Abstract
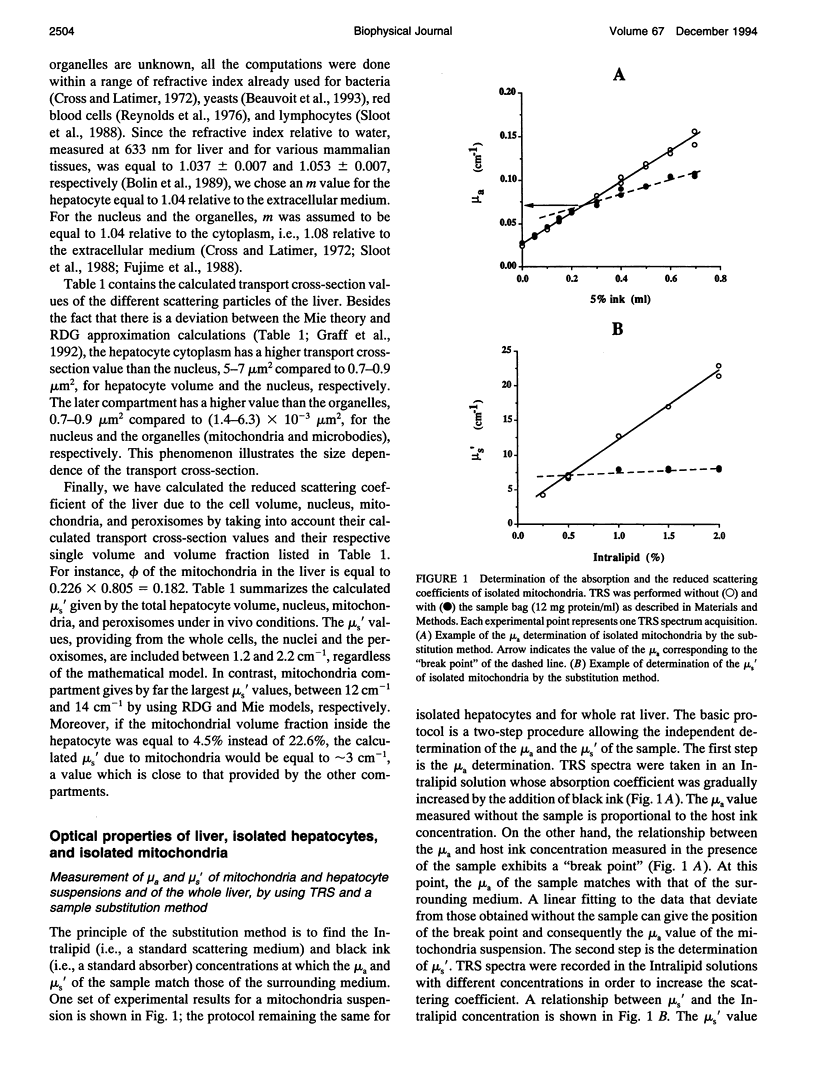
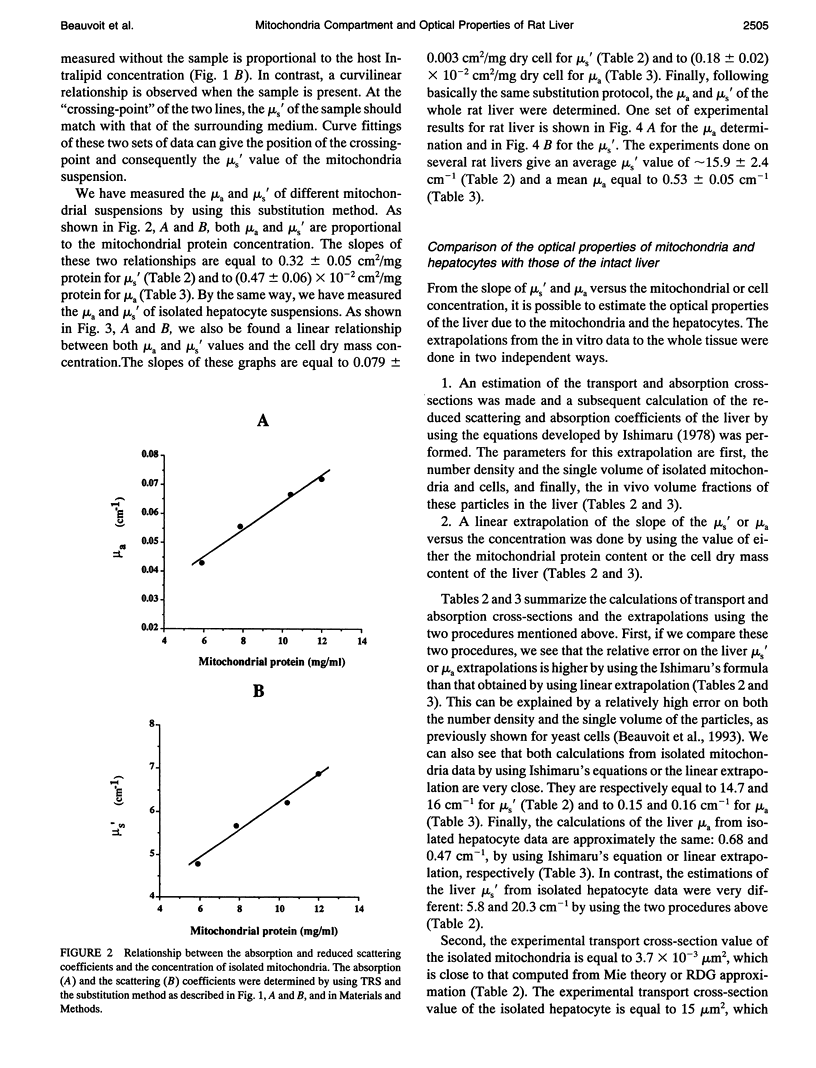
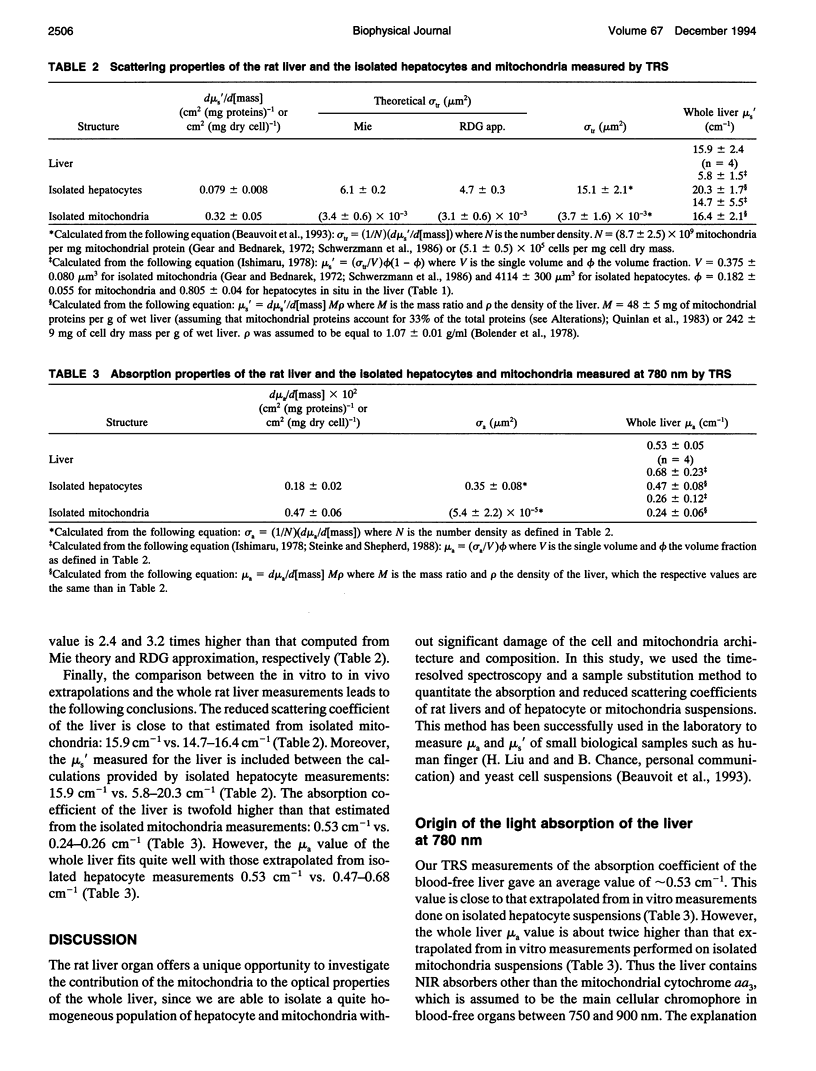
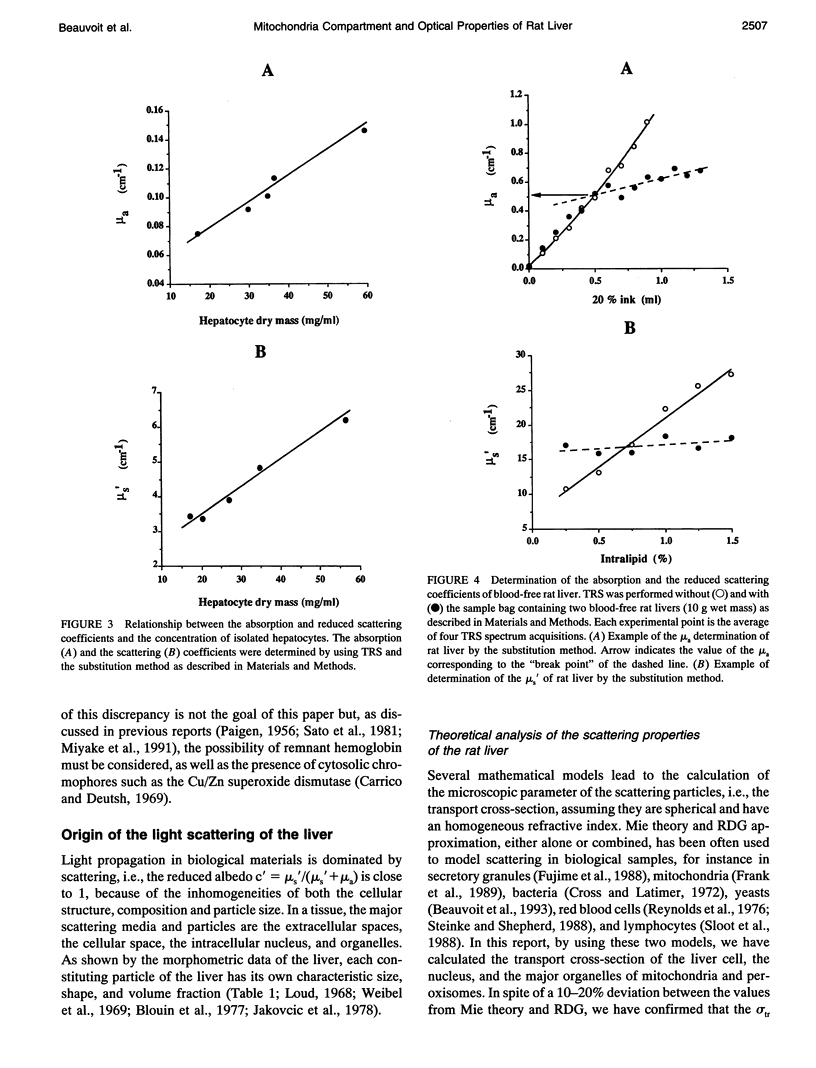
The purpose of this work was to analyze the contribution of the mitochondria to the optical properties, i.e., light absorption and scattering, of the blood-free rat liver. Firstly, a theoretical model of the reduced scattering coefficient of the liver was performed by using the Mie theory, the Rayleigh-Debye-Gans approximation, and the electron microscopy descriptions of the liver ultrastructure. Compared with the hepatocyte volume, the nucleus and the peroxisomes, the mitochondria compartment, accounting for 22% of the liver cell volume, seemed to be the predominant factor for the light scattering of the liver. Second, by using time-resolved spectroscopy and a sample substitution method, we have measured the absorption and reduced scattering coefficients of blood-free perfused rat livers, isolated hepatocyte suspensions, and isolated mitochondria suspensions. A subsequent extrapolation of the isolated mitochondria data to the in vivo mitochondrial content and a comparison with the whole liver measurements lead to the following conclusions: 1) the mitochondria account for about 50% of the liver absorption coefficient at 780 nm (mu a = 0.25 cm-1 extrapolated from isolated mitochondria vs. 0.53 +/- 0.05 cm-1 measured for the liver); and 2) the mitochondrial compartment is the primary factor for the light scattering in the rat liver (mu s' = 15.5 cm-1 extrapolated from the isolated mitochondria versus 15.9 +/- 2.4 cm-1 measured for the liver), demonstrating the relevancy of our preliminary theoretical study.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blouin A., Bolender R. P., Weibel E. R. Distribution of organelles and membranes between hepatocytes and nonhepatocytes in the rat liver parenchyma. A stereological study. J Cell Biol. 1977 Feb;72(2):441–455. doi: 10.1083/jcb.72.