Abstract
Prolonged growth of murine embryonic stem (ES) cells lacking the telomerase reverse transcriptase, mTert, results in a loss of telomere DNA and an increased incidence of end-to-end fusions and aneuploidy. Furthermore, loss of only one copy of mTert also results in telomere shortening intermediate between wild-type (wt) and mTert-null ES cells [Liu, Y., Snow, B. E., Hande, M. P., Yeung, D., Erdmann, N. J., Wakeham, A., Itie, A., Siderovski, D. P., Lansdorp, P. M., Robinson, M. O. & Harrington, L. (2000) Curr. Biol. 10, 1459–1462]. Unexpectedly, although average telomere length in mTert+/− ES cells declined to a similar level as mTert-null ES cells, mTert+/− ES cell lines retained a minimal telomeric DNA signal at all chromosome ends. Consequently, no end-to-end fusions and genome instability were observed in the latest passages of mTert+/− ES cell lines. These data uncover a functional distinction between the dosage-dependent function of telomerase in average telomere-length maintenance and the selective maintenance of critically short telomeres in cells heterozygous for mTert. In normal and tumor cells, we suggest that telomerase activity insufficient to maintain a given average telomere length may, nonetheless, provide a protective advantage from end-to-end fusion and genome instability.
Eukaryotic telomerase is a ribonucleoprotein enzyme complex that adds telomere DNA repeats at chromosome ends. All telomerases seem to contain two critical components for enzymatic activity; the telomerase RNA, which provides the template for telomeric DNA synthesis, and a catalytic protein with similarity to reverse transcriptases, called TERT (telomerase reverse transcriptase; ref. 1). In Saccharomyces cerevisiae, plants, and mice, disruption of the telomerase RNA or TERT abolishes telomerase activity and leads to telomere shortening, which leads, in turn, to complete loss of telomere DNA, end-to-end fusions and genome instability (2–9). Telomerase or telomere-associated components were first characterized in ciliates and yeast, however little is known about the precise role of the several mammalian telomerase-associated proteins identified to date (1, 10).
Murine embryonic stem (ES) cells and tissues disrupted for TERT lack telomerase activity and, upon prolonged culture, exhibit loss of telomere DNA, increased aneuploidy, and end-to-end fusions (11). This phenotype parallels that of mice and ES cells disrupted for the essential RNA component of murine telomerase, mTR (3, 12–14). In mTR-null mice bred together for successive generations, telomere DNA attrition eventually leads to genome instability, loss of proliferative potential, and an increased incidence of spontaneous tumors in older mice (9, 15–18). Similarly, in S. cerevisiae, ablation of telomerase activity leads to telomere erosion and an eventual cessation of cell division (4–6, 19), and genetic instability is markedly increased (20, 21). Recently, we have shown that mTert exhibits haploinsufficiency for telomere-length maintenance in vivo, because mTert+/− ES cells exhibit telomere shortening at an intermediate rate compared with mTert-null ES cells (11).
Evidence in yeast and human cells suggests that the ability of telomerase to promote cell proliferation can be uncoupled from net lengthening of telomeres (10). However, until recently, it was not possible to functionally distinguish between the importance of overall telomere lengthening or maintenance vs. the maintenance of minimal telomeric DNA at critically eroded chromosome ends (22). Because mTert+/− ES cells are haploinsufficient for telomere-length maintenance but retain telomerase activity, we were able to test the consequences of telomere-length erosion in cells differing only in the presence or absence of telomerase activity. Despite an average telomere length similar to mTert-null ES cells at which end-to-end fusions are observed, mTert+/− ES cells maintain a minimal telomere DNA signal at all chromosome ends and do not exhibit end-to-end fusions. These data point to a functional distinction between telomerase activity levels that are necessary for the maintenance of a given average telomere length and telomerase activity levels that are insufficient for telomere-length maintenance but can nonetheless be recruited to replenish critically short chromosome ends.
Methods
Murine ES Cell Culture.
The generation of the mTert-targeting vector and the mTert+/− and mTert-null ES cells were described (11). ES cell culture was carried out as described (23). All ES cell culture and sample preparation for the assays below were performed at the Ontario Cancer Institute/Amgen Institute.
Cell Lysate Preparation and Telomerase Assays.
