Abstract
1. The unidirectional influx of amino acids into the guinea-pig syncytiotrophoblast was measured using a single circulation paired-tracer dilution technique which allows separate characterization of both fetal and maternal interfaces. An in situ preparation perfused through the fetal circulation was used to examine the fetal side, while an isolated preparation perfused through both the fetal and maternal circulations was used to study both interfaces simultaneously.
2. On the fetal side the maximal uptake (Umax) determined at tracer concentrations was high for the short-chain neutral amino acid alanine (76%) and the long-chain neutrals, leucine (75%), phenylalanine (90%) and tyrosine (82%) and for the basic amino acid lysine (65%). In contrast, Umax was negligible for α-methylaminoisobutyric acid and taurine, a β-amino acid.
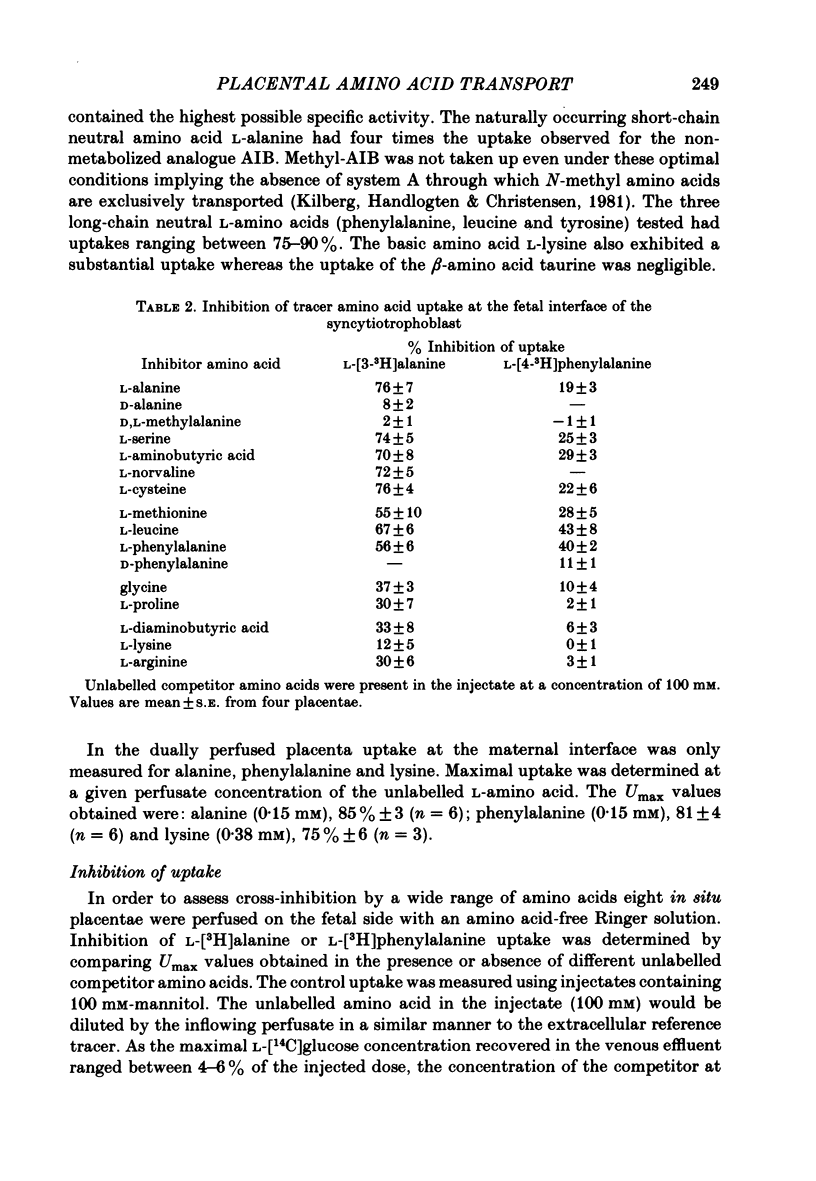
3. The uptake of alanine and phenylalanine on the fetal side was inhibited by both short-chain (alanine, serine, cysteine) and long-chain (phenylalanine, methionine, leucine) neutral amino acids. d-alanine had no effect on l-alanine uptake whereas d-phenylalanine significantly inhibited that of l-phenylalanine. Diaminobutyric acid, lysine and arginine were effective inhibitors of alanine uptake but had no effect on phenylalanine uptake.
