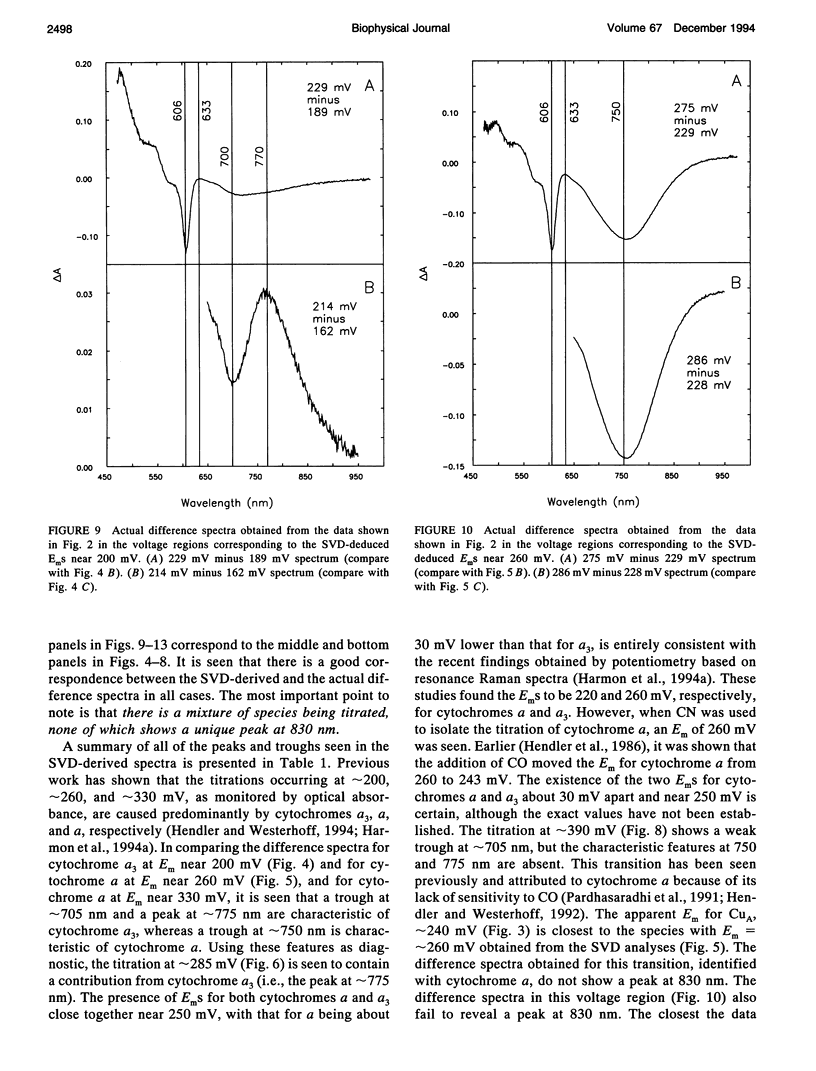
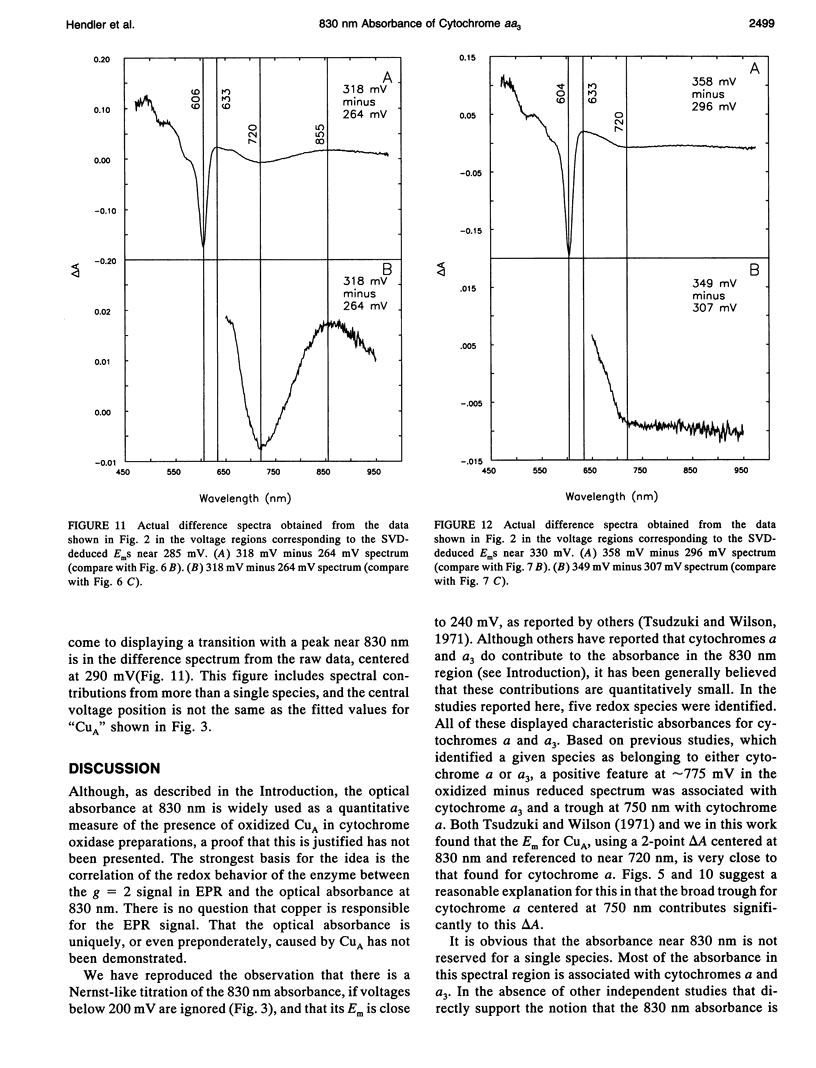
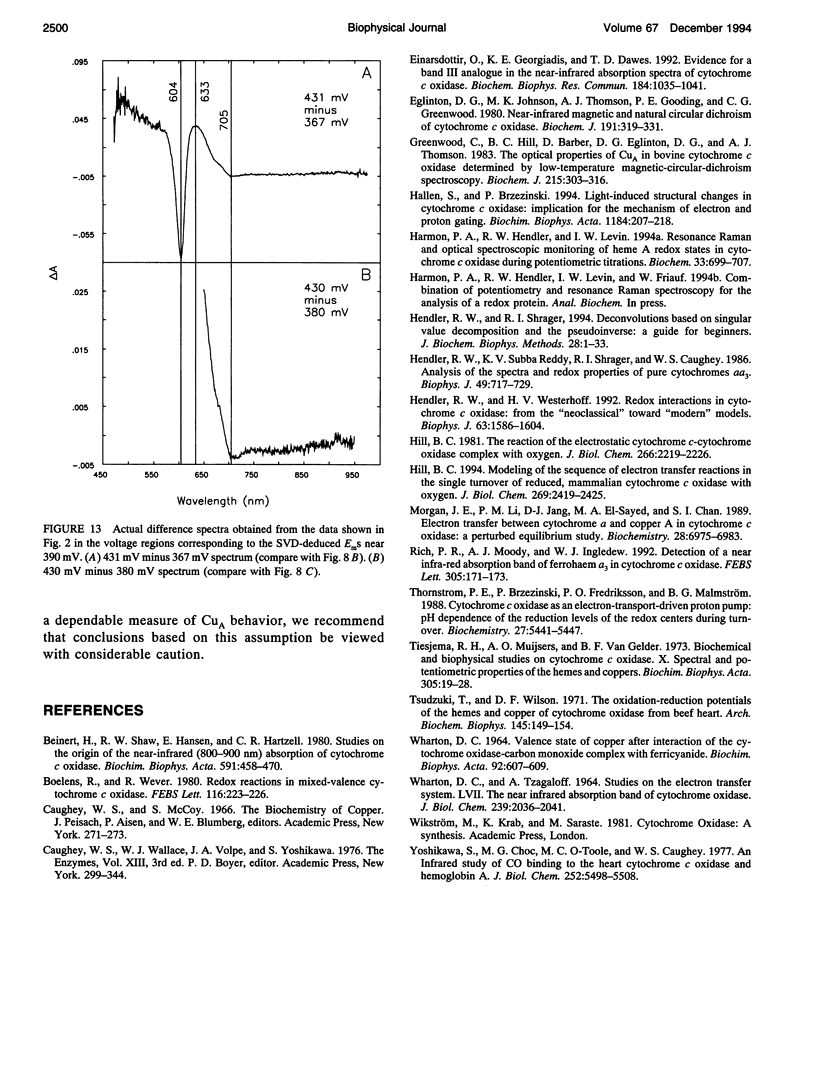
Abstract
Singular value decomposition (SVD) was used to deconvolute the spectral changes occurring in the near infrared region during potentiometric titrations of cytochrome aa3. Overall oxidized minus reduced difference spectra revealed a broad absorbance feature centered near 830 nm with an apparent Em near 250 mV. However, SVD did not isolate any spectral species with an absorbance centered near 830 nm. It was found that the spectral changes occurring in the wavelength region from 650 to 950 nm were associated mainly with cytochromes a and a3. It was concluded that the absorbance at 830 nm should not be used as an independent measure of the concentration of CuA in cytochrome aa3.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beinert H., Shaw R. W., Hansen R. E., Hartzell C. R. Studies on the origin of the near-infrared (800-900 nm) absorption of cytochrome c oxidase. Biochim Biophys Acta. 1980 Jul 8;591(2):458–470. doi: 10.1016/0005-2728(80)90176-0. [DOI] [PubMed] [Google Scholar]
- Boelens R., Wever R. Redox reactions in mixed-valence cytochrome c oxidase. FEBS Lett. 1980 Jul 28;116(2):223–226. doi: 10.1016/0014-5793(80)80649-1. [DOI] [PubMed] [Google Scholar]