Cells lysed in a buffer containing 0.5% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS; Intergen, Purchase, NY) were prepared as described (24). Telomerase activity was assayed by using the telomere repeat amplification protocol (TRAP) according to the manufacturer's instructions (Intergen; ref. 24). The PCR was typically performed for 20 cycles, and activity was determined to be within the linear range of the assay by using serial dilutions of cell extract. The telomerase extension products were normalized to the internal PCR standard with NIH IMAGE QUANT analysis.
Quantitative Reverse Transcription (RT)-PCR Analysis of Mouse TERT.
Total RNA was extracted from wild type, mTert+/− or mTert-null ES cells by using Trizol (Life Technologies, Rockville, MD), according to the manufacturer's instructions. The level of mTert mRNA in ES cells was determined by quantitative RT-PCR analysis (Taqman) with an Applied Biosystems Prism 7700 Sequence Detection System (Perkin–Elmer) according to the manufacturer's instructions and normalized to murine glyceraldehyde-3-phosphate dehydrogenase (Gapdh) mRNA. The sequences of the mTert PCR primers are 5′-TGGTGGAGGTTGTTGCCAA-3′ (forward), 5′-CCACTGCATACTGGCGGATAC-3′(reverse), and 5′-TGATCAGGCACTCGGAGAGCACGTAC-3′ (probe). The sequences of the Gapdh PCR primers are 5′-GAACGGGAAGCTCACTGGC-3′ (forward), 5′-ACCACCTTCTTGATGTCATCATACTT-3′ (reverse), and 5′-TGGCCTTCCGTGTTCCTACCCCC-3′ (probe).
Telomere Length Analysis.
The average telomere fluorescence in ES cells was measured by flow cytometric fluorescence in situ hybridization (Flow-FISH) as described (25), with minor modifications (11). Quantitative telomeric FISH (Q-FISH) was performed as described (26), also with minor modifications. Images were recorded with a C4742–95 12-bit camera (Hamamatsu, Middlesex, NJ) on a Leitz DMRBE fluorescence microscope (Leica Microsystems, Richmond Hill, Ontario, Canada) equipped with N2.1 and A type filter cubes (Leica Microsystems) for Cy3 and 4′,6-diamidino-2-phenylindole (DAPI), respectively, and a 100W mercury lamp (Osram, Mississauga, Ontario, Canada). Image acquisition and camera control were performed with OPENLAB v.2.2.5 (Improvision, Lexington, MA). Images were acquired with a 100× PL-FLUOTAR objective lens (Leica). Approximately 10 images from each genotype were analyzed with TFL-TELO software (kindly provided by P. Lansdorp, Terry Fox Laboratory, Vancouver) (27, 28).
Results
Progressive Loss of Telomeric DNA upon Prolonged Culture of mTert+/− ES Cells.
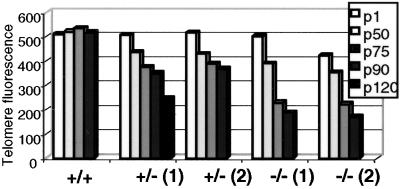
Because mTert+/− ES cells exhibit telomere erosion, we anticipated the mTert+/− ES clones would eventually phenocopy the telomere loss observed in mTert-null ES cells. We followed the growth of two mTert+/− ES clones and two mTert-null ES cell clones up to or beyond 90 passages (p90; ≈560 cell doublings). By using Flow-FISH, which measures the total telomere DNA content in a cell population (see Methods), we observed that all mTert+/− and mTert-null ES cell clones progressively lost telomeric DNA with continued growth in culture (Fig. 1, ref. 11). As expected, mTert-null ES cells exhibited a marked loss in telomere DNA; at p90, an average of nine chromosome ends per metaphase lacked a detectable telomere DNA signal (Fig. 2, Table 1). In particular, the telomeric DNA content in late passages (p90-p120) of mTert+/− ES cells eventually reached a similar overall telomere DNA content to mTert-null ES cells at p50–p75 (Fig. 1). However, despite the end-to-end fusions observed in mTert-null ES cells at this total telomeric DNA content, we found no evidence of chromosome ends without detectable telomere DNA in either of the mTert+/− ES cell lines (Fig. 2, Table 1).
Figure 1.
Flow-FISH analysis of telomeric DNA content in ES cell populations. Average relative telomere fluorescence upon continued passage (p1-p120) of wt, two independently derived mTert+/− ES cell lines, and two mTert−/− ES cell lines. Representative, average telomere fluorescence intensity at each passage is presented for each cell line and not averaged between samples because of variability in initial telomere lengths in each cell line.
Figure 2.
