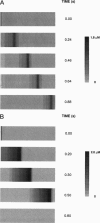
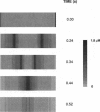
Abstract
Cytosolic Ca2+ waves occur in a number of cell types either spontaneously or after stimulation by hormones, neurotransmitters, or treatments promoting Ca2+ influx into the cells. These waves can be broadly classified into two types. Waves of type 1, observed in cardiac myocytes or Xenopus oocytes, correspond to the propagation of sharp bands of Ca2+ throughout the cell at a rate that is high enough to permit the simultaneous propagation of several fronts in a given cells. Waves of type 2, observed in hepatocytes, endothelial cells, or various kinds of eggs, correspond to the progressive elevation of cytosolic Ca2+ throughout the cell, followed by its quasi-homogeneous return down to basal levels. Here we analyze the propagation of these different types of intracellular Ca2+ waves in a model based on Ca(2+)-induced Ca2+ release (CICR). The model accounts for transient or sustained waves of type 1 or 2, depending on the size of the cell and on the values of the kinetic parameters that measure Ca2+ exchange between the cytosol, the extracellular medium, and intracellular stores. Two versions of the model based on CICR are considered. The first version involves two distinct Ca2+ pools sensitive to inositol 1,4,5-trisphosphate (IP3) and Ca2+, respectively, whereas the second version involves a single pool sensitive both to Ca2+ and IP3 behaving as co-agonists for Ca2+ release. Intracellular Ca2+ waves occur in the two versions of the model based on CICR, but fail to propagate in the one-pool model at subthreshold levels of IP3. For waves of type 1, we investigate the effect of the spatial distribution of Ca(2+)-sensitive Ca2+ stores within the cytosol, and show that the wave fails to propagate when the distance between the stores exceeds a critical value on the order of a few microns. We also determine how the period and velocity of the waves are affected by changes in parameters measuring stimulation, Ca2+ influx into the cell, or Ca2+ pumping into the stores. For waves of type 2, the numerical analysis indicates that the best qualitative agreement with experimental observations is obtained for phase waves. Finally, conditions are obtained for the occurrence of "echo" waves that are sometimes observed in the experiments.
Full text
PDF

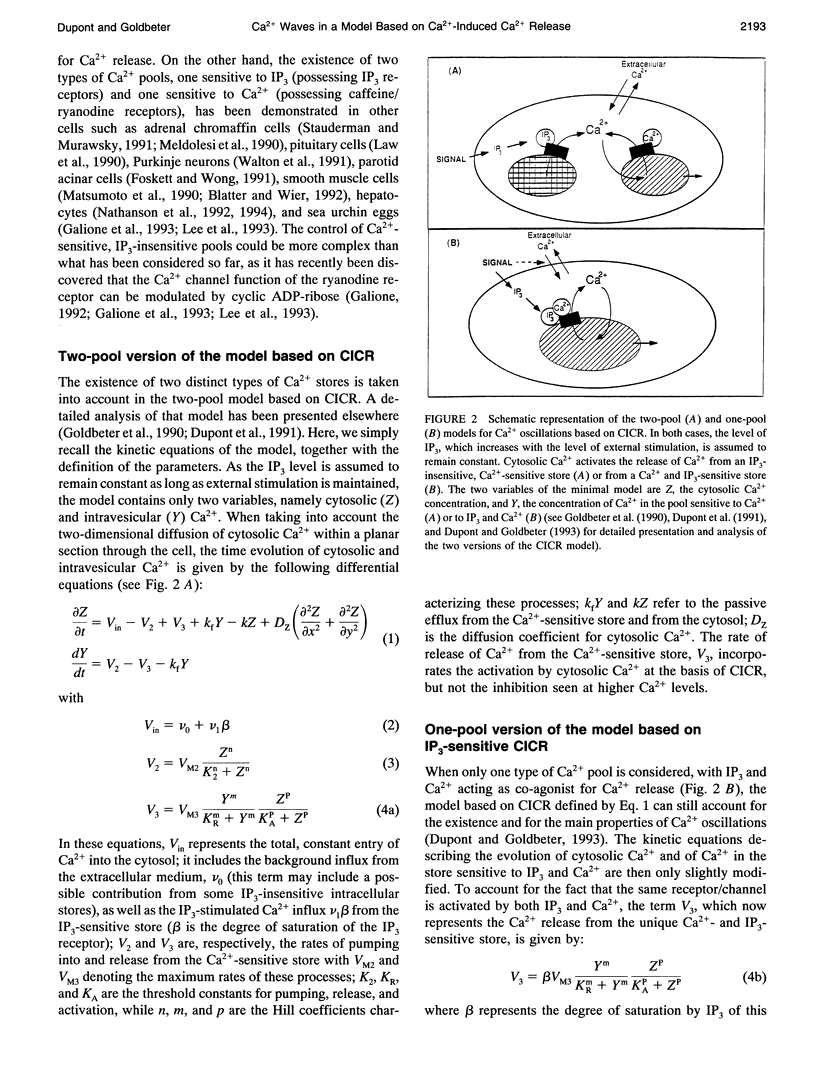
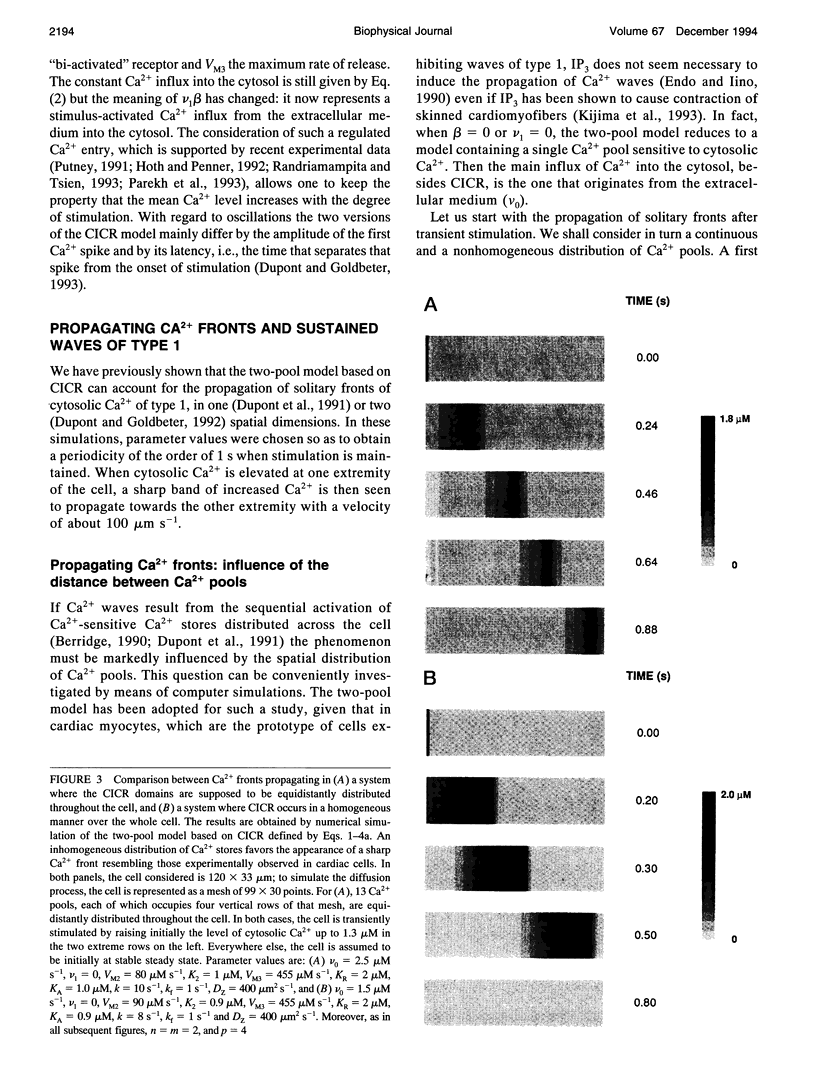
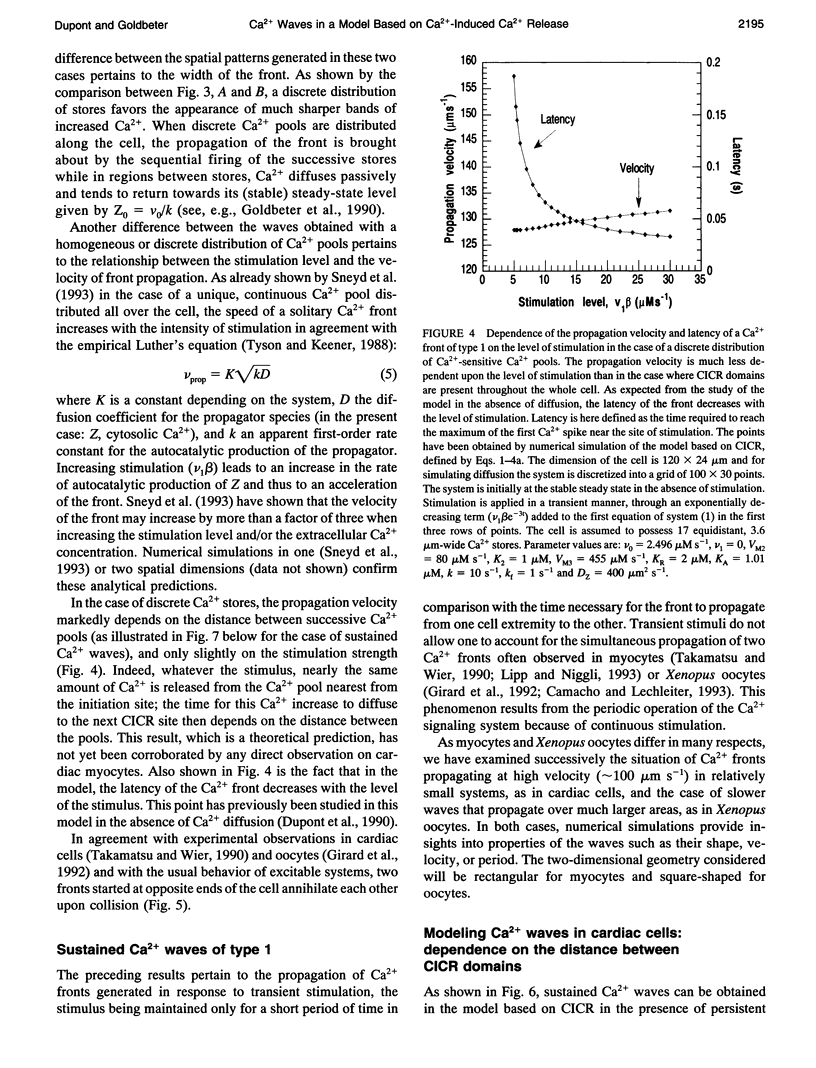