- Eglinton D. G., Johnson M. K., Thomson A. J., Gooding P. E., Greenwood C. Near-infrared magnetic and natural circular dichroism of cytochrome c oxidase. Biochem J. 1980 Nov 1;191(2):319–331. doi: 10.1042/bj1910319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einarsdóttir O., Georgiadis K. E., Dawes T. D. Evidence for a band III analogue in the near-infrared absorption spectra of cytochrome c oxidase. Biochem Biophys Res Commun. 1992 Apr 30;184(2):1035–1041. doi: 10.1016/0006-291x(92)90695-h. [DOI] [PubMed] [Google Scholar]
- Greenwood C., Hill B. C., Barber D., Eglinton D. G., Thomson A. J. The optical properties of CuA in bovine cytochrome c oxidase determined by low-temperature magnetic-circular-dichroism spectroscopy. Biochem J. 1983 Nov 1;215(2):303–316. doi: 10.1042/bj2150303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallén S., Brzezinski P. Light-induced structural changes in cytochrome c oxidase: implication for the mechanism of electron and proton gating. Biochim Biophys Acta. 1994 Mar 8;1184(2-3):207–218. doi: 10.1016/0005-2728(94)90225-9. [DOI] [PubMed] [Google Scholar]
- Harmon P. A., Hendler R. W., Levin I. W. Resonance Raman and optical spectroscopic monitoring of heme a redox states in cytochrome c oxidase during potentiometric titrations. Biochemistry. 1994 Jan 25;33(3):699–707. doi: 10.1021/bi00169a011. [DOI] [PubMed] [Google Scholar]
- Hendler R. W., Reddy K. V., Shrager R. I., Caughey W. S. Analysis of the spectra and redox properties of pure cytochromes aa3. Biophys J. 1986 Mar;49(3):717–729. doi: 10.1016/S0006-3495(86)83698-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendler R. W., Shrager R. I. Deconvolutions based on singular value decomposition and the pseudoinverse: a guide for beginners. J Biochem Biophys Methods. 