Abstract
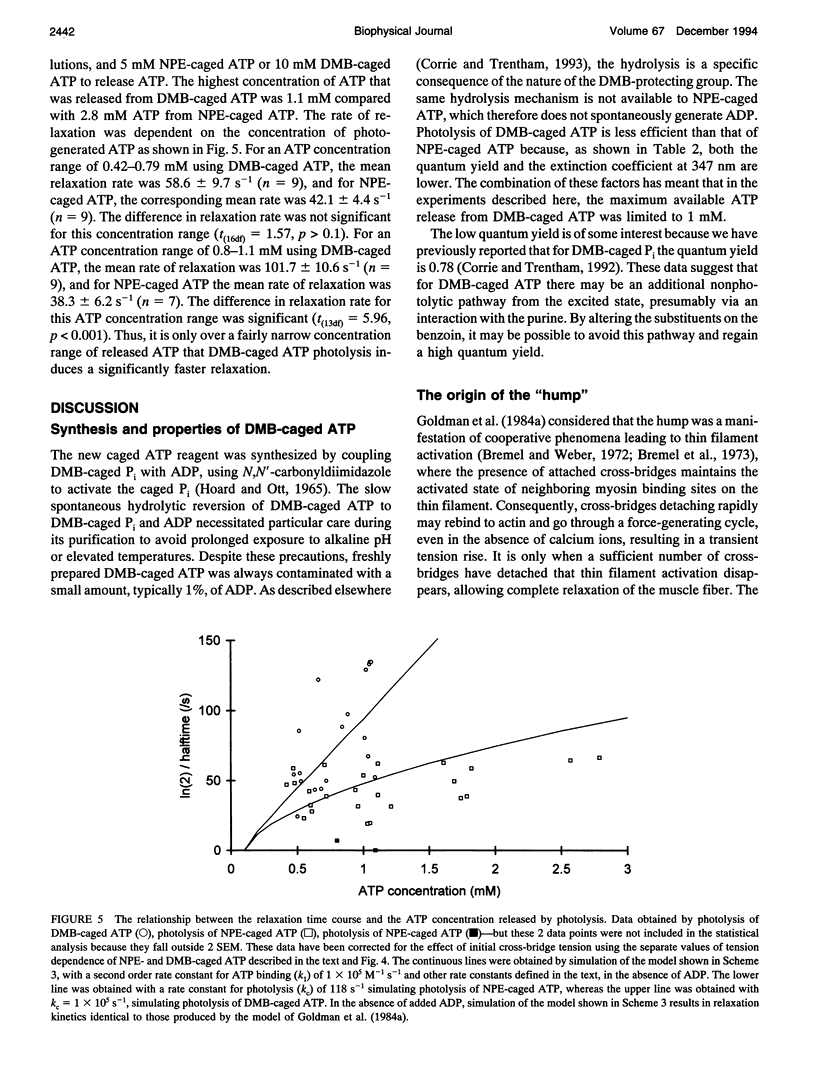
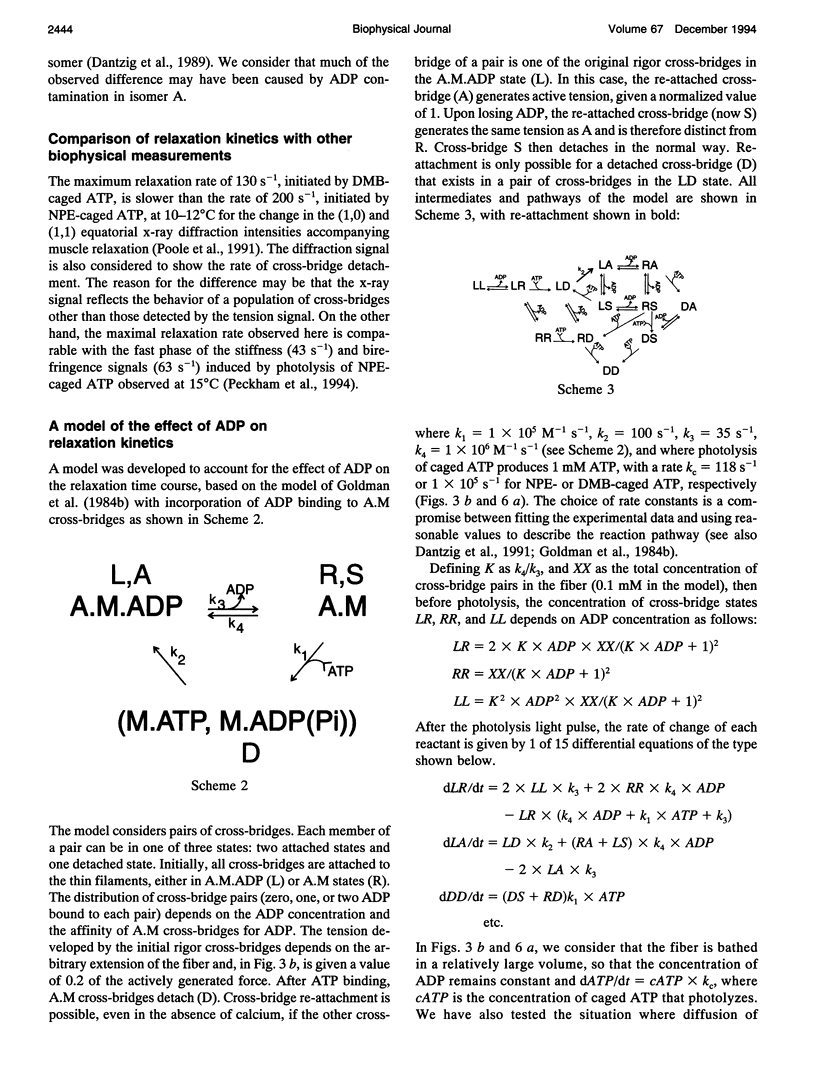
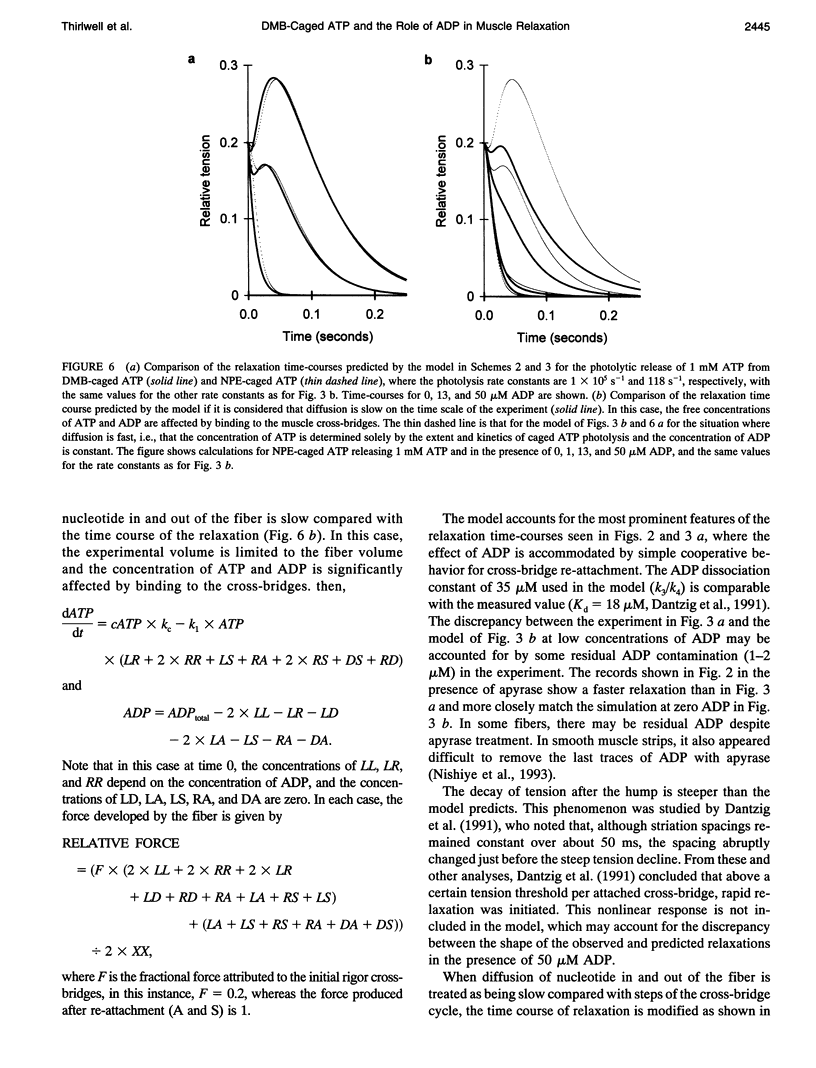
The complex time course of tension decay was investigated in fast-twitch permeabilized rabbit muscle fibers when they were relaxed from the rigor state using photochemical generation of ATP. A novel caged ATP compound, the P3-3',5'-dimethoxybenzoin ester of ATP (DMB-caged ATP), as well as the P3-1-(2-nitrophenyl)ethyl ester of ATP (NPE-caged ATP), have been used. DMB-caged ATP photolyzes at least three orders of magnitude more rapidly than NPE-caged ATP. The role of ADP on relaxation kinetics from rigor was examined by using apyrase to remove ADP from the rigor muscle solutions. The presence of Pi-sensitive states was investigated from the effect of Pi on relaxation. Rigor tension was varied enabling the influence of tension on the relaxation to be examined. The time course of relaxation was faster with DMB-caged ATP compared with NPE-caged ATP for concentrations of ATP released by photolysis greater than 0.7 mM. Most of the complexity in the relaxation tension records was caused by ADP. In the absence of ADP, tension decayed monotonically after photochemical release of ATP in a process whose rate was unaffected by Pi. In the presence of ADP, relaxation was more complex and tension passed through a maximum. A model invoking cooperative interactions involving ADP-containing myosin heads provides a reasonable description of the data.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLINKS J. R. INFLUENCE OF OSMOTIC STRENGTH ON CROSS-SECTION AND VOLUME OF ISOLATED SINGLE MUSCLE FIBRES. J Physiol. 1965 Mar;177:42–57. doi: 10.1113/jphysiol.1965.sp007574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremel R. D., Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nat New Biol. 1972 Jul 26;238(82):97–101. doi: 10.1038/newbio238097a0. [DOI] [PubMed] [Google Scholar]
- Chase P. B., Kushmerick M. J. Effects of pH on contraction of rabbit fast and slow skeletal muscle fibers. Biophys J. 1988 Jun;53(6):935–946. doi: 10.1016/S0006-3495(88)83174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Pate E. The effects of ADP and phosphate on the contraction of muscle fibers. Biophys J. 1985 Nov;48(5):789–798. doi: 10.1016/S0006-3495(85)83837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzig J. A., Hibberd M. G., Trentham D. R., Goldman Y. E. Cross-bridge kinetics in the presence of MgADP investigated by photolysis of caged ATP in rabbit psoas muscle fibres. J Physiol. 