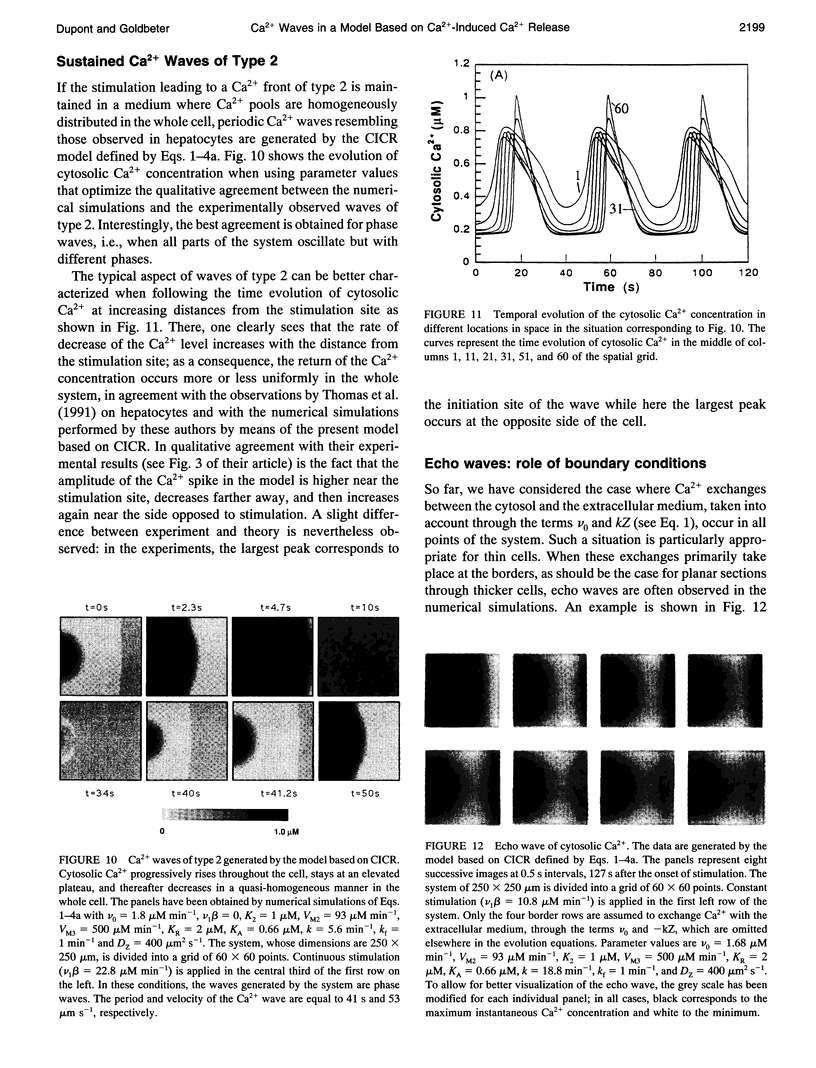




Images in this article
Selected References
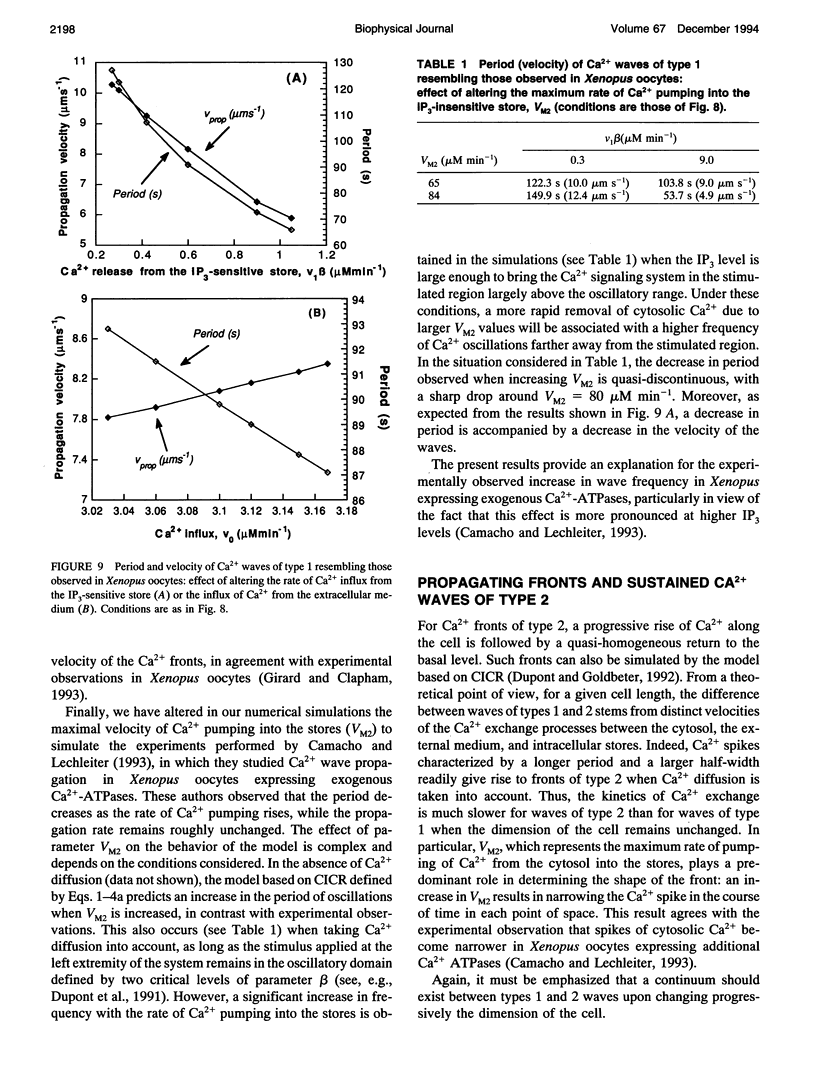
These references are in PubMed. This may not be the complete list of references from this article.
- Allbritton N. L., Meyer T., Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992 Dec 11;258(5089):1812–1815. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
- Amundson J., Clapham D. Calcium waves. Curr Opin Neurobiol. 1993 Jun;3(3):375–382. doi: 10.1016/0959-4388(93)90131-h. [DOI] [PubMed] [Google Scholar]
- Atri A., Amundson J., Clapham D., Sneyd J. A single-pool model for intracellular calcium oscillations and waves in the Xenopus laevis oocyte. Biophys J. 1993 Oct;65(4):1727–1739. doi: 10.1016/S0006-3495(93)81191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backx P. H., de Tombe P. P., Van Deen J. H., Mulder B. J., ter Keurs H. E. A model of propagating calcium-induced calcium release mediated by calcium diffusion. J Gen Physiol. 1989 May;93(5):963–977. doi: 10.1085/jgp.93.5.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Calcium oscillations. J Biol Chem. 1990 Jun 15;265(17):9583–9586. [PubMed] [Google Scholar]
- Berridge M. J., Dupont G. Spatial and temporal signalling by calcium. Curr Opin Cell Biol. 1994 Apr;6(2):267–274. doi: 10.1016/0955-0674(94)90146-5. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Galione A. Cytosolic calcium oscillators. FASEB J. 1988 Dec;2(15):3074–3082. doi: 10.1096/fasebj.2.15.2847949. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Watras J., Ehrlich B. E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991 Jun 27;351(6329):751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Blatter L. A., Wier W. G. Agonist-induced [Ca2+]i waves and Ca(2+)-induced Ca2+ release in mammalian vascular smooth muscle cells. Am J Physiol. 1992 Aug;263(2 Pt 2):H576–H586. doi: 10.1152/ajpheart.1992.263.2.H576. [DOI] [PubMed] [Google Scholar]
- Boitano S., Dirksen E. R., Sanderson M. J. Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science. 1992 Oct 9;258(5080):292–295. doi: 10.1126/science.1411526. [DOI] [PubMed] [Google Scholar]
- Busa W. B., Ferguson J. E., Joseph S. K., Williamson J. R., Nuccitelli R. Activation of frog (Xenopus laevis) eggs by inositol trisphosphate. I. Characterization of Ca2+ release from intracellular stores. J Cell Biol. 1985 Aug;101(2):677–682. doi: 10.1083/jcb.101.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho P., Lechleiter J. D. Increased frequency of calcium waves in Xenopus laevis oocytes that express a calcium-ATPase. Science. 1993 Apr 9;260(5105):226–229. doi: 10.1126/science.8385800. [DOI] [PubMed] [Google Scholar]