1994 Jan;28(1):1–33. doi: 10.1016/0165-022x(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Hendler R. W., Westerhoff H. V. Redox interactions in cytochrome c oxidase: from the "neoclassical" toward "modern" models. Biophys J. 1992 Dec;63(6):1586–1604. doi: 10.1016/S0006-3495(92)81748-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill B. C. Modeling the sequence of electron transfer reactions in the single turnover of reduced, mammalian cytochrome c oxidase with oxygen. J Biol Chem. 1994 Jan 28;269(4):2419–2425. [PubMed] [Google Scholar]
- Hill B. C. The reaction of the electrostatic cytochrome c-cytochrome oxidase complex with oxygen. J Biol Chem. 1991 Feb 5;266(4):2219–2226. [PubMed] [Google Scholar]
- Morgan J. E., Li P. M., Jang D. J., el-Sayed M. A., Chan S. I. Electron transfer between cytochrome a and copper A in cytochrome c oxidase: a perturbed equilibrium study. Biochemistry. 1989 Aug 22;28(17):6975–6983. doi: 10.1021/bi00443a030. [DOI] [PubMed] [Google Scholar]
- Rich P. R., Moody A. J., Ingledew W. J. Detection of a near infra-red absorption band of ferrohaem a3 in cytochrome c oxidase. FEBS Lett. 1992 Jul 6;305(3):171–173. doi: 10.1016/0014-5793(92)80659-5. [DOI] [PubMed] [Google Scholar]