1991 Jan;432:639–680. doi: 10.1113/jphysiol.1991.sp018405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood A. B., Wood D. S., Bock K. L., Sorenson M. M. Chemically skinned mammalian skeletal muscle. I. The structure of skinned rabbit psoas. Tissue Cell. 1979;11(3):553–566. doi: 10.1016/0040-8166(79)90062-4. [DOI] [PubMed] [Google Scholar]
- Ferenczi M. A., Goldman Y. E., Simmons R. M. The dependence of force and shortening velocity on substrate concentration in skinned muscle fibres from Rana temporaria. J Physiol. 1984 May;350:519–543. doi: 10.1113/jphysiol.1984.sp015216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi M. A. Phosphate burst in permeable muscle fibers of the rabbit. Biophys J. 1986 Sep;50(3):471–477. doi: 10.1016/S0006-3495(86)83484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuglsang A., Khromov A., Török K., Somlyo A. V., Somlyo A. P. Flash photolysis studies of relaxation and cross-bridge detachment: higher sensitivity of tonic than phasic smooth muscle to MgADP. J Muscle Res Cell Motil. 1993 Dec;14(6):666–677. doi: 10.1007/BF00141563. [DOI] [PubMed] [Google Scholar]
- Goldman Y. E., Hibberd M. G., McCray J. A., Trentham D. R. Relaxation of muscle fibres by photolysis of caged ATP. Nature. 1982 Dec 23;300(5894):701–705. doi: 10.1038/300701a0. [DOI] [PubMed] [Google Scholar]
- Goldman Y. E., Hibberd M. G., Trentham D. R. Initiation of active contraction by photogeneration of adenosine-5'-triphosphate in rabbit psoas muscle fibres. J Physiol. 1984 Sep;354:605–624. doi: 10.1113/jphysiol.1984.sp015395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E., Hibberd M. G., Trentham D. R. Relaxation of rabbit psoas muscle fibres from rigor by photochemical generation of adenosine-5'-triphosphate. J Physiol. 1984 Sep;354:577–604. doi: 10.1113/jphysiol.1984.sp015394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E., Simmons R. M. Control of sarcomere length in skinned muscle fibres of Rana temporaria during mechanical transients. J Physiol. 1984 May;350:497–518. doi: 10.1113/jphysiol.1984.sp015215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOARD D. E., OTT D. G. CONVERSION OF MONO- AND OLIGODEOXYRIBONUCLEOTIDES TO 5-TRIPHOSPHATES. J Am Chem Soc. 1965 Apr 20;87:1785–1788. doi: 10.1021/ja01086a031. [DOI] [PubMed] [Google Scholar]