FISH analysis of ES cell metaphase preparations. (Upper) Representative metaphase preparations of wild-type ES cells at p75 (+/+ p75), and mTert+/− and mTert−/− ES cells at p90 (+/− p90, −/− p90). (Lower) The same images were overexposed to demonstrate that mTert+/− cells retain detectable telomere signals at all chromosome ends. Chromosome ends with no telomeric DNA signal (arrows) and end-to-end fusions (*) are indicated.
Table 1.
Chromosomal abnormalities in mTert+/− and mTert null ES cells
| Cell line | Metaphases analyzed | Aneuploidy (%)* | End-to-end fusions (per metaphase) | Signal-free ends† (per metaphase) |
|---|---|---|---|---|
| WT p1 | 50 | 5 (10) | 0 | 0 |
| WT p30 | 51 | 7 (13) | 0.02 | 0 |
| WT p50 | 49 | 19 (39) | 0 | 0 |
| WT p75 | 52 | 30 (58) | 0 | 0 |
| HET1 p1 | 50 | 4 (8) | 0 | 0 |
| HET1 p30 | 50 | 11 (22) | 0 | 0 |
| HET1 p50 | 50 | 23 (46) | 0.02 | 0 |
| HET1 p75 | 50 | 33 (66) | 0 | 0 |
| HET1 p90 | 42 | 23 (55)‡ | 0 | 0 |
| HET1 p120 | 25 | not done | 0 | 0 |
| HET2 p1 | 50 | 5 (10) | 0 | 0 |
| HET2 p50 | 50 | 20 (40) | 0 | 0 |
| HET2 p75 | 50 | 28 (56) | 0 | 0 |
| HET2 p90 | 50 | 31 (62)‡ | 0 | 0 |
| KO p1 | 50 | 7 (14) | 0 | 0 |
| KO p30 | 50 | 23 (46) | 0.04 | 0 |
| KO p50 | 50 | 30 (60) | 0.52 | 3.5 |
| KO p75 | 50 | 38 (76) | 1 | 5.3 |
| KO p90 | 51 | 41 (80)‡ | 2.5 | 9.0 |
WT, wild type. HET1 and HET2 are independently derived mTert+/− ES cells. KO, mTert−/−.
Aneuploidy is expressed as the percentage of metaphases with other than 40 chromosomes.
Overexposed FISH images were used to assess whether chromosome ends lacked a telomeric DNA signal.
A χ2 test between KO p90 and HET p90 yielded a statistical difference of P < 0.01 for HET1 and P < 0.05 for HET2. No statistical significance in % aneuploidy was found between WT and HET ES cells, or between KO and HET ES cells at a comparable average telomere length (e.g., HET p90 and KO p50).
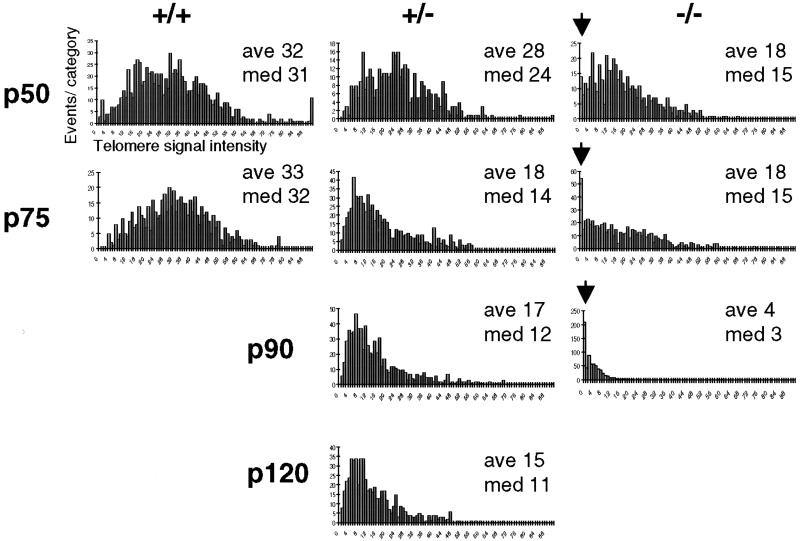
To determine precisely the telomere-length distributions in the ES cell populations, we performed Q-FISH, a technique which measures the telomeric DNA signal at each chromosome end in individual metaphase cells (27). Consistent with the Flow-FISH analysis, the telomere-length distribution in wt ES cells remained constant (or slightly increased) upon prolonged culture, whereas mTert+/− and mTert-null ES cells showed a continual decline in telomere signal with increasing passage (Fig. 3). Comparison of mTert+/− and mTert-null ES cells at a similar average telomere length revealed an important difference between the two genotypes (Fig. 3, compare +/−, p90 and p120 to −/−, p50 and p75). In mTert-null ES cells, chromosome ends with telomeric DNA signals below the minimal threshold of detection were evident as early as p50 and increased with successive passages (Fig. 3, arrows). However, such ‘signal-free’ chromosome ends were not observed in mTert+/− ES cells even at the latest passages tested (Fig. 3, p90 and p120). Within this growth period, removal of one allele of mTert is insufficient to maintain wild-type average telomere length but is sufficient to prevent the loss of detectable telomere signals at chromosome ends.
