Abstract
Introduction
Nipocalimab is a high-affinity, fully human, immunoglobulin G (IgG) 1 monoclonal antibody that inhibits the neonatal Fc receptor. Nipocalimab is under development for the treatment of various IgG autoantibody- and alloantibody-mediated diseases. This study assessed the safety, pharmacokinetics (PK), and pharmacodynamics (PD) of a single dose of nipocalimab in healthy Chinese volunteers.
Methods
In this phase I, open-label study, healthy volunteers received single doses of intravenous (IV) nipocalimab at 15, 30, or 45 mg/kg. The primary objective was to assess the PK following a single administration of nipocalimab. Secondary objectives included the PD effects of nipocalimab on change from baseline in total serum IgG levels, safety, and tolerability.
Results
A total of 30 healthy Chinese volunteers (mean age 31.0 years, 93.3% men) received single doses of IV nipocalimab. Following a single infusion of nipocalimab, mean exposure increased as the dose of nipocalimab increased. Maximum serum nipocalimab concentrations increased proportionally with doses, whereas the area under the concentration–time curve increased by greater than a dose-proportional manner. Nipocalimab led to dose-dependent reductions in serum IgG levels from baseline; this decrease was sustained over a longer period of time with higher dose levels. Nipocalimab was generally well tolerated, with an acceptable safety profile, across all three doses; most of the treatment-emergent adverse events (TEAEs) were mild. Higher doses of nipocalimab were not associated with increased frequency of TEAEs.
Conclusion
Our findings add to the evidence on the safety, tolerability, and PD of nipocalimab in the Chinese population, and support for the treatment of pathogenic IgG-mediated diseases in this population.
Trial registration
ClinicalTrials.gov NCT05151692.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40120-025-00763-5.
Keywords: Autoimmune disease, Chinese, Nipocalimab
Key Summary Points
| Why carry out this study? |
| Nipocalimab has shown promising efficacy and safety in patients with certain autoimmune diseases (including generalized myasthenia gravis) in the global population. |
| However, the pharmacokinetics, pharmacodynamics (PD), and safety data are lacking in the Chinese population. |
| What was learned from this study? |
| Single administration of nipocalimab showed consistent PD profiles, with no new safety signal in healthy Chinese volunteers. |
| Our findings support further development of nipocalimab for the treatment of pathogenic immunoglobulin G-mediated diseases in the Chinese population. |
Introduction
Autoantibody-mediated and alloantibody-mediated diseases comprise over 80 autoimmune/alloimmune conditions across a diverse range of therapeutic areas [1, 2], including autoimmune neuromuscular disorders such as generalized myasthenia gravis (gMG), chronic inflammatory demyelinating polyneuropathy (CIDP), and idiopathic inflammatory myopathies [3]. Autoantibody- and alloantibody-mediated diseases are typically characterized by the presence of pathogenic immunoglobulin G (IgG)-based autoantibodies, which mediate tissue destruction or impairment by directly binding against target structures or via IgG immune complex-induced complement activation or inflammatory phagocyte recruitment [4–7]. In the context of autoimmune neuromuscular disorders, a combination of humoral and cell-mediated immune mechanisms may lead to nerve injury and the resulting impairment of neuromuscular function [3]. Commonly used therapies for autoimmune neuromuscular disorders primarily involve the use of intravenous (IV) immunoglobulins, corticosteroids, and plasmapheresis [3]. However, these therapies may lack adequate efficacy, are nonspecific, and are associated with adverse events (AEs) [8–12].
Neonatal Fc receptor (FcRn), an endosomal IgG transporter, has previously been demonstrated to contribute to the long half-life (t1/2) of IgG and preserve its levels in the systemic circulation [6, 13, 14]. FcRn promotes recycling of IgG from endosomes and its release into the bloodstream, thereby preventing lysosomal degradation of IgG [6, 15, 16]. Therefore, FcRn presents a potential drug target for the treatment of autoantibody-mediated diseases. Targeted blockade of IgG–FcRn binding has been shown to suppress IgG recycling and accelerate degradation of pathogenic and total IgG [17]. Inhibition of FcRn has been shown to result in IgG lowering overall while preserving immune function without causing broad immunosuppression functions [13, 14, 18]. The extent of the reduction in IgG levels achieved with FcRn inhibitors was similar to the levels achieved following plasma exchange and was associated with therapeutic benefit in IgG-mediated immune diseases [19–21].
Nipocalimab is a high-affinity, fully human IgG1 monoclonal antibody that selectively binds to the IgG binding site on endogenous FcRn to block the formation of IgG1–FcRn complexes and facilitate IgG clearance. Nipocalimab shows a unique ability to bind to FcRn with picomolar affinity regardless of whether it is at the endosomal pH of 6.0 or extracellular pH of 7.4, therefore enabling rapid full occupancy of FcRn throughout the recycling pathway [22]. These distinctive features thus confer the potential for rapid and sustained clearance of IgG and disease response [18, 23]. Nipocalimab has been shown to cause rapid and sustained reductions of IgG and IgG autoantibodies in preclinical studies. Nipocalimab has a known safety and pharmacokinetic–pharmacodynamic (PK–PD) profile from previous phase I studies and proven efficacy in specific populations with IgG-mediated autoimmune diseases, including myasthenia gravis, hemolytic disease of the fetus and newborn, and Sjögren’s disease [23–25]. However, these studies involved predominantly non-Asian populations; PK/PD and safety data are still lacking in the Chinese population.