4. On the maternal side uptake of alanine, phenylalanine and lysine was measured. Over a wide range of concentrations self-inhibition of alanine influx was similar to the cross-inhibition observed with phenylalanine. In contrast, the influx of phenylalanine, which was strongly self-inhibited, was only partially cross-inhibited by alanine.
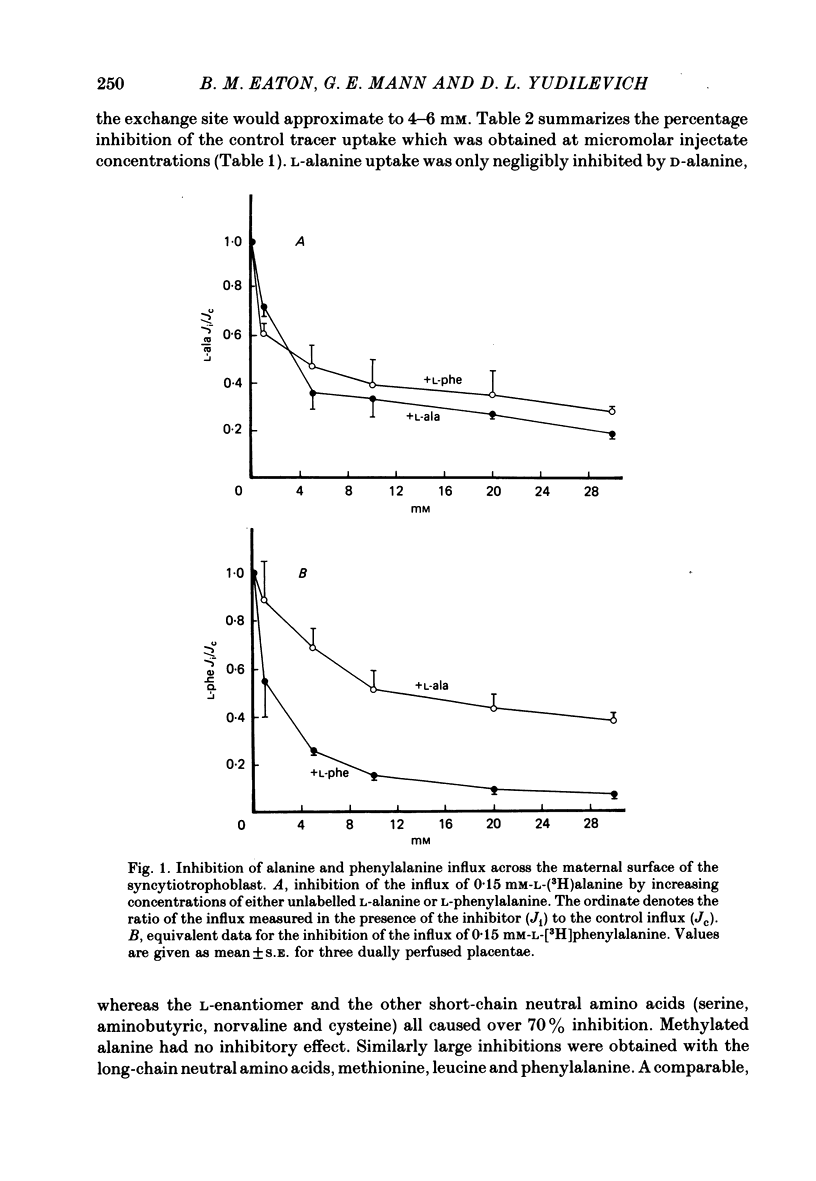