Figure 3.
Q-FISH analysis of individual ES cell metaphase preparations. The respective genotype is indicated at the top, and the passage number is at the left. Data were accumulated by using approximately 10 metaphases for each histogram. The ‘events per category’, or number of telomeres within a given range of telomeric DNA intensities, was plotted against the telomere DNA signal intensity with arbitrary units (0 = no telomeric DNA signal, and increasing in increments of arbitrary telomere fluorescence units to 90). Average and median telomere lengths are shown for each histogram (ave, med, respectively). Note that the decrease in mean telomere length appears greater by Q-FISH than by Flow-FISH in the latest passages in mTert−/− ES cells (compare p90 −/−, Fig. 1 and 3). We believe this difference could reflect higher nonspecific (i.e., nontelomeric) fluorescence with the Flow-FISH method, combined with reduced telomere DNA signal intensities in Q-FISH under the image conditions required to capture a wide variation in telomeric DNA signal in a near-linear range.
Genome Instability in Late Passage mTert−/− ES Cells but Not in mTert+/− ES Cells.
Previously, we showed that mTert-null ES cells at p50 harbored approximately one chromosome fusion per metaphase (11). By p75–p90, mTert-null ES cells contained an average of 2.5 end-to-end fusions per metaphase and showed a significant increase in aneuploidy compared with wt ES cells (Fig. 2, Table 1). In contrast, in two independently derived mTert+/− ES cell lines, we found only 1 end-to-end fusion in more than 450 metaphase spreads analyzed (Fig. 2, Table 1). At p90, the aneuploidy in mTert-null ES cells showed a statistical difference between the wt and mTert+/− ES cell lines (Table 1). However, when compared between samples with a similar average telomere length, there was no statistical difference between mTert-null ES and mTert+/− ES cells (Table 1). Thus, the increase in aneuploidy in late passage mTert-null ES cell lines is consistent with the increased frequency of signal-free ends and end-to-end fusions in these cells and also is consistent with the notion that end-to-end fusions would lead to increased chromosome breakage and loss.
Telomerase Activity in mTert+/− ES Cells.
Unexpectedly, we did not observe any difference in telomerase activity levels by TRAP between mTert+/− or wt ES cells, regardless of the passage number analyzed (ref. 11, Fig. 4 Upper, 5). An inability to detect a decrease in telomerase activity also was observed in extracts prepared from mTR+/−-derived tissues when compared with wt mice (52). It is possible that limitations in the sensitivity and linearity of the in vitro telomerase assays might obscure subtle changes in the level of telomerase enzyme activity. In an independently derived mTert+/− mouse, Yuan et al. (29) were able to detect a decrease in telomerase activity by using an in vitro telomerase elongation assay termed “stretch-PCR”. By using this and other published telomerase assays, we could not detect a difference in telomerase activity between our wt or mTert+/− ES cells (data not shown; refs. 29 and 30).
Figure 4.
Telomerase activity levels in mTert+/− ES cells. (Upper) Telomerase activity in wild-type or mTert+/− ES cells. TRAP was performed for 20 PCR cycles on 1.0, 0.5, 0.25, and 0.125 μg of cell extracts prepared from wt and mTert+/− ES cell extracts at the indicated passage numbers. An internal PCR standard for the TRAP is shown at bottom right with an arrow. The asterisk (*) indicates a nonspecific product in mouse extracts that is resistant to RNase A treatment. (Bottom) The telomerase extension products were normalized to internal standard with nih image quant analysis; SDs (error bars) were calculated based on three separate experiments.
Figure 5.