We therefore conducted this phase I study to evaluate the PK, PD, safety, tolerability, and immunogenicity of nipocalimab following a single IV administration in healthy Chinese adult participants.
Methods
Participants
Eligible participants were healthy adults aged 18–55 years, with a body mass index ≥ 18.0 kg/m2 and ≤ 27.9 kg/m2; body weight ≥ 50 kg and ≤ 110 kg, serum IgG level above the lower limit of normal at screening, and no clinically significant medical conditions based on physical examination, medical history, vital signs, 12-lead electrocardiogram (ECG), and clinical laboratory tests at screening. A negative pregnancy test was required for women of childbearing potential, and contraceptive measures were required for both men and women in the study per the protocol.
Key exclusion criteria included the presence or history of clinically significant medical illness or malignancy; use of any prescription or nonprescription medications (except those allowed in the protocol) 14 days prior to first dosing; any history or existing drug or drug-related allergies, hypersensitivity, or drug intolerance; presence of active/acute/chronic infection; serum albumin below the lower limit of normal at screening; receipt of a live vaccine within 12 weeks prior to screening or need to receive a live vaccine during the study/within 12 weeks of the last dose of the study drug; or had a Bacille Calmette-Guérin vaccination within 1 year of the first administration of the study drug. A full list of eligibility criteria is provided in Table S1 in the Supplementary Materials.
Ethical Approval
The study protocol and its amendment were reviewed and approved by an independent ethics committee at the study site (Peking University Third Hospital Medical Science Research Ethics Committee). The study was conducted in compliance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines, and with relevant law and regulatory requirements. All participants had provided written informed consent.
Study Design
This was a single-dose, sequential, open-label, non-randomized phase I study conducted at a single study site in China. The study comprised a screening phase (up to 28 days before administration of the study drug), a 5-day inpatient study phase (single IV doses of nipocalimab were given on day 1), and an outpatient follow-up phase until day 57 (Fig. S1 in the Supplementary Materials).
The three doses of nipocalimab (15, 30, and 45 mg/kg) were selected based on the doses likely to be studied in phase II and/or phase III trials for various autoimmune conditions, including gMG. Based on PK data of previous phase I studies [18, 22], in which nipocalimab was administered up to 60 mg/kg at single doses or up to 30 mg/kg at multiple doses, nipocalimab was well tolerated, with no serious AEs or deaths reported. In a phase II study, nipocalimab 60 mg/kg every 2 weeks also showed that nipocalimab was well tolerated in patients with gMG [23]. PK results for nipocalimab at a once-weekly dose of 30 mg/kg and population PK modeling indicated limited accumulation [18]. Based on these PK and safety findings, no PK accumulation or significant safety issues were expected for the selected doses in healthy Chinese participants. Escalation to the next dose level was permitted only upon demonstration of adequate safety and tolerability from the previous cohort, as assessed by the principal investigator. Nipocalimab was formulated at 30 mg/mL as a solution in a single-use vial, and the actual drug dose for IV administration at a fixed infusion rate over 30 min was determined based on the participant’s weight at check-in (on day −1).
Outcomes
The primary objective of this study was to assess the PK of nipocalimab following single IV administration of nipocalimab in healthy Chinese adult participants. Secondary objectives included safety and tolerability, PD evaluation, and immunogenicity of nipocalimab. Blood FcRn receptor occupancy following single IV administration of nipocalimab was assessed as an exploratory outcome.
PK Assessments
PK analysis of nipocalimab was performed on blood samples collected at the start and end of the infusion on day 1, at prespecified time points within day 1, and at days 2, 3, 4,5, 8, 10, 12, 15, 18, 22, and 29.
Serum concentrations of nipocalimab were analyzed using electrochemiluminescence immunoassay (LabCorp, Shanghai, China), validated in terms of accuracy, specificity, and sensitivity. The detection limits for nipocalimab ranged between 10 and 1280 ng/mL.
Serum PK measures included area under the serum concentration–time curve (AUC) from start of infusion at time 0 to the time of the last measurable concentration (AUClast), AUC from time 0 extrapolated to infinity (AUCinf), maximum observed serum concentration (Cmax), last measurable serum concentration (Clast), time to reach the maximum observed serum concentration (Tmax), time of last measurable serum concentration (Tlast), t1/2, clearance (CL), and volume of distribution (Vz).