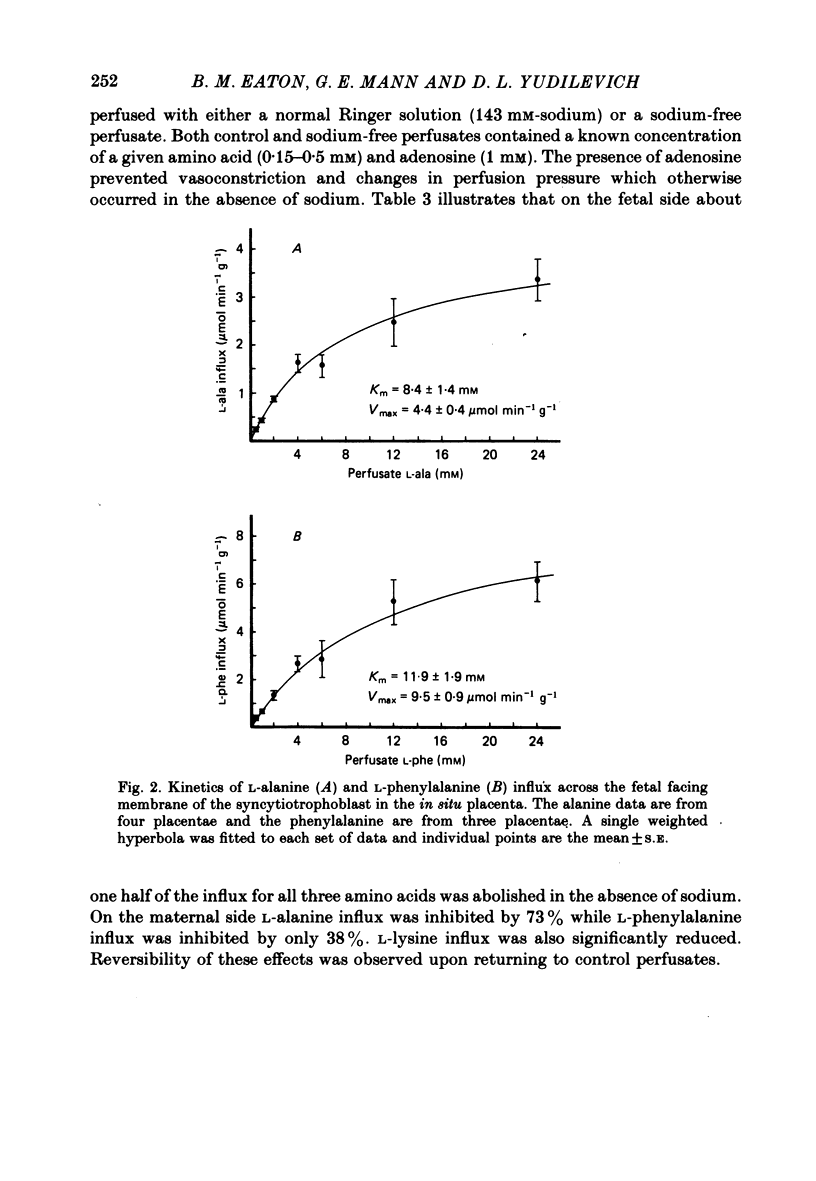
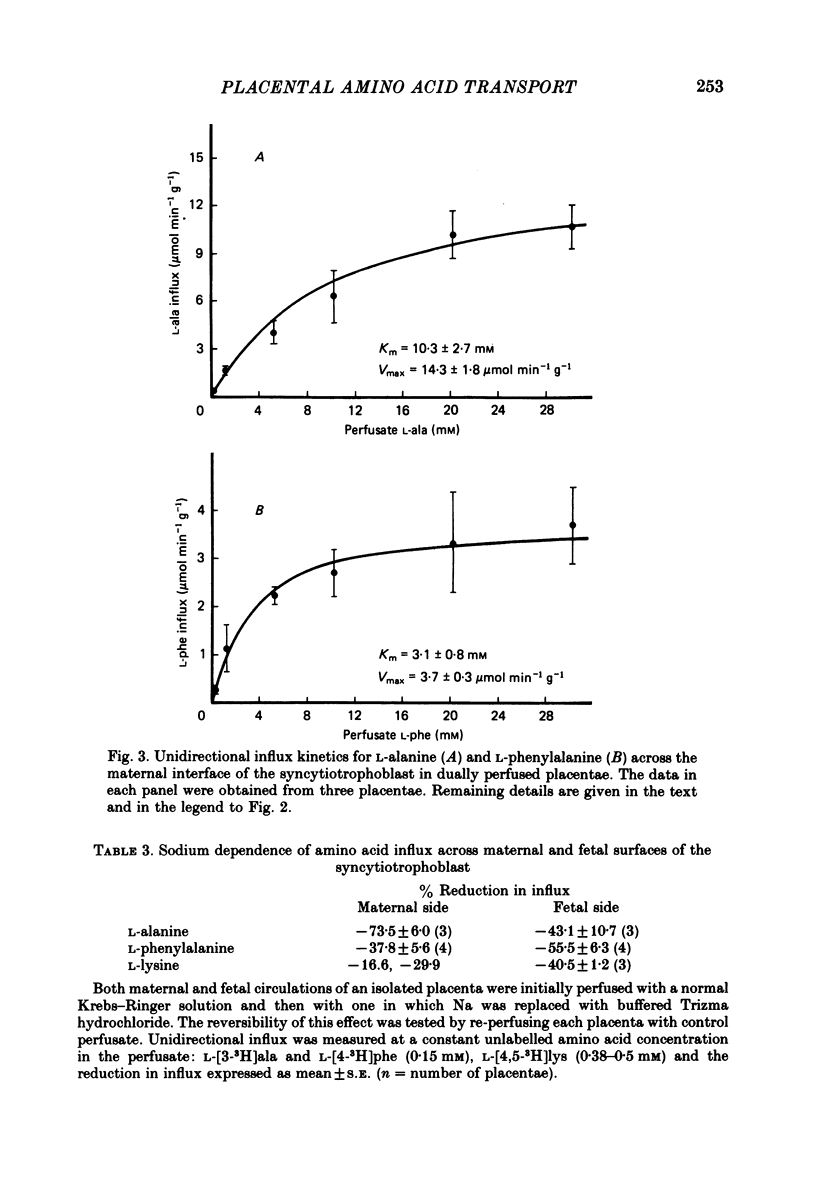
5. Influx of alanine and phenylalanine was measured at various perfusate concentrations and was found to be saturable on both maternal and fetal sides. The data were fitted to a single hyperbola and, on the maternal side, the Km for alanine (10.3±2.7 mm, mean±s.e., n = 3) was three-fold higher than the value measured for phenylalanine (3·1±0·8 mm). On the fetal side the Km values for alanine (8·4±1·4 mm, n = 4) and phenylalanine (11·9±1·9 mm, n = 3) were similar.
6. The uptake of alanine, phenylalanine and lysine appeared to be highly sodium-dependent accounting for 40-70% of the total influx. However, the inhibited fractions were found to be different on the two sides of the placenta.