Quantitation of mTert mRNA levels in wild-type. mTert+/− and mTert-null ES cells at early passage (A), p50 (B), and late passage (C). The relative copies of mTert mRNA were calculated based on a standard curve obtained by using quantitative RT-PCR (Taqman) analysis and then normalized to the level of Gapdh mRNA in the corresponding samples. The passage number and genotype of each sample is indicated above. Average ratios of mTert to Gapdh are shown above each data bar. In the wt p50 sample, there were reproducibly decreased levels of Gapdh mRNA, leading to the apparently higher ratio of mTert/Gapdh (data not shown) (D). Analysis of telomerase activity levels in late passages of ES cells. The respective genotypes and passage numbers are shown above. TRAP was performed for 20 cycles on 1.0, 0.5, 0.25, and 0.125 μg of cell extracts of the indicated genotype. An internal PCR standard for the TRAP is shown at bottom left with an arrow. The asterisk (*) indicates a nonspecific product in mouse extracts that is resistant to RNase A treatment.
Therefore, we measured mTert mRNA levels in mTert+/− ES cells by quantitative RT-PCR (see Methods) and observed an approximately one-half reduction when compared to wt cells (Fig. 5 A and B). However, in the latest passage of one mTert+/− ES cell line (p120), we observed an increase in mTert mRNA levels compared with wt cells (Fig. 5C). At p90, an ≈1.5-fold increase in expression of mTert (relative to previous passages) also was detected in the second mTert+/− ES cell line (data not shown). Regardless of passage number, we did not detect any difference in telomerase activity between wt and mTert+/− ES cells (Fig. 4, Fig. 5D). Given the potential limitations of the in vitro telomerase assay, it is difficult to ascertain whether the increase in mTert mRNA expression at very late passages translates into an increase in telomerase activity. Regardless of the levels of telomerase activity at passages 90–120 in mTert+/− ES cells, it is clear that telomere length continued to decline compared with previous passages, and no end-to-end fusions were observed. We were unable to measure the levels of endogenous mTERT protein because of its low abundance (data not shown). By using RT-PCR, we also observed comparable levels of mTR in all genotypes (wt, +/−, and −/−; data not shown).
Discussion
Maintenance of Critically Short Telomeres Is Separable from Telomere-Length Maintenance.
In telomerase-positive mTert+/− ES cells, we have established that maintenance of a given average telomere length can be uncoupled from maintenance of minimal telomeric DNA at critically short chromosome ends. These data further suggest that critically short chromosome ends, and not average short telomere length, lead to end-to-end fusions and concomitant genome instability in mTert-deficient ES cells. In ES cells heterozygous for mTR, haploinsufficiency in telomere-length maintenance was not detected (12). However, recent genetic analysis of mTR+/− mice crossed with mTR−/− sixth generation mice with short telomeres established that critically short chromosome ends could be selectively elongated in the mTR+/− offspring but not in mTR−/− littermates with similar average telomere lengths (22, 31). Hemann et al. (22) also demonstrated that loss of telomere function occurred specifically on chromosomes with critically short telomeres. In interspecies crosses between mTR+/− mice with long telomeres and mTR+/− mice with short telomeres, minimal telomeric DNA was maintained at all chromosome ends in mTR+/− offspring, despite an inability to elongate telomeres to the average length observed in wild-type interspecies littermates (52).
As yet, there exists only indirect evidence for dosage-sensitive telomerase function in human cells. Low levels of telomerase activity in human diploid fibroblasts allow cell proliferation at an average telomere length comparable to that of senescent primary fibroblasts, suggesting that selective elongation of critically short telomeres might mask a ‘senescence-inducing’ arrest (32). Limiting amounts of telomerase activity also might account for the extension of lifespan and reduction in genome instability of virally transformed human cells that are incapable of net telomere lengthening (33). Finally, one can speculate that in some human cell types such as hematopoietic cells, low levels of telomerase activity might prove beneficial for the maintenance of genome stability, despite the inability to maintain an equilibrium average telomere length (34).
Telomerase Seems to Be Recruited to the Shortest Telomeres.