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolender R. P., Paumgartner D., Losa G., Muellener D., Weibel E. R. Intergrated stereological and biochemical studies of hepatocytic membranes. I. Membrane recoveries in subcellular fractions. J Cell Biol. 1978 May;77(2):565–583. doi: 10.1083/jcb.77.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico R. J., Deutsch H. F. Isolation of human hepatocuprein and cerebrocuprein. Their identity with erythrocuprein. J Biol Chem. 1969 Nov 25;244(22):6087–6093. [PubMed] [Google Scholar]
- Chance B., Leigh J. S., Miyake H., Smith D. S., Nioka S., Greenfeld R., Finander M., Kaufmann K., Levy W., Young M. Comparison of time-resolved and -unresolved measurements of deoxyhemoglobin in brain. Proc Natl Acad Sci U S A. 1988 Jul;85(14):4971–4975. doi: 10.1073/pnas.85.14.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Nioka S., Kent J., McCully K., Fountain M., Greenfeld R., Holtom G. Time-resolved spectroscopy of hemoglobin and myoglobin in resting and ischemic muscle. Anal Biochem. 1988 Nov 1;174(2):698–707. doi: 10.1016/0003-2697(88)90076-0. [DOI] [PubMed] [Google Scholar]
- Chen L. B. Fluorescent labeling of mitochondria. Methods Cell Biol. 1989;29:103–123. doi: 10.1016/s0091-679x(08)60190-9. [DOI] [PubMed] [Google Scholar]
- David H., Krause W., Behrisch D. Morphometrical characterization of isolated rat hepatocytes. Biomed Biochim Acta. 1990;49(7):563–571. [PubMed] [Google Scholar]
- Frank K. H., Kessler M., Appelbaum K., Albrecht H. P., Mauch E. D. Measurements of angular distributions of Rayleigh and Mie scattering events in biological models. Phys Med Biol. 1989 Dec;34(12):1901–1916. doi: 10.1088/0031-9155/34/12/012. [DOI] [PubMed] [Google Scholar]
- Fujime S., Takasaki-Ohsita M., Miyamoto S. Dynamic light scattering from polydisperse suspensions of large spheres. Characterization of isolated secretory granules. Biophys J. 1988 Dec;54(6):1179–1183. doi: 10.1016/S0006-3495(88)83054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta K., Ohno S., Gibo Y., Kiyosawa K., Furuta S. Three-dimensional ultrastructure of normal rat hepatocytes by quick-freezing and deep-etching method. J Gastroenterol Hepatol. 1992 Sep-Oct;7(5):486–490. doi: 10.1111/j.1440-1746.1992.tb01025.x. [DOI] [PubMed] [Google Scholar]