- Thörnström P. E., Brzezinski P., Fredriksson P. O., Malmström B. G. Cytochrome c oxidase as an electron-transport-driven proton pump: pH dependence of the reduction levels of the redox centers during turnover. Biochemistry. 1988 Jul 26;27(15):5441–5447. doi: 10.1021/bi00415a009. [DOI] [PubMed] [Google Scholar]
- Tiesjema R. H., Muijsers A. O., van Gelder B. F. Biochemical and biophysical studies on cytochrome c oxidase. X. Spectral and potentiometric properties of the hemes and coppers. Biochim Biophys Acta. 1973 Apr 27;305(1):19–28. doi: 10.1016/0005-2728(73)90227-2. [DOI] [PubMed] [Google Scholar]
- Tsudzuki T., Wilson D. F. The oxidation-reduction potentials of the hemes and copper of cytochrome oxidase from beef heart. Arch Biochem Biophys. 1971 Jul;145(1):149–154. doi: 10.1016/0003-9861(71)90021-x. [DOI] [PubMed] [Google Scholar]
- WHARTON D. C., TZAGOLOFF A. STUDIES ON THE ELECTRON TRANSFER SYSTEM. LVII. THE NEAR INFRARED ABSORPTION BAND OF CYTOCHROME OXIDASE. J Biol Chem. 1964 Jun;239:2036–2041. [PubMed] [Google Scholar]
- WHARTON D. C. VALENCE STATE OF COPPER AFTER INTERACTION OF THE CYTOCHROME OXIDASE-CARBON MONOXIDE COMPLEX WITH FERRICYANIDE. Biochim Biophys Acta. 1964 Dec 23;92:607–609. doi: 10.1016/0926-6569(64)90022-7. [DOI] [PubMed] [Google Scholar]
- Yoshikawa S., Choc M. G., O'Toole M. C., Caughey W. S. An infrared study of CO binding to heart cytochrome c oxidase and hemoglobin A. Implications re O2 reactions. J Biol Chem. 1977 Aug 10;252(15):5498–5508. [PubMed] [Google Scholar]