PD Assessments
PD analysis of serum total IgG was conducted using blood samples collected at the start and end of the nipocalimab infusion, at days 2, 3, 4,5, 8, 10, 12, 15, 18, 22, 29, and 43, and at the end of the study on day 57. Serum levels of IgG were analyzed using Optilite IgG kit assays (The Binding Site Inc., San Diego, CA, USA).
Whole blood samples for analysis of receptor occupancy in circulating monocytes were collected at similar time points as those for serum total IgG, with additional collection time points at 2 h and 8 h post-infusion on day 1. Receptor occupancy was determined using a flow cytometry method (United-Power Pharma Tech Co., Ltd, Beijing, China). Full receptor occupancy was defined as ≥ 90% occupied blood FcRn (or < 10% of unoccupied receptors).
Immunogenicity Assessments
Binding antibodies to nipocalimab (hereafter referred to as anti-drug antibodies [ADA]) were evaluated in serum samples collected at the start of the infusion and at days 15, 29, and 57, using electrochemiluminescence immunoassay (LabCorp, Shanghai, China).
Safety and Tolerability Assessments
Safety and tolerability of nipocalimab were monitored throughout until the end of the study at day 57. Key safety measures included the frequency and severity of AEs, serious AEs (SAEs), and treatment-emergent AEs (TEAEs), as well as clinical laboratory evaluations, vital signs, and an ECG. AEs were coded according to the Medical Dictionary for Regulatory Activities system organ class and preferred terms for each dose group.
Adverse events of special interest (AESIs) were also recorded, which included hypoalbuminemia (albumin levels < 20 g/L or < 2.0 g/dL) and severe, medically significant, or immediately life-threatening infections requiring IV anti-infective, operative, or invasive intervention requiring hospitalization or prolonged hospitalization. For clinical laboratory evaluations, blood samples for serum chemistry and hematology analysis and a random urine sample for urinalysis were collected per the prespecified schedule in the protocol.
Statistical Analysis
The sample size was planned based on typical sample sizes in phase I studies, with no formal sample size and power calculation. A total of 30 healthy Chinese participants were planned and enrolled across the three dose cohorts, with 10 participants in each cohort, assuming a drop-out rate of 20%.
Participant characteristics, serum concentrations of nipocalimab, and derived PK and PD parameters were summarized using descriptive statistics. PK analysis was performed in all participants who had received one dose of the study drug and had at least one estimated PK parameter. PD analysis was conducted in all participants who received one dose of the study drug and had at least one post-dose PD measurement. Incidence of ADA was summarized based on the immunogenicity population, defined as participants who had received one dose of the study drug and had at least one post-dose sample. Safety analysis was based on the safety population, which included all participants who had received one dose of the study drug.
PK measures were determined using non-compartmental analysis (Phoenix™ WinNonlin® version 8.1.1 Certara LP, Princeton, NJ, USA). All statistical analyses were performed using SAS version 9.4 software (SAS Institute, Cary, NC, USA).
Results
Participant Characteristics
Between May 30, 2022, and September 09, 2022, 30 participants received one dose of nipocalimab and were included in the PK, PD, and safety analyses. Twenty-nine (96.7%) participants completed the study; one participant in the 15-mg/kg nipocalimab group was lost to follow-up after day 15.
In general, baseline demographic and key clinical characteristics were similar across treatment cohorts (Table 1). The mean age was 31.0 years, 93.3% were men, mean body weight was 67.3 kg, mean baseline body mass index was 23.1 kg/m2, and mean baseline serum IgG ranged between 10.3 and 11.2 g/L (Table 1).
Table 1.
Participant baseline demographics and characteristics
| 15 mg/kg (n = 10) | 30 mg/kg (n = 10) | 45 mg/kg (n = 10) | Total (N = 30) | |
|---|---|---|---|---|