- Charles A. C., Merrill J. E., Dirksen E. R., Sanderson M. J. Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron. 1991 Jun;6(6):983–992. doi: 10.1016/0896-6273(91)90238-u. [DOI] [PubMed] [Google Scholar]
- Cheek T. R., McGuinness O. M., Vincent C., Moreton R. B., Berridge M. J., Johnson M. H. Fertilisation and thimerosal stimulate similar calcium spiking patterns in mouse oocytes but by separate mechanisms. Development. 1993 Sep;119(1):179–189. doi: 10.1242/dev.119.1.179. [DOI] [PubMed] [Google Scholar]
- Cheer A., Vincent J. P., Nuccitelli R., Oster G. Cortical activity in vertebrate eggs. I: The activation waves. J Theor Biol. 1987 Feb 21;124(4):377–404. doi: 10.1016/s0022-5193(87)80217-5. [DOI] [PubMed] [Google Scholar]
- Cheng H., Lederer W. J., Cannell M. B. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993 Oct 29;262(5134):740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Cuthbertson K. S., Chay T. R. Modelling receptor-controlled intracellular calcium oscillators. Cell Calcium. 1991 Feb-Mar;12(2-3):97–109. doi: 10.1016/0143-4160(91)90012-4. [DOI] [PubMed] [Google Scholar]
- De Young G. W., Keizer J. A single-pool inositol 1,4,5-trisphosphate-receptor-based model for agonist-stimulated oscillations in Ca2+ concentration. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9895–9899. doi: 10.1073/pnas.89.20.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisle S., Welsh M. J. Inositol trisphosphate is required for the propagation of calcium waves in Xenopus oocytes. J Biol Chem. 1992 Apr 25;267(12):7963–7966. [PubMed] [Google Scholar]
- Donahue B. S., Abercrombie R. F. Free diffusion coefficient of ionic calcium in cytoplasm. Cell Calcium. 1987 Dec;8(6):437–448. doi: 10.1016/0143-4160(87)90027-3. [DOI] [PubMed] [Google Scholar]
- Dupont G., Berridge M. J., Goldbeter A. Latency correlates with period in a model for signal-induced Ca2+ oscillations based on Ca2(+)-induced Ca2+ release. Cell Regul. 1990 Oct;1(11):853–861. doi: 10.1091/mbc.1.11.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont G., Berridge M. J., Goldbeter A. Signal-induced Ca2+ oscillations: properties of a model based on Ca(2+)-induced Ca2+ release. Cell Calcium. 1991 Feb-Mar;12(2-3):73–85. doi: 10.1016/0143-4160(91)90010-c. [DOI] [PubMed] [Google Scholar]