- Hibberd M. G., Dantzig J. A., Trentham D. R., Goldman Y. E. Phosphate release and force generation in skeletal muscle fibers. Science. 1985 Jun 14;228(4705):1317–1319. doi: 10.1126/science.3159090. [DOI] [PubMed] [Google Scholar]
- Hibberd M. G., Goldman Y. E., Trentham D. R. Laser-induced photogeneration of ATP: a new approach to the study of chemical kinetics of muscle contraction. Curr Top Cell Regul. 1984;24:357–364. doi: 10.1016/b978-0-12-152824-9.50038-1. [DOI] [PubMed] [Google Scholar]
- Horiuti K., Sakoda T., Yamada K. Mechanical response to photolytic ATP pulses of skinned muscle fibres pre-activated with a small pulse of ATP. J Muscle Res Cell Motil. 1993 Jun;14(3):335–340. doi: 10.1007/BF00123098. [DOI] [PubMed] [Google Scholar]
- Houadjeto M., Travers F., Barman T. Ca(2+)-activated myofibrillar ATPase: transient kinetics and the titration of its active sites. Biochemistry. 1992 Feb 11;31(5):1564–1569. doi: 10.1021/bi00120a038. [DOI] [PubMed] [Google Scholar]
- Kawai M., Wray J. S., Zhao Y. The effect of lattice spacing change on cross-bridge kinetics in chemically skinned rabbit psoas muscle fibers. I. Proportionality between the lattice spacing and the fiber width. Biophys J. 1993 Jan;64(1):187–196. doi: 10.1016/S0006-3495(93)81356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Cooke R., Pate E. A model of stress relaxation in cross-bridge systems: effect of a series elastic element. Am J Physiol. 1993 Jul;265(1 Pt 1):C279–C288. doi: 10.1152/ajpcell.1993.265.1.C279. [DOI] [PubMed] [Google Scholar]
- MOLNAR J., LORAND L. Studies on apyrases. Arch Biochem Biophys. 1961 May;93:353–363. doi: 10.1016/0003-9861(61)90278-8. [DOI] [PubMed] [Google Scholar]
- Martin H., Barsotti R. J. Relaxation from rigor of skinned trabeculae of the guinea pig induced by laser photolysis of caged ATP. Biophys J. 1994 Apr;66(4):1115–1128. doi: 10.1016/S0006-3495(94)80892-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisescu D. G. Kinetics of reaction in calcium-activated skinned muscle fibres. Nature. 1976 Aug 12;262(5569):610–613. doi: 10.1038/262610a0. [DOI] [PubMed] [Google Scholar]
