Abstract