- Dupont G., Goldbeter A. One-pool model for Ca2+ oscillations involving Ca2+ and inositol 1,4,5-trisphosphate as co-agonists for Ca2+ release. Cell Calcium. 1993 Apr;14(4):311–322. doi: 10.1016/0143-4160(93)90052-8. [DOI] [PubMed] [Google Scholar]
- Dupont G., Goldbeter A. Oscillations and waves of cytosolic calcium: insights from theoretical models. Bioessays. 1992 Jul;14(7):485–493. doi: 10.1002/bies.950140711. [DOI] [PubMed] [Google Scholar]
- Endo M., Iino M. Properties of calcium release channels of the intracellular calcium store in muscle cells. Adv Second Messenger Phosphoprotein Res. 1990;24:122–127. [PubMed] [Google Scholar]
- Fewtrell C. Ca2+ oscillations in non-excitable cells. Annu Rev Physiol. 1993;55:427–454. doi: 10.1146/annurev.ph.55.030193.002235. [DOI] [PubMed] [Google Scholar]
- Finch E. A., Turner T. J., Goldin S. M. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991 Apr 19;252(5004):443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- Foskett J. K., Wong D. Free cytoplasmic Ca2+ concentration oscillations in thapsigargin-treated parotid acinar cells are caffeine- and ryanodine-sensitive. J Biol Chem. 1991 Aug 5;266(22):14535–14538. [PubMed] [Google Scholar]
- Galione A. Ca(2+)-induced Ca2+ release and its modulation by cyclic ADP-ribose. Trends Pharmacol Sci. 1992 Aug;13(8):304–306. doi: 10.1016/0165-6147(92)90096-o. [DOI] [PubMed] [Google Scholar]
- Galione A., McDougall A., Busa W. B., Willmott N., Gillot I., Whitaker M. Redundant mechanisms of calcium-induced calcium release underlying calcium waves during fertilization of sea urchin eggs. Science. 1993 Jul 16;261(5119):348–352. doi: 10.1126/science.8392748. [DOI] [PubMed] [Google Scholar]
- Girard S., Clapham D. Acceleration of intracellular calcium waves in Xenopus oocytes by calcium influx. Science. 1993 Apr 9;260(5105):229–232. doi: 10.1126/science.8385801. [DOI] [PubMed] [Google Scholar]
- Girard S., Lückhoff A., Lechleiter J., Sneyd J., Clapham D. Two-dimensional model of calcium waves reproduces the patterns observed in Xenopus oocytes. Biophys J. 1992 Feb;61(2):509–517. doi: 10.1016/S0006-3495(92)81855-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbeter A., Dupont G., Berridge M. J. Minimal model for signal-induced Ca2+ oscillations and for their frequency encoding through protein phosphorylation. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1461–1465. doi: 10.1073/pnas.87.