- Nishiye E., Somlyo A. V., Török K., Somlyo A. P. The effects of MgADP on cross-bridge kinetics: a laser flash photolysis study of guinea-pig smooth muscle. J Physiol. 1993 Jan;460:247–271. doi: 10.1113/jphysiol.1993.sp019470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham M., Ferenczi M. A., Irving M. A birefringence study of changes in myosin orientation during relaxation of skinned muscle fibers induced by photolytic ATP release. Biophys J. 1994 Sep;67(3):1141–1148. doi: 10.1016/S0006-3495(94)80581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole K. J., Maeda Y., Rapp G., Goody R. S. Dynamic X-ray diffraction measurements following photolytic relaxation and activation of skinned rabbit psoas fibres. Adv Biophys. 1991;27:63–75. doi: 10.1016/0065-227x(91)90008-2. [DOI] [PubMed] [Google Scholar]
- Rosenfeld S. S., Taylor E. W. The dissociation of 1-N6-ethenoadenosine diphosphate from regulated actomyosin subfragment 1. J Biol Chem. 1987 Jul 25;262(21):9994–9999. [PubMed] [Google Scholar]
- Rosenfeld S. S., Taylor E. W. The mechanism of regulation of actomyosin subfragment 1 ATPase. J Biol Chem. 1987 Jul 25;262(21):9984–9993. [PubMed] [Google Scholar]
- Schoenberg M. Equilibrium muscle crossbridge behavior: the interaction of myosin crossbridges with actin. Adv Biophys. 1993;29:55–73. doi: 10.1016/0065-227x(93)90005-p. [DOI] [PubMed] [Google Scholar]
- Shimizu H., Fujita T., Ishiwata S. Regulation of tension development by MgADP and Pi without Ca2+. Role in spontaneous tension oscillation of skeletal muscle. Biophys J. 1992 May;61(5):1087–1098. doi: 10.1016/S0006-3495(92)81918-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. W., Reid G. P., Trentham D. R. Synthesis and properties of caged nucleotides. Methods Enzymol. 1989;172:288–301. doi: 10.1016/s0076-6879(89)72019-x. [DOI] [PubMed] [Google Scholar]
- Webb M. R. A continuous spectrophotometric assay for inorganic phosphate and for measuring phosphate release kinetics in biological systems. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4884–4887. doi: 10.1073/pnas.89.11.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]