In S. cerevisiae, several proteins including Est1p, Cdc13p, Stn1p, and Ten1p play a pivotal role in the recruitment of yeast telomerase to telomeres (35, 36). Telomere lengthening is promoted particularly when telomeres are short and binding sites for the double-stranded telomere binding protein RAP1 are limiting (37–40). Analogous, as yet uncharacterized mammalian factors might actively recruit mammalian telomerase specifically to short chromosome ends. Alternatively, inhibition of telomerase elongation may be relieved as telomeres erode because of a loss of telomere binding sites for factors such as TRF1 that inhibit telomerase function (41, 42). Other modifications that might occur at critically short telomeres include increased tankyrase activity, which antagonizes TRF1 binding to telomere DNA (43, 44), changes in the structure of telomere termini (e.g., the t-loop) (45), or changes in telomeric chromatin upon telomere shortening (46).
Evidence for Dosage Sensitivity in the Telomerase Complex.
Further analysis will be required to determine whether haploinsufficiency of mTert in ES cells eventually leads to a short equilibrium telomere length, an increase in genome instability, or a compensatory increase in expression of mTert RNA and telomerase activity. Given the extensive breeding times required for loss of telomere function even in telomerase-null mice, it may be difficult to establish an intermediate rate of telomere shortening in crosses between mTert+/− mice. However, there is evidence for ‘additive haploinsufficiency’ in telomerase components in S. cerevisiae. Lundblad and coworkers (5, 19) observed that the progeny of diploid yeast heterozygous for more than one telomerase component show accelerated telomere shortening and senescence compared with progeny from a single heterozygote diploid. In humans, a reduction in the expression of the human telomerase RNA has been reported in individuals affected with dyskeratosis congenita (DKC), which correlates with shorter telomeres in these individuals (47). In a recent report, three families with autosomal dominant DKC possess a linked mutation in one allele of human telomerase RNA, which also seems to result in telomere shortening (48). It is not clear whether this mutation leads to haploinsufficiency or inhibits by dominant-negative interference (48). Our data support the idea that dosage effects in telomerase enzyme components may be a significant cause of telomere-length reduction in vivo.
The purpose of such a finely tuned balance between telomerase components and telomere-length equilibrium is unclear. Optimal telomerase activity levels may serve to limit cell proliferation, to counterbalance factors that inhibit telomere lengthening, and to meet cellular requirements for ongoing telomere synthesis. Rapid tumor cell death has been observed upon telomerase inhibition in some tumor types with short telomeres (49–51). In these human tumor cells, survival at very short average telomere lengths may depend upon the continuous ability of telomerase to selectively elongate critically short chromosome ends and repress end-to-end fusions. Thus, even partial inhibition of telomerase in some tumor types may prove an effective anti-cancer strategy.
Acknowledgments
We thank N. Erdmann and D. Bouchard for expert technical assistance, X. Zhang for analysis of mTR levels, C. W. Greider, M. Hemann, and R. Hodes for sharing unpublished data, and M. Tyers and members of the Harrington laboratory for critical comments on the manuscript. L.H. acknowledges the support of the Canadian Institutes of Health Research (MT14340) and the National Institutes of Health (AG8422117).
Abbreviations
- TERT
telomerase reverse transcriptase
- ES
embryonic stem cells
- TRAP
telomere repeat amplification protocol
- TR
telomerase RNA
- Flow-FISH
flow cytometry fluorescence in situ hybridization
- Q-FISH
quantitative FISH
- wt
wild type
- p
passage