7. The results of uptake, cross-inhibition and Na+-dependency experiments suggest the presence of an alanine-serine-cysteine (ASC) type system and a leucine (L) type system with markedly overlapping specificities at both the fetal and maternal interfaces. Separate kinetic characterization of a two carrier system was not possible under the conditions of these experiments. However, kinetic parameters for the over-all transport of alanine and phenylalanine were measured.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyd C. A., Lund E. K. L-proline transport by brush border membrane vesicles prepared from human placenta. J Physiol. 1981 Jun;315:9–19. doi: 10.1113/jphysiol.1981.sp013728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante J. C., Mann G. E., Yudilevich D. L. Specificity of neutral amino acid uptake at the basolateral side of the epithelium in the cat salivary gland in situ. J Physiol. 1981;313:65–79. doi: 10.1113/jphysiol.1981.sp013651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H. N. Exploiting amino acid structure to learn about membrane transport. Adv Enzymol Relat Areas Mol Biol. 1979;49:41–101. doi: 10.1002/9780470122945.ch2. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- Dancis J., Money W. L., Springer D., Levitz M. Transport of amino acids by placenta. Am J Obstet Gynecol. 1968 Jul 15;101(6):820–829. doi: 10.1016/0002-9378(68)90038-0. [DOI] [PubMed] [Google Scholar]
- Eaton B. M., Yudilevich D. L. Uptake and asymmetric efflux of amino acids at maternal and fetal sides of placenta. Am J Physiol. 1981 Sep;241(3):C106–C112. doi: 10.1152/ajpcell.1981.241.3.C106. [DOI] [PubMed] [Google Scholar]
- Enders R. H., Judd R. M., Donohue T. M., Smith C. H. Placental amino acid uptake. III. Transport systems for neutral amino acids. Am J Physiol. 1976 Mar;230(3):706–710. doi: 10.1152/ajplegacy.1976.230.3.706. [DOI] [PubMed] [Google Scholar]
- Guidotti G. G., Borghetti A. F., Gazzola G. C. The regulation of amino acid transport in animal cells. Biochim Biophys Acta. 1978 Dec 15;515(4):329–366. doi: 10.1016/0304-4157(78)90009-6. [DOI] [PubMed] [Google Scholar]
- Hayashi S., Sanada K., Sagawa N., Yamada N., Kido K. Umbilical vein-artery differences of plasma amino acids in the last trimester of human pregnancy. Biol Neonate. 1978;34(1-2):11–18. doi: 10.1159/000241099. [DOI] [PubMed] [Google Scholar]
- Hill P. M., Young M. Net placental transfer of free amino acids against varying concentrations. J Physiol. 1973 Dec;235(2):409–422. doi: 10.1113/jphysiol.1973.sp010393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. J., Mann G. E., Smaje L. H. The role of cyclic nucleotides and related compounds in nerve-mediated vasodilatation in the cat submandibular gland. Br J Pharmacol. 1980 Mar;68(3):485–497. doi: 10.1111/j.1476-5381.1980.tb14563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilberg M. S., Christensen H. N., Handlogten M. E. Cysteine as a system-specific substrate for transport system ASC in rat hepatocytes. Biochem Biophys Res Commun. 1979 May 28;88(2):744–751. doi: 10.1016/0006-291x(79)92110-7. [DOI] [PubMed] [Google Scholar]
- Kilberg M. S., Handlogten M. E., Christensen H. N. Characteristics of system ASC for transport of neutral amino acids in the isolated rat hepatocyte. J Biol Chem. 1981 Apr 10;256(7):3304–3312. [PubMed] [Google Scholar]
- Leichtweiss H. P., Schröder H. L-Lactate and D-Lactate carriers on the fetal and the maternal side of the trophoblast in the isolated guinea pig placenta. Pflugers Arch. 1981 Apr;390(1):80–85. doi: 10.1007/BF00582716. [DOI] [PubMed] [Google Scholar]