- Gear A. R., Bednarek J. M. Direct counting and sizing of mitochondria in solution. J Cell Biol. 1972 Aug;54(2):325–345. doi: 10.1083/jcb.54.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackenbrock C. R. Ultrastructural bases for metabolically linked mechanical activity in mitochondria. I. Reversible ultrastructural changes with change in metabolic steady state in isolated liver mitochondria. J Cell Biol. 1966 Aug;30(2):269–297. doi: 10.1083/jcb.30.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P. The regulation of the matrix volume of mammalian mitochondria in vivo and in vitro and its role in the control of mitochondrial metabolism. Biochim Biophys Acta. 1989 Mar 23;973(3):355–382. doi: 10.1016/s0005-2728(89)80378-0. [DOI] [PubMed] [Google Scholar]
- Hoffmann H. P., Avers C. J. Mitochondrion of yeast: ultrastructural evidence for one giant, branched organelle per cell. Science. 1973 Aug 24;181(4101):749–751. doi: 10.1126/science.181.4101.749. [DOI] [PubMed] [Google Scholar]
- Jakovcic S., Swift H. H., Gross N. J., Rabinowitz M. Biochemical and stereological analysis of rat liver mitochondria in different thyroid states. J Cell Biol. 1978 Jun;77(3):887–901. doi: 10.1083/jcb.77.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Chen L. B. Localization of mitochondria in living cells with rhodamine 123. Proc Natl Acad Sci U S A. 1980 Feb;77(2):990–994. doi: 10.1073/pnas.77.2.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemasters J. J., DiGuiseppi J., Nieminen A. L., Herman B. Blebbing, free Ca2+ and mitochondrial membrane potential preceding cell death in hepatocytes. Nature. 1987 Jan 1;325(6099):78–81. doi: 10.1038/325078a0. [DOI] [PubMed] [Google Scholar]