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth M., Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992 Jan 23;355(6358):353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Jacob R. Imaging cytoplasmic free calcium in histamine stimulated endothelial cells and in fMet-Leu-Phe stimulated neutrophils. Cell Calcium. 1990 Feb-Mar;11(2-3):241–249. doi: 10.1016/0143-4160(90)90075-6. [DOI] [PubMed] [Google Scholar]
- Jaffe L. F. Classes and mechanisms of calcium waves. Cell Calcium. 1993 Nov;14(10):736–745. doi: 10.1016/0143-4160(93)90099-r. [DOI] [PubMed] [Google Scholar]
- Jaffe L. F. Sources of calcium in egg activation: a review and hypothesis. Dev Biol. 1983 Oct;99(2):265–276. doi: 10.1016/0012-1606(83)90276-2. [DOI] [PubMed] [Google Scholar]
- Jafri M. S., Vajda S., Pasik P., Gillo B. A membrane model for cytosolic calcium oscillations. A study using Xenopus oocytes. Biophys J. 1992 Jul;63(1):235–246. doi: 10.1016/S0006-3495(92)81583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Li Y. X., Miyashita Y. Subcellular distribution of Ca2+ release channels underlying Ca2+ waves and oscillations in exocrine pancreas. Cell. 1993 Aug 27;74(4):669–677. doi: 10.1016/0092-8674(93)90514-q. [DOI] [PubMed] [Google Scholar]
- Kijima Y., Saito A., Jetton T. L., Magnuson M. A., Fleischer S. Different intracellular localization of inositol 1,4,5-trisphosphate and ryanodine receptors in cardiomyocytes. J Biol Chem. 1993 Feb 15;268(5):3499–3506. [PubMed] [Google Scholar]
- Kort A. A., Capogrossi M. C., Lakatta E. G. Frequency, amplitude, and propagation velocity of spontaneous Ca++-dependent contractile waves in intact adult rat cardiac muscle and isolated myocytes. Circ Res. 1985 Dec;57(6):844–855. doi: 10.1161/01.res.57.6.844. [DOI] [PubMed] [Google Scholar]
- Kuba K., Takeshita S. Simulation of intracellular Ca2+ oscillation in a sympathetic neurone. J Theor Biol. 1981 Dec 21;93(4):1009–1031. doi: 10.1016/0022-5193(81)90352-0. [DOI] [PubMed] [Google Scholar]
- Kushmerick M. J., Podolsky R. J. Ionic mobility in muscle cells. Science. 1969 Dec 5;166(3910):1297–1298. doi: 10.1126/science.166.3910.1297. [DOI] [PubMed] [Google Scholar]
- Law G. J., Pachter J. A., Thastrup O., Hanley M. R., Dannies P. S. Thapsigargin, but not caffeine, blocks the ability of thyrotropin-releasing hormone to release Ca2+ from an intracellular store in GH4C1 pituitary cells. Biochem J. 1990 Apr 15;267(2):359–364. doi: 10.1042/bj2670359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechleiter J. D., Clapham D. E. Molecular mechanisms of intracellular calcium excitability in X. laevis oocytes. Cell. 1992 Apr 17;69(2):283–294. doi: 10.1016/0092-8674(92)90409-6. [DOI] [PubMed] [Google Scholar]