- Leichtweiss H. P., Schröder H. Uber den Glucosetransport durch die isolierte, beiderseits künstlich perfundierte Meerschweinchenplacenta. Pflugers Arch. 1971;325(2):139–148. doi: 10.1007/BF00587004. [DOI] [PubMed] [Google Scholar]
- Longo L. D., Yuen P., Gusseck D. J. Anaerobic, glycogen-dependent transport of amino acids by the placenta. Nature. 1973 Jun 29;243(5409):531–533. doi: 10.1038/243531a0. [DOI] [PubMed] [Google Scholar]
- MONEY W. L., DANCIS J. Technique for the in situ study of placental transport in the pregnant guinea pig. Am J Obstet Gynecol. 1960 Aug;80:209–214. doi: 10.1016/0002-9378(60)90114-9. [DOI] [PubMed] [Google Scholar]
- Miller R. K., Berndt W. O. Characterization of neutral amino acid accumulation by human term placental slices. Am J Physiol. 1974 Dec;227(6):1236–1242. doi: 10.1152/ajplegacy.1974.227.6.1236. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Connor J. D., Crawford I. L. Permeability changes in the blood-brain barrier: causes and consequences. CRC Crit Rev Toxicol. 1975 Jan;3(2):159–199. doi: 10.3109/10408447509079857. [DOI] [PubMed] [Google Scholar]
- Paterson J. Y., Sepúlveda F. V., Smith M. W. Distinguishing transport systems having overlapping specificities for neutral and basic amino acids in the rabbit ileum. J Physiol. 1981;319:345–354. doi: 10.1113/jphysiol.1981.sp013912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipps A. F., Holzman I. R., Teng C., Battaglia F. C. Tissue concentrations of free amino acids in term human placentas. Am J Obstet Gynecol. 1978 Aug 15;131(8):881–887. doi: 10.1016/s0002-9378(16)33136-2. [DOI] [PubMed] [Google Scholar]
- Reynolds M. L., Young M. The transfer of free alpha-amino nitrogen across the placental membrane in the guinea-pig. J Physiol. 1971 May;214(3):583–597. doi: 10.1113/jphysiol.1971.sp009450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzycki S. M., Kelley L. K., Smith C. H. Placental amino acid uptake. IV. Transport microvillous membrane vesicles. Am J Physiol. 1978 Jan;234(1):C27–C35. doi: 10.1152/ajpcell.1978.234.1.C27. [DOI] [PubMed] [Google Scholar]
- Schneider H., Möhlen K. H., Dancis J. Transfer of amino acids across the in vitro perfused human placenta. Pediatr Res. 1979 Apr;13(4 Pt 1):236–240. doi: 10.1203/00006450-197904000-00005. [DOI] [PubMed] [Google Scholar]
- Sepúlveda F. V., Smith M. W. Discrimination between different entry mechanisms for neutral amino acids in rabbit ileal mucosa. J Physiol. 1978 Sep;282:73–90. doi: 10.1113/jphysiol.1978.sp012449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. H., Adcock E. W., 3rd, Teasdale F., Meschia G., Battaglia F. C. Placental amino acid uptake: tissue preparation, kinetics, and preincubation effect. Am J Physiol. 1973 Mar;224(3):558–564. doi: 10.1152/ajplegacy.1973.224.3.558. [DOI] [PubMed] [Google Scholar]
- Yudilevich D. L., Eaton B. M. Amino acid carriers at maternal and fetal surfaces of the placenta by single circulation paired-tracer dilution. Kinetics of phenylalanine transport. Biochim Biophys Acta. 1980 Feb 28;596(2):315–319. doi: 10.1016/0005-2736(80)90364-8. [DOI] [PubMed] [Google Scholar]
- Yudilevich D. L., Eaton B. M., Short A. H., Leichtweiss H. P. Glucose carriers at maternal and fetal sides of the trophoblast in guinea pig placenta. Am J Physiol. 1979 Nov;237(5):C205–C212. doi: 10.1152/ajpcell.1979.237.5.C205. [DOI] [PubMed] [Google Scholar]
- van Dijk J. P., van Kreel B. K. Transport and accumulation of alpha-aminoisobutyric acid (A.I.B.) in the guinea pig placenta. Pflugers Arch. 1978 Nov 30;377(3):217–224. doi: 10.1007/BF00584275. [DOI] [PubMed] [Google Scholar]