- Liu H., Miwa M., Beauvoit B., Wang N. G., Chance B. Characterization of absorption and scattering properties of small-volume biological samples using time-resolved spectroscopy. Anal Biochem. 1993 Sep;213(2):378–385. doi: 10.1006/abio.1993.1435. [DOI] [PubMed] [Google Scholar]
- Loud A. V. A quantitative stereological description of the ultrastructure of normal rat liver parenchymal cells. J Cell Biol. 1968 Apr;37(1):27–46. doi: 10.1083/jcb.37.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa I., Aoi H., Sando N., Kuroiwa T. Fluorescence microscopic studies of mitochondrial nucleoids during meiosis and sporulation in the yeast, Saccharomyces cerevisiae. J Cell Sci. 1984 Mar;66:21–38. doi: 10.1242/jcs.66.1.21. [DOI] [PubMed] [Google Scholar]
- Miyake H., Nioka S., Zaman A., Smith D. S., Chance B. The detection of cytochrome oxidase heme iron and copper absorption in the blood-perfused and blood-free brain in normoxia and hypoxia. Anal Biochem. 1991 Jan;192(1):149–155. doi: 10.1016/0003-2697(91)90200-d. [DOI] [PubMed] [Google Scholar]
- PAIGEN K. Hemoglobin as the red pigment of microsomes. Biochim Biophys Acta. 1956 Feb;19(2):297–299. doi: 10.1016/0006-3002(56)90431-0. [DOI] [PubMed] [Google Scholar]
- Pfaller W., Willinger C., Stoll B., Hallbrucker C., Lang F., Häussinger D. Structural reaction pattern of hepatocytes following exposure to hypotonicity. J Cell Physiol. 1993 Feb;154(2):248–253. doi: 10.1002/jcp.1041540206. [DOI] [PubMed] [Google Scholar]
- Quinlan P. T., Thomas A. P., Armston A. E., Halestrap A. P. Measurement of the intramitochondrial volume in hepatocytes without cell disruption and its elevation by hormones and valinomycin. Biochem J. 1983 Aug 15;214(2):395–404. doi: 10.1042/bj2140395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber B. F., Somogyi R., Stucki J. W. Hormone-induced intracellular calcium oscillations and mitochondrial energy supply in single hepatocytes. Biochim Biophys Acta. 1990 Jul 25;1018(2-3):190–193. doi: 10.1016/0005-2728(90)90246-z. [DOI] [PubMed] [Google Scholar]