- Lechleiter J., Girard S., Peralta E., Clapham D. Spiral calcium wave propagation and annihilation in Xenopus laevis oocytes. Science. 1991 Apr 5;252(5002):123–126. doi: 10.1126/science.2011747. [DOI] [PubMed] [Google Scholar]
- Lee H. C., Aarhus R., Walseth T. F. Calcium mobilization by dual receptors during fertilization of sea urchin eggs. Science. 1993 Jul 16;261(5119):352–355. doi: 10.1126/science.8392749. [DOI] [PubMed] [Google Scholar]
- Lipp P., Niggli E. Microscopic spiral waves reveal positive feedback in subcellular calcium signaling. Biophys J. 1993 Dec;65(6):2272–2276. doi: 10.1016/S0006-3495(93)81316-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P., Niggli E. Modulation of Ca2+ release in cultured neonatal rat cardiac myocytes. Insight from subcellular release patterns revealed by confocal microscopy. Circ Res. 1994 May;74(5):979–990. doi: 10.1161/01.res.74.5.979. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Kanaide H., Shogakiuchi Y., Nakamura M. Characteristics of the histamine-sensitive calcium store in vascular smooth muscle. Comparison with norepinephrine- or caffeine-sensitive stores. J Biol Chem. 1990 Apr 5;265(10):5610–5616. [PubMed] [Google Scholar]
- Meldolesi J., Madeddu L., Pozzan T. Intracellular Ca2+ storage organelles in non-muscle cells: heterogeneity and functional assignment. Biochim Biophys Acta. 1990 Nov 12;1055(2):130–140. doi: 10.1016/0167-4889(90)90113-r. [DOI] [PubMed] [Google Scholar]
- Meyer T. Cell signaling by second messenger waves. Cell. 1991 Feb 22;64(4):675–678. doi: 10.1016/0092-8674(91)90496-l. [DOI] [PubMed] [Google Scholar]
- Meyer T., Holowka D., Stryer L. Highly cooperative opening of calcium channels by inositol 1,4,5-trisphosphate. Science. 1988 Apr 29;240(4852):653–656. doi: 10.1126/science.2452482. [DOI] [PubMed] [Google Scholar]
- Meyer T., Stryer L. Calcium spiking. Annu Rev Biophys Biophys Chem. 1991;20:153–174. doi: 10.1146/annurev.bb.20.060191.001101. [DOI] [PubMed] [Google Scholar]
- Meyer T., Stryer L. Molecular model for receptor-stimulated calcium spiking. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5051–5055. doi: 10.1073/pnas.85.14.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov S. L. Theoretical analysis of Ca wave propagation along the surface of intracellular stores. J Theor Biol. 1990 Sep 7;146(1):87–97. doi: 10.1016/s0022-5193(05)80045-1. [DOI] [PubMed] [Google Scholar]
