner F. R., Kung C. A large-conductance mechanosensitive channel in E. coli encoded by mscL alone. Nature. 1994 Mar 17;368(6468):265–268. doi: 10.1038/368265a0. [DOI] [PubMed] [Google Scholar]
- Sukharev S. I., Martinac B., Arshavsky V. Y., Kung C. Two types of mechanosensitive channels in the Escherichia coli cell envelope: solubilization and functional reconstitution. Biophys J. 1993 Jul;65(1):177–183. doi: 10.1016/S0006-3495(93)81044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOSTESON D. C., SHEA E., DARLING R. C. Potassium and sodium of red blood cells in sickle cell anemia. J Clin Invest. 1952 Apr;31(4):406–411. doi: 10.1172/JCI102623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran-Son-Tay R., Sutera S. P., Zahalak G. I., Rao P. R. Membrane stress and internal pressure in a red blood cell freely suspended in a shear flow. Biophys J. 1987 Jun;51(6):915–924. doi: 10.1016/S0006-3495(87)83419-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Zee J., Van Steveninck J., Koster J. F., Dubbelman T. M. Inhibition of enzymes and oxidative damage of red blood cells induced by t-butylhydroperoxide-derived radicals. Biochim Biophys Acta. 1989 Apr 14;980(2):175–180. doi: 10.1016/0005-2736(89)90397-0. [DOI] [PubMed] [Google Scholar]
- Weaver F. E., Polster H., Febboriello P., Sheetz M. P., Schmid-Schonbein H., Koppel D. E. Normal band 3-cytoskeletal interactions are maintained on tanktreading erythrocytes. Biophys J. 1990 Dec;58(6):1427–1436. doi: 10.1016/S0006-3495(90)82488-7. [DOI] [PMC free article] [PubMed] [Google Scholar]