- Sato N., Matsumura T., Shichiri M., Kamada T., Abe H., Hagihara B. Hemoperfusion, rate of oxygen consumption and redox levels of mitochondrial cytochrome c (+c1) in liver in situ of anesthetized rat measured by reflectance spectrophotometry. Biochim Biophys Acta. 1981 Jan 14;634(1):1–10. doi: 10.1016/0005-2728(81)90122-5. [DOI] [PubMed] [Google Scholar]
- Schwerzmann K., Cruz-Orive L. M., Eggman R., Sänger A., Weibel E. R. Molecular architecture of the inner membrane of mitochondria from rat liver: a combined biochemical and stereological study. J Cell Biol. 1986 Jan;102(1):97–103. doi: 10.1083/jcb.102.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevick E. M., Chance B., Leigh J., Nioka S., Maris M. Quantitation of time- and frequency-resolved optical spectra for the determination of tissue oxygenation. Anal Biochem. 1991 Jun;195(2):330–351. doi: 10.1016/0003-2697(91)90339-u. [DOI] [PubMed] [Google Scholar]
- Sloot P. M., Hoekstra A. G., Figdor C. G. Osmotic response of lymphocytes measured by means of forward light scattering: theoretical considerations. Cytometry. 1988 Nov;9(6):636–641. doi: 10.1002/cyto.990090620. [DOI] [PubMed] [Google Scholar]
- Smiley S. T., Reers M., Mottola-Hartshorn C., Lin M., Chen A., Smith T. W., Steele G. D., Jr, Chen L. B. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3671–3675. doi: 10.1073/pnas.88.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandler B., Hoppel C. L. Studies on giant mitochondria. Ann N Y Acad Sci. 1986;488:65–81. doi: 10.1111/j.1749-6632.1986.tb46548.x. [DOI] [PubMed] [Google Scholar]
- Weibel E. R., Stäubli W., Gnägi H. R., Hess F. A. Correlated morphometric and biochemical studies on the liver cell. I. Morphometric model, stereologic methods, and normal morphometric data for rat liver. J Cell Biol. 1969 Jul;42(1):68–91. doi: 10.1083/jcb.42.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L. Metastasis. Cell Biophys. 1993 Aug-Dec;23(1-3):1–2. doi: 10.1007/BF02796506. [DOI] [PubMed] [Google Scholar]