- Miyazaki S., Yuzaki M., Nakada K., Shirakawa H., Nakanishi S., Nakade S., Mikoshiba K. Block of Ca2+ wave and Ca2+ oscillation by antibody to the inositol 1,4,5-trisphosphate receptor in fertilized hamster eggs. Science. 1992 Jul 10;257(5067):251–255. doi: 10.1126/science.1321497. [DOI] [PubMed] [Google Scholar]
- Moses R. L., Delcarpio J. B. Sarcoplasmic reticulum and intermediate filament organization in cultured neonatal cardiac muscle cells. Studies with reduced osmium ferrocyanide. Cell Tissue Res. 1983;228(3):489–496. doi: 10.1007/BF00211470. [DOI] [PubMed] [Google Scholar]
- Nathanson M. H., Padfield P. J., O'Sullivan A. J., Burgstahler A. D., Jamieson J. D. Mechanism of Ca2+ wave propagation in pancreatic acinar cells. J Biol Chem. 1992 Sep 5;267(25):18118–18121. [PubMed] [Google Scholar]
- Parekh A. B., Terlau H., Stühmer W. Depletion of InsP3 stores activates a Ca2+ and K+ current by means of a phosphatase and a diffusible messenger. Nature. 1993 Aug 26;364(6440):814–818. doi: 10.1038/364814a0. [DOI] [PubMed] [Google Scholar]
- Parker I., Yao Y. Regenerative release of calcium from functionally discrete subcellular stores by inositol trisphosphate. Proc Biol Sci. 1991 Dec 23;246(1317):269–274. doi: 10.1098/rspb.1991.0154. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990 Nov-Dec;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Randriamampita C., Tsien R. Y. Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 1993 Aug 26;364(6440):809–814. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- Rooney T. A., Thomas A. P. Intracellular calcium waves generated by Ins(1,4,5)P3-dependent mechanisms. Cell Calcium. 1993 Nov;14(10):674–690. doi: 10.1016/0143-4160(93)90094-m. [DOI] [PubMed] [Google Scholar]
- Sneyd J., Girard S., Clapham D. Calcium wave propagation by calcium-induced calcium release: an unusual excitable system. Bull Math Biol. 1993 Mar;55(2):315–344. doi: 10.1007/BF02460886. [DOI] [PubMed] [Google Scholar]
- Somogyi R., Stucki J. W. Hormone-induced calcium oscillations in liver cells can be explained by a simple one pool model. J Biol Chem. 1991 Jun 15;266(17):11068–11077. [PubMed] [Google Scholar]
- Speksnijder J. E., Sardet C., Jaffe L. F. Periodic calcium waves cross ascidian eggs after fertilization. Dev Biol. 1990 Nov;142(1):246–249. doi: 10.1016/0012-1606(90)90168-i. [DOI] [PubMed] [Google Scholar]