| Age, years | 30.1 (4.7) | 30.4 (6.5) | 32.5 (7.3) | 31.0 (6.2) |
| Sex, n (%) | ||||
| Female | 0 | 0 | 2 (20.0) | 2 (6.7) |
| Male | 10 (100.0) | 10 (100.0) | 8 (80.0) | 28 (93.3) |
| Weight, kg | 64.0 (8.6) | 71.8 (8.7) | 66.2 (8.5) | 67.3 (8.9) |
| Height, cm | 170.8 (7.2) | 173.1 (4.9) | 167.7 (7.3) | 170.5 (6.7) |
| BMI, kg/m2 | 22.0 (2.8) | 23.9 (2.0) | 23.6 (2.9) | 23.1 (2.7) |
| IgG, g/L | 10.6 (2.3) | 10.3 (1.6) | 11.2 (2.5) | 10.7 (2.1) |
| Albumin | 47.4 (1.7) | 46.0 (2.4) | 45.5 (1.9) | 46.3 (2.1) |
Data are mean (standard deviation) unless otherwise specified
BMI body mass index, IgG immunoglobulin G
PK
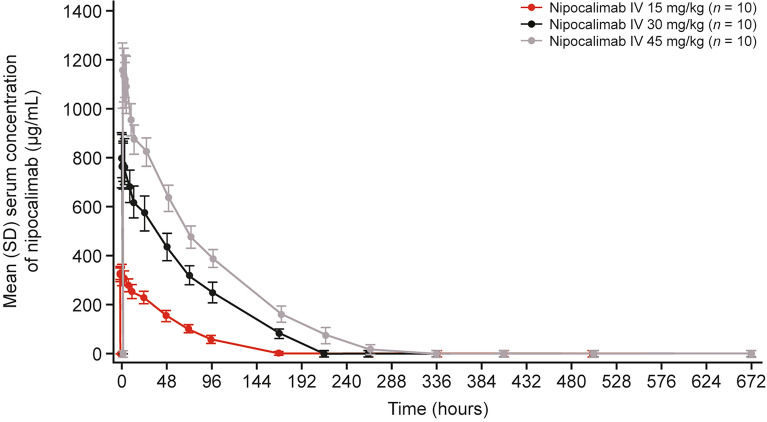
Nipocalimab showed a similar pattern of serum concentration–time profiles following a single infusion across all treatment doses (Fig. 1). The maximum mean serum concentrations were achieved within 15 min after the end of infusion across all dose cohorts, with a greater peak achieved following higher dose levels. Thereafter, the serum concentrations gradually decreased in a non-linear dose-dependent manner, indicating a target-mediated drug disposition. Nipocalimab remained detectable in at least 50% (5/10) of participants up to day 10 (~ 216 h post-dose) for the 15-mg/kg cohort, day 12 (~ 264 h post-dose) for the 30-mg/kg cohort, and day 45 (~ 336 h post-dose) for the 45-mg/kg cohort.
Fig. 1.
Serum concentration–time profiles of nipocalimab. IV, intravenous
As the doses of nipocalimab increased, mean exposure (Cmax and AUCs) also increased (Table 2). Median Tmax values following nipocalimab infusion ranged from 0.5 to 0.8 h (i.e., 2.4–15.0 min after end of infusion) across dose cohorts. Mean T1/2 was 14.3 h, 9.3 h, and 14.1 h for 15-, 30-, and 45-mg/kg nipocalimab doses, respectively. Arithmetic mean CL and Vz values ranged from 0.032 to 0.055 L/h and 0.5 to 1.1 L, respectively, across all dose cohorts. While the dose-normalized mean Cmax appeared to be comparable across the different dose cohorts, the dose-normalized mean AUCs were higher in the 30- and 45-mg/kg dose cohorts, indicating a dose-proportional increase in Cmax, whereas the increase in AUC was considered to be greater than dose proportional for nipocalimab doses ranging from 15 to 45 mg/kg.
Table 2.
Serum PK parameters of nipocalimab
| 15 mg/kg (n = 10) | 30 mg/kg (n = 10)b | 45 mg/kg (n = 10)b | |
|---|---|---|---|
| Cmax, μg/mL | 337 (31) | 814 (97) | 1172 (115) |
| Tmaxa, h | 0.8 (0.5–1.5) | 0.5 (0.5–2.5) | 0.6 (0.5–2.5) |
| Clast, μg/mL | 2.42 (4.70) | 0.02 (0.01) | 0.14 (0.35) |
| Tlasta, h |
180.6 (144.4–337.2) |
263.9 (263.6–672.5) |
336.1 (264.0–336.4) |
| AUClast, μg h/mL | 17,505 (2345) | 58,784 (7934) | 91,738 (9344) |
| AUC∞, μg h/mL | 17,574 (2404) | 60,220 (6901) | 92,963 (9023) |
| t1/2, h | 14.3 (5.0) | 9.3 (3.4) | 14.1 (8.6) |
| Vz, L | 1.1 (0.4) | 0.5 (0.2) | 0.7 (0.5) |
| CL, L/h | 0.055 (0.006) | 0.036 (0.005) | 0.032 (0.004) |
Data are mean (standard deviation) unless otherwise specified
aData are median (range)
bn = 9 for AUC∞, t1/2, Vz, and CL
AUCinf AUC from time 0 extrapolated to infinity, AUClast area under the serum concentration–time curve from start of infusion at time 0 to the time of the last measurable concentration, CL clearance, Clast last measurable serum concentration, Cmax maximum observed serum concentration, PK pharmacokinetics, t1/2 half-life, Tlast time of last measurable serum concentration, Tmax time to reach the maximum observed serum concentration, Vz volume of distribution
PD
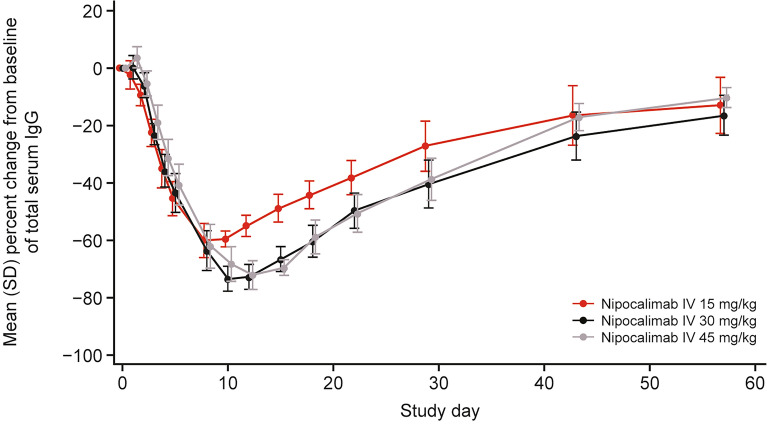
Decreases from baseline in mean total serum IgG levels were observed across all dose cohorts following nipocalimab infusion, with these declines lasting for a longer duration with higher dose levels (Fig. 2). The serum IgG levels reached a maximum decrease of 60.0% (day 8), 73.4% (day 10), and 72.1% (day 12) from baseline following a single infusion of 15, 30, and 45 mg/kg nipocalimab, respectively. By the end of the study at day 57, mean serum IgG levels had returned to over 80% of the baseline levels (ranging from 83.6% to 89.7% of baseline) across all dose cohorts.