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Nugent C I, Lundblad V. Genes Dev. 1998;12:1073–1085. doi: 10.1101/gad.12.8.1073. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura T M, Morin G B, Chapman K B, Weinrich S L, Andrews W H, Lingner J, Harley C B, Cech T R. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- 3.Blasco M A, Lee H W, Hande M P, Samper E, Lansdorp P M, DePinho R A, Greider C W. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 4.Singer M S, Gottschling D E. Science. 1994;266:404–409. doi: 10.1126/science.7545955. [DOI] [PubMed] [Google Scholar]
- 5.Lendvay T S, Morris D K, Sah J, Balasubramanian B, Lundblad V. Genetics. 1996;144:1399–1412. doi: 10.1093/genetics/144.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lingner J, Hughes T R, Shevchenko A, Mann M, Lundblad V, Cech T R. Science. 1997;276:561–567. doi: 10.1126/science.276.5312.561. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald M S, Riha K, Gao F, Ren S, McKnight T D, Shippen D E. Proc Natl Acad Sci USA. 1999;96:14813–14818. doi: 10.1073/pnas.96.26.14813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riha K, McKnight T D, Griffing L R, Shippen D E. Science. 2001;291:1797–1800. doi: 10.1126/science.1057110. [DOI] [PubMed] [Google Scholar]
- 9.Lee H W, Blasco M A, Gottlieb G J, Horner J W, 2nd, Greider C W, DePinho R A. Nature (London) 1998;392:569–574. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- 10.Blackburn E H. Cell. 2001;106:661–673. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Snow B E, Hande M P, Yeung D, Erdmann N J, Wakeham A, Itie A, Siderovski D P, Lansdorp P M, Robinson M O, Harrington L. Curr Biol. 2000;10:1459–1462. doi: 10.1016/s0960-9822(00)00805-8. [DOI] [PubMed] [Google Scholar]
- 12.Niida H, Matsumoto T, Satoh H, Shiwa M, Tokutake Y, Furuichi Y, Shinkai Y. Nat Genet. 1998;19:203–206. doi: 10.1038/580. [DOI] [PubMed] [Google Scholar]
- 13.Niida H, Shinkai Y, Hande M P, Matsumoto T, Takehara S, Tachibana M, Oshimura M, Lansdorp P M, Furuichi Y. Mol Cell Biol. 2000;20:4115–4127. doi: 10.1128/mcb.20.11.4115-4127.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hande M P, Samper E, Lansdorp P, Blasco M A. J Cell Biol. 1999;144:589–601. doi: 10.1083/jcb.144.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrera E, Samper E, Martin-Caballero J, Flores J M, Lee H W, Blasco M A. EMBO J. 1999;18:2950–2960. doi: 10.1093/emboj/18.11.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrera E, Samper E, Blasco M A. EMBO J. 1999;18:1172–1181. doi: 10.1093/emboj/18.5.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudolph K L, Chang S, Lee H W, Blasco M, Gottlieb G J, Greider C, DePinho R A. Cell. 1999;96:701–712. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- 18.Herrera E, Martinez A C, Blasco M A. EMBO J. 2000;19:472–481. doi: 10.1093/emboj/19.3.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lingner J, Cech T R, Hughes T R, Lundblad V. Proc Natl Acad Sci USA. 1997;94:11190–11195. doi: 10.1073/pnas.94.21.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hackett J A, Feldser D M, Greider C W. Cell. 2001;106:275–286. doi: 10.1016/s0092-8674(01)00457-3. [DOI] [PubMed] [Google Scholar]
- 21.Myung K, Chen C, Kolodner R D. Nature (London) 2001;411:1073–1076. doi: 10.1038/35082608. [DOI] [PubMed] [Google Scholar]
- 22.Hemann M T, Strong M A, Hao L Y, Greider C W. Cell. 2001;107:67–77. doi: 10.1016/s0092-8674(01)00504-9. [DOI] [PubMed] [Google Scholar]
- 23.Hakem R, de la Pompa J L, Sirard C, Mo R, Woo M, Hakem A, Wakeham A, Potter J, Reitmair A, Billia F, et al. Cell. 1996;85:1009–1023. doi: 10.1016/s0092-8674(00)81302-1. [DOI] [PubMed] [Google Scholar]
- 24.Kim N W, Piatyszek M A, Prowse K R, Harley C B, West M D, Ho P L, Coviello G M, Wright W E, Weinrich S L, Shay J W. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 25.Rufer N, Dragowska W, Thornbury G, Roosnek E, Lansdorp P M. Nat Biotechnol. 1998;16:743–747. doi: 10.1038/nbt0898-743. [DOI] [PubMed] [Google Scholar]
- 26.Zijlmans J M, Martens U M, Poon S S, Raap A K, Tanke H J, Ward R K, Lansdorp P M. Proc Natl Acad Sci USA. 1997;94:7423–7428. doi: 10.1073/pnas.94.14.7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lansdorp P M, Verwoerd N P, van de Rijke F M, Dragowska V, Little M T, Dirks R W, Raap A K, Tanke H J. Hum Mol Genet. 1996;5:685–691. doi: 10.1093/hmg/5.5.685. [DOI] [PubMed] [Google Scholar]