- Stauderman K. A., Murawsky M. M. The inositol 1,4,5-trisphosphate-forming agonist histamine activates a ryanodine-sensitive Ca2+ release mechanism in bovine adrenal chromaffin cells. J Biol Chem. 1991 Oct 15;266(29):19150–19153. [PubMed] [Google Scholar]
- Stern M. D. Theory of excitation-contraction coupling in cardiac muscle. Biophys J. 1992 Aug;63(2):497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swillens S., Mercan D. Computer simulation of a cytosolic calcium oscillator. Biochem J. 1990 Nov 1;271(3):835–838. doi: 10.1042/bj2710835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu T., Wier W. G. Calcium waves in mammalian heart: quantification of origin, magnitude, waveform, and velocity. FASEB J. 1990 Mar;4(5):1519–1525. doi: 10.1096/fasebj.4.5.2307330. [DOI] [PubMed] [Google Scholar]
- Thomas A. P., Renard D. C., Rooney T. A. Spatial and temporal organization of calcium signalling in hepatocytes. Cell Calcium. 1991 Feb-Mar;12(2-3):111–126. doi: 10.1016/0143-4160(91)90013-5. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Tsien R. Y. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- Wakui M., Osipchuk Y. V., Petersen O. H. Receptor-activated cytoplasmic Ca2+ spiking mediated by inositol trisphosphate is due to Ca2(+)-induced Ca2+ release. Cell. 1990 Nov 30;63(5):1025–1032. doi: 10.1016/0092-8674(90)90505-9. [DOI] [PubMed] [Google Scholar]
- Wakui M., Potter B. V., Petersen O. H. Pulsatile intracellular calcium release does not depend on fluctuations in inositol trisphosphate concentration. Nature. 1989 May 25;339(6222):317–320. doi: 10.1038/339317a0. [DOI] [PubMed] [Google Scholar]
- Walton P. D., Airey J. A., Sutko J. L., Beck C. F., Mignery G. A., Südhof T. C., Deerinck T. J., Ellisman M. H. Ryanodine and inositol trisphosphate receptors coexist in avian cerebellar Purkinje neurons. J Cell Biol. 1991 Jun;113(5):1145–1157. doi: 10.1083/jcb.113.5.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Agonist-induced oscillations in cytoplasmic free calcium concentration in single rat hepatocytes. Cell Calcium. 1987 Feb;8(1):79–100. doi: 10.1016/0143-4160(87)90038-8. [DOI] [PubMed] [Google Scholar]
- Yagodin S. V., Holtzclaw L., Sheppard C. A., Russell J. T. Nonlinear propagation of agonist-induced cytoplasmic calcium waves in single astrocytes. J Neurobiol. 1994 Mar;25(3):265–280. doi: 10.1002/neu.480250307. [DOI] [PubMed] [Google Scholar]