Fig. 2.
Percentage change in IgG over time. IgG, immunoglobulin G
Following nipocalimab administration, rapid full occupancy of FcRn was observed for almost all participants (93.3%, 28/30) across doses. Onset of saturation occurred between days 1 and 3, with the receptors remaining nearly or fully saturated for the subsequent 3–6 days, 5–10 days, and 8–11 days for 15, 30, and 45 mg/kg nipocalimab, respectively, following onset of saturation.
Safety
Across all three dose cohorts, a total of 23 (76.7%) participants reported at least one TEAE during the study, with eight (80.0%), eight (80.0%), and seven (70.0%) participants experiencing TEAEs in the 15-, 30-, and 45-mg/kg cohorts, respectively (Table 3). All TEAEs were mild, except for an episode of presyncope in the 15-mg/kg IV treatment cohort, which was moderate in severity (Table S2 in the Supplementary Materials). This participant had decreased blood pressure (BP) during PK blood sampling 1 h following nipocalimab infusion, and reported pain when the IV indwelling catheter was adjusted. At the end of blood sampling, the participant complained of dizziness, showing pallor and cold limbs; BP was 49/29 mmHg and heart rate was 60 beats per minute. The event was managed by immediately placing the participant in a supine position, taking an ECG, and oxygen saturation and BP monitoring. The episode eventually resolved without any further intervention and was considered to be unrelated to the study drug. The most commonly reported TEAEs (in ≥ 10% of all participants) were increased blood triglycerides (n = 10, 33.3%), increased blood glucose (n = 6, 20.0%), increased blood uric acid (n = 6, 20.0%), and increased blood creatine phosphokinase (n = 4, 13.3%) (Table 3). All these TEAEs were mild, with most of the increases in values being transient, and the participants recovered without any treatment; none of the TEAEs were considered to be related to the study drug (Table S2 in the Supplementary Materials).
Table 3.
TEAEs and most frequent TEAEs (in ≥ 10% of all participants) by dosing cohort
| Summary of TEAEs, n (%) | 15 mg/kg (n = 10) |
30 mg/kg (n = 10) |
45 mg/kg (n = 10) |
Total (N = 30) |
|---|---|---|---|---|
| At least 1 TEAE | 8 (80.0) | 8 (80.0) | 7 (70.0) | 23 (76.7) |
| TRAEsa | 0 | 0 | 2 (20.0) | 2 (6.7) |
| Most frequent TEAEs (in ≥ 10% of all participants) by preferred terms | ||||
| Increased blood triglycerides | 1 (10.0) | 6 (60.0) | 3 (30.0) | 10 (33.3) |
| Increased blood glucose | 4 (40.0) | 0 | 2 (20.0) | 6 (20.0) |
| Increased blood uric acid | 2 (20.0) | 2 (20.0) | 2 (20.0) | 6 (20.0) |
| Increased blood creatine phosphokinase | 0 | 2 (20.0) | 2 (20.0) | 4 (13.3) |
aAs assessed by investigator
TEAE treatment-emergent adverse event, TRAE treatment-related adverse event
Treatment-related TEAEs occurred in only two (6.7%) participants in total (Table 3), one of which was increased blood cholesterol and the other was decreased lymphocyte count. Both events were reported in the 45-mg/kg treatment cohort, were mild, and resolved by the end of the study.
Mean albumin values remained within the reference range (40–55 g/L) in all groups at all time points. Decreased albumin of grade 1 occurred in 10 (33.3%) participants (three in the 30-mg/kg group and seven in the 45-mg/kg group). None of these abnormalities were considered TEAEs.
No participants had markedly abnormal laboratory values in cholesterol, high-density lipoprotein cholesterol, or low-density lipoprotein cholesterol during the study. Three participants experienced an increase in blood triglycerides (from grade 1 at baseline to grade 2 post-baseline), although all were mild and not considered related to the study drug. Increased blood cholesterol was reported in only one participant, who recovered without any treatment; this event was mild and assessed by the investigator as being related to the study drug. There were no clinically meaningful abnormal trends for mean percentage changes from baseline in lipid parameters; no AEs related to increased high-density lipoprotein or low-density lipoprotein cholesterol and no treatment-related cardiovascular AEs were reported in participants with abnormal lipid values. None of the participants with elevated lipids required initiation of therapy for dyslipidemia.