- 28.Poon S S, Martens U M, Ward R K, Lansdorp P M. Cytometry. 1999;36:267–278. doi: 10.1002/(sici)1097-0320(19990801)36:4<267::aid-cyto1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 29.Yuan X, Ishibashi S, Hatakeyama S, Saito M, Nakayama J, Nikaido R, Haruyama T, Watanabe Y, Iwata H, Iida M, et al. Genes Cells. 1999;4:563–572. doi: 10.1046/j.1365-2443.1999.00284.x. [DOI] [PubMed] [Google Scholar]
- 30.Prowse K R, Avilion A A, Greider C W. Proc Natl Acad Sci USA. 1993;90:1493–1497. doi: 10.1073/pnas.90.4.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samper E, Flores J M, Blasco M A. EMBO Rep. 2001;2:800–807. doi: 10.1093/embo-reports/kve174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ouellette M M, Liao M, Herbert B S, Johnson M, Holt S E, Liss H S, Shay J W, Wright W E. J Biol Chem. 2000;275:10072–10076. doi: 10.1074/jbc.275.14.10072. [DOI] [PubMed] [Google Scholar]
- 33.Zhu J, Wang H, Bishop J M, Blackburn E H. Proc Natl Acad Sci USA. 1999;96:3723–3728. doi: 10.1073/pnas.96.7.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaziri H, Dragowska W, Allsopp R C, Thomas T E, Harley C B, Lansdorp P M. Proc Natl Acad Sci USA. 1994;91:9857–9860. doi: 10.1073/pnas.91.21.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evans S K, Lundblad V. J Cell Sci. 2000;19:3357–3364. doi: 10.1242/jcs.113.19.3357. [DOI] [PubMed] [Google Scholar]
- 36.Grandin N, Damon C, Charbonneau M. EMBO J. 2001;20:1173–1183. doi: 10.1093/emboj/20.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marcand S, Wotton D, Gilson E, Shore D. Ciba Found Symp. 1997;211:76–93. doi: 10.1002/9780470515433.ch6. [DOI] [PubMed] [Google Scholar]
- 38.Marcand S, Brevet V, Gilson E. EMBO J. 1999;18:3509–3519. doi: 10.1093/emboj/18.12.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ray A, Runge K W. Mol Cell Biol. 1998;18:1284–1295. doi: 10.1128/mcb.18.3.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ray A, Runge K W. Mol Cell Biol. 1999;19:31–45. doi: 10.1128/mcb.19.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Steensel B, de Lange T. Nature (London) 1997;385:740–743. doi: 10.1038/385740a0. [DOI] [PubMed] [Google Scholar]
- 42.Smogorzewska A, van Steensel B, Bianchi A, Oelmann S, Schaefer M R, Schnapp G, de Lange T. Mol Cell Biol. 2000;20:1659–1668. doi: 10.1128/mcb.20.5.1659-1668.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith S, Giriat I, Schmitt A, de Lange T. Science. 1998;282:1484–1487. doi: 10.1126/science.282.5393.1484. [DOI] [PubMed] [Google Scholar]
- 44.Smith S, de Lange T. Curr Biol. 2000;10:1299–1302. doi: 10.1016/s0960-9822(00)00752-1. [DOI] [PubMed] [Google Scholar]
- 45.Griffith J D, Comeau L, Rosenfield S, Stansel R M, Bianchi A, Moss H, de Lange T. Cell. 1999;97:503–514. doi: 10.1016/s0092-8674(00)80760-6. [DOI] [PubMed] [Google Scholar]
- 46.Shore D. Curr Opin Genet Dev. 2001;11:189–198. doi: 10.1016/s0959-437x(00)00178-7. [DOI] [PubMed] [Google Scholar]
- 47.Mitchell J R, Wood E, Collins K. Nature (London) 1999;402:551–555. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- 48.Vulliamy T, Marrone A, Goldman F, Dearlove A, Bessler M, Mason P J, Dokal I. Nature (London) 2001;413:432–435. doi: 10.1038/35096585. [DOI] [PubMed] [Google Scholar]
- 49.Saretzki G, Ludwig A, von Zglinicki T, Runnenbaum I B. Cancer Gene Ther. 2001;10:827–834. doi: 10.1038/sj.cgt.7700383. [DOI] [PubMed] [Google Scholar]
- 50.Kim M M, Rivera M A, Botchkina I L, Shalaby R, Thor A D, Blackburn E H. Proc Natl Acad Sci USA. 2001;98:7982–7987. doi: 10.1073/pnas.131211098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang X, Mar V, Zhou W, Harrington L, Robinson M O. Genes Dev. 1999;13:2388–2399. doi: 10.1101/gad.13.18.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hathcock K S, Hemann M T, Opperman K K, Strong M A, Greider C W, Hodes R J. Proc Natl Acad Sci USA. 2002;99:3591–3596. doi: 10.1073/pnas.012549799. [DOI] [PMC free article] [PubMed] [Google Scholar]