There were no SAEs or AEs leading to study discontinuation. No injection-site or infusion-related reactions were detected during the study. There were also no AESIs, COVID-19-associated AEs, or major adverse cardiovascular events observed. No deaths were reported during the study. There were no clinically significant changes in vital signs, ECGs, or physical examination attributable to nipocalimab. No noteworthy mean changes from baseline to the end of the study were observed in hematology, clinical chemistry, or urinalysis parameters during the study.
A total of eight participants (26.7%) tested positive for ADA to nipocalimab, with peak titers ranging from 1:10 to 1:80.
Discussion
Nipocalimab, administered as single infusion doses ranging from 15 to 45 mg/kg, was well tolerated, with no new safety concerns, in healthy Chinese adult participants in this phase I study. Nipocalimab demonstrated a dose-dependent PK profile, with increased mean exposure at higher dose levels. In parallel, increasing doses were associated with prolonged periods of IgG reductions. Rapid full occupancy of FcRn receptors was also observed across all three nipocalimab doses. These findings suggest that the safety, tolerability, and PD of nipocalimab in healthy Chinese adult participants are generally consistent with those reported in previous phase I studies, thus adding to the evidence on the safety and PK/PD of nipocalimab in a population often underrepresented in previous global studies [18, 26].
Based on the serum concentration–time profile following a single infusion of nipocalimab, the nonlinear dose-dependent decrease in serum nipocalimab concentration may be attributed to target-mediated drug disposition, a finding consistent with previous phase I studies [22, 26]. The overall PK parameters also showed dose-dependent changes, with a trend toward higher Cmax, greater AUC, and lower CL at higher doses of nipocalimab, which was in line with other phase I studies [22, 26]. In our study, the mean t1/2 of nipocalimab in Chinese participants ranged from 9.3 to 14.3 h across the three dose cohorts of 15–45 mg/kg. Among healthy Japanese volunteers receiving single infusions of nipocalimab, t1/2 ranged from 16.1 to 39.7 h across doses of 10–60 mg/kg [22]. At a similar dose of 30 mg/kg nipocalimab, drug exposure (Cmax and AUC) among Chinese individuals in our study appeared to be higher than in Japanese volunteers [22], although comparison between populations requires further validation from future studies designed specifically to address population-based differences. In other phase I studies involving predominantly non-Asian populations, t1/2 ranged between 7.8 and 33.7 h for nipocalimab doses of 3–60 mg/kg [26] or 48.8–55.0 h for doses of 30–60 mg/kg at various infusion rates [26]. Nonetheless, it should be noted that direct comparison of PK between studies is challenging due to variations in sample size, formulation, and bioanalytical methods used in different studies.
In the present study, nipocalimab led to dose-dependent decreases in serum IgG levels, with increasingly prolonged durations of IgG reduction with higher nipocalimab doses. Serum IgG reached maximum reductions of 60.0%, 73.4%, and 72.1% from baseline on days 8, 10, and 12 following 15-, 30-, and 45-mg/kg nipocalimab infusions, respectively. These findings are largely consistent with those observed in the healthy Japanese and Caucasian populations [18, 22]. Following a single IV administration of 30 mg/kg nipocalimab, the maximum IgG reductions are generally consistent, at 73.4%, 68.9%, and ~ 75.0% in Chinese, Japanese, and Caucasian healthy participants, respectively [18, 22].
The rapid decline in serum IgG after nipocalimab administration (onset within 24 h post-infusion) observed in our study aligns with the mechanism that nipocalimab rapidly and fully saturates FcRn to facilitate IgG recycling and degradation [18]. In addition, the maximal IgG reductions plateaued at approximately 72.1% of baseline levels with the 45-mg/kg nipocalimab dose, which is close to serum IgG levels observed in individuals and the preclinical model lacking FcRn, at ~ 80–90% below baseline, indicating blockade of FcRn. While data on nipocalimab effects on other subtypes of immunoglobulins were not collected, previous phase I studies have demonstrated the selectivity of nipocalimab towards IgG reductions, with no observable effects on the levels of other immunoglobulins such as immunoglobulin A, immunoglobulin E, and immunoglobulin M. The tolerability and consistent PD effects could potentially provide a window of safety in nipocalimab dosing in further clinical studies and support further assessment of nipocalimab in the Chinese population with autoimmune diseases characterized by pathogenic IgG autoantibodies, including gMG.
ADA were observed in 26.7% participants in total; however, the presence of ADA did not appear to affect Cmax or AUCs. Nonetheless, it should be noted that the observation in this study is only after a single dose of nipocalimab, and further study with multiple dosing will shed more light on the potential effects of ADA on PK and efficacy of nipocalimab.
Nipocalimab was generally safe and well tolerated by the Chinese healthy participants, and no new safety signals were observed in this study relative to previous phase I studies [18, 22, 26]. All TEAEs were mild, except for one case of presyncope of moderate severity in the 15-mg/kg IV treatment cohort. The only two treatment-related AEs reported were one case of increased blood cholesterol and one case of decreased lymphocyte count; both were mild and occurred in the 45-mg/kg dose cohort. There were no SAEs, AESIs, or AEs leading to study discontinuation or deaths during the study. A slight decrease in serum albumin was observed, but mean values remained within the normal range; no AEs associated with albumin reduction were reported and there were no events of hypoalbuminemia. No clinically meaningful abnormal trends were observed for mean percentage changes in lipid parameters; there were no treatment-related cardiovascular AEs or requirement for dyslipidemia treatment in participants with abnormal lipid values. No clinically significant changes in vital signs, ECGs, or physical examination occurred. The tolerability profile of nipocalimab may be attributed to its high selectivity due to its high affinity for FcRn [18].
Study limitations include the small sample size, relatively short treatment duration, and inclusion of only healthy individuals with no significant comorbidities or concomitant medications, which may not be representative of patients with IgG-mediated autoimmune or alloimmune diseases. Additionally, only a single infusion of nipocalimab was assessed, and therefore further studies with repeated dosing of nipocalimab in specific population with IgG-mediated auto/alloimmune conditions are warranted to provide better understanding of the impact of nipocalimab among Chinese individuals.
Conclusion
Single doses of nipocalimab in healthy Chinese adult participants demonstrated PD profiles that are consistent with findings from previous phase I studies, including those involving mainly non-Asian participants and another on Japanese healthy volunteers. Nipocalimab, given as IV infusion at doses between 15 and 45 mg/kg, was well tolerated and showed an acceptable safety profile, with no new safety findings. Following a single infusion of nipocalimab, Cmax increased in a dose-proportional manner while AUC increased in a greater than dose-proportional manner. Nipocalimab led to dose-dependent lowering of serum IgG levels from baseline, with this decrease sustained over a longer period of time with higher dose levels, which coincides with full receptor occupancy. These data add to the evidence on the safety and PK/PD of nipocalimab in the Chinese population, who were underrepresented in previous studies of nipocalimab. Further studies involving Chinese participants with specific IgG autoantibody-mediated disease are currently ongoing, including gMG, warm autoimmune hemolytic anemia, and chronic inflammatory demyelinating polyneuropathy.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the participants and their families for making the study possible, and also the investigators and clinical study teams who conducted the study.
Medical Writing and Editorial Assistance
Medical writing assistance was provided by Pearl Toh, PhD, of Parexel, and was funded by Johnson & Johnson, Shanghai, China.
Author Contributions
Haiyan Li, Juanfang Liu, Xiaohong Wang, Weilong Zhao, Lili Zhang, and Zhongqi Dong contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Juanfang Liu, Weilong Zhao, Lili Zhang, Xiaoye Niu, Jingyao Liu, and Zhongqi Dong. The first draft of the manuscript was written by Juanfang Liu, Weilong Zhao, Lili Zhang, and Zhongqi Dong, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Johnson & Johnson (China) Investment Ltd., who also funded the Rapid Service Fee.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
Juanfang Liu, Weilong Zhao, Lili Zhang, and Zhongqi Dong are current employees of Johnson & Johnson. Haiyan Li, Xiaohong Wang, Xiaoye Niu, and Jingyao Liu have nothing to disclose.
Ethical Approval
The study protocol and its amendment were reviewed and approved by an independent ethics committee at the study site (Peking University Third Hospital Medical Science Research Ethics Committee). The study was conducted in compliance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines, and with relevant law and regulatory requirements. All participants had provided written informed consent.
Footnotes
Prior Presentation: The data in this manuscript have been presented in part at the China National Conference of Neurology 2023 [Z Dong, X Wang, L Zhang, et al. A phase I study to evaluate the neonatal Fc receptor antagonist nipocalimab in healthy Chinese adult participants. Abstract presented at China National Conference of Neurology 2023; September 7–10, 2023; Chengdu, China; PO-0643].
Haiyan Li and Juanfang Liu are co-first authors.
References
- 1.Eggert M, Zettl UK, Neeck G. Autoantibodies in autoimmune diseases. Curr Pharm Des. 2010;16:1634–43. [DOI] [PubMed] [Google Scholar]
- 2.Ludwig RJ, Vanhoorelbeke K, Leypoldt F, et al. Mechanisms of autoantibody-induced pathology. Front Immunol. 2017;8:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kraker J, Zivkovic SA. Autoimmune neuromuscular disorders. Curr Neuropharmacol. 2011;9:400–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thurman JM, Panzer SE, Le Quintrec M. The role of complement in antibody mediated transplant rejection. Mol Immunol. 2019;112:240–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brodsky RA. Warm autoimmune hemolytic anemia. Reply N Engl J Med. 2019;381:1881–2. [DOI] [PubMed] [Google Scholar]
- 6.Pyzik M, Sand KMK, Hubbard JJ, Andersen JT, Sandlie I, Blumberg RS. The neonatal Fc receptor (FcRn): a misnomer? Front Immunol. 2019;10:1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilhus NE, Tzartos S, Evoli A, Palace J, Burns TM, Verschuuren J. Myasthenia gravis. Nat Rev Dis Primers. 2019;5:30. [DOI] [PubMed] [Google Scholar]
- 8.Jager U, Barcellini W, Broome CM, et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: recommendations from the first international consensus meeting. Blood Rev. 2020;41: 100648. [DOI] [PubMed] [Google Scholar]
- 9.Bacci ED, Coyne KS, Poon JL, Harris L, Boscoe AN. Understanding side effects of therapy for myasthenia gravis and their impact on daily life. BMC Neurol. 2019;19:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmaldienst S, Mullner M, Goldammer A, et al. Intravenous immunoglobulin application following immunoadsorption: benefit or risk in patients with autoimmune diseases? Rheumatology (Oxford). 2001;40:513–21. [DOI] [PubMed] [Google Scholar]
- 11.Giacomelli R, Afeltra A, Alunno A, et al. International consensus: What else can we do to improve diagnosis and therapeutic strategies in patients affected by autoimmune rheumatic diseases (rheumatoid arthritis, spondyloarthritides, systemic sclerosis, systemic lupus erythematosus, antiphospholipid syndrome and Sjogren’s syndrome)?: The unmet needs and the clinical grey zone in autoimmune disease management. Autoimmun Rev. 2017;16:911–24. [DOI] [PubMed] [Google Scholar]
- 12.Tony HP, Burmester G, Schulze-Koops H, et al. Safety and clinical outcomes of rituximab therapy in patients with different autoimmune diseases: experience from a national registry (GRAID). Arthritis Res Ther. 2011;13:R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peter HH, Ochs HD, Cunningham-Rundles C, et al. Targeting FcRn for immunomodulation: benefits, risks, and practical considerations. J Allergy Clin Immunol. 2020;146(479–91): e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blumberg LJ, Humphries JE, Jones SD, et al. Blocking FcRn in humans reduces circulating IgG levels and inhibits IgG immune complex-mediated immune responses. Sci Adv. 2019;5: eaax9586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Mathieu M, Brezski RJ. IgG Fc engineering to modulate antibody effector functions. Protein Cell. 2018;9:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–25. [DOI] [PubMed] [Google Scholar]
- 17.Alfaidi N, Karmastaji S, Matic A, Bril V. FcRn inhibitor therapies in neurologic diseases. CNS Drugs. 2024;38:425–41. [DOI] [PubMed] [Google Scholar]
- 18.Ling LE, Hillson JL, Tiessen RG, et al. M281, an anti-FcRn antibody: pharmacodynamics, pharmacokinetics, and safety across the full range of IgG reduction in a first-in-human study. Clin Pharmacol Ther. 2019;105:1031–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pyzik M, Kozicky LK, Gandhi AK, Blumberg RS. The therapeutic age of the neonatal Fc receptor. Nat Rev Immunol. 2023;23:415–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohkubo A, Okado T, Kurashima N, et al. Removal characteristics of immunoglobulin G subclasses by conventional plasma exchange and selective plasma exchange. Ther Apher Dial. 2015;19:361–6. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan AA. Therapeutic plasma exchange: a technical and operational review. J Clin Apher. 2013;28:3–10. [DOI] [PubMed] [Google Scholar]
- 22.Matsushima N, Shibata S, Leu JH, et al. Pharmacokinetics and pharmacodynamics of nipocalimab, a neonatal Fc receptor blocker, in healthy Japanese volunteers. Clin Drug Investig. 2024;44:587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antozzi C, Guptill J, Bril V, et al. Safety and efficacy of nipocalimab in patients with generalized myasthenia gravis: results from the randomized phase 2 Vivacity-MG Study. Neurology. 2024;102: e207937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moise KJ Jr, Ling LE, Oepkes D, et al. Nipocalimab in early-onset severe hemolytic disease of the fetus and newborn. N Engl J Med. 2024;391:526–37. [DOI] [PubMed] [Google Scholar]
- 25.Gottenberg JE, Sivils K, Campbell K, et al. Efficacy and safety of nipocalimab, an anti-FcRn monoclonal antibody, in primary Sjogren’s disease: Results from a phase 2, multicenter, randomized, placebo-controlled, double-blind study (DAHLIAS). Ann Rheum Dis. 2024;83(supplement 1):240. [Google Scholar]
- 26.Leu JH, Vermeulen A, Abbes C, Arroyo S, Denney WS, Ling LE. Pharmacokinetics and pharmacodynamics across infusion rates of intravenously administered nipocalimab: results of a phase 1, placebo-controlled study. Front Neurosci. 2024;18:1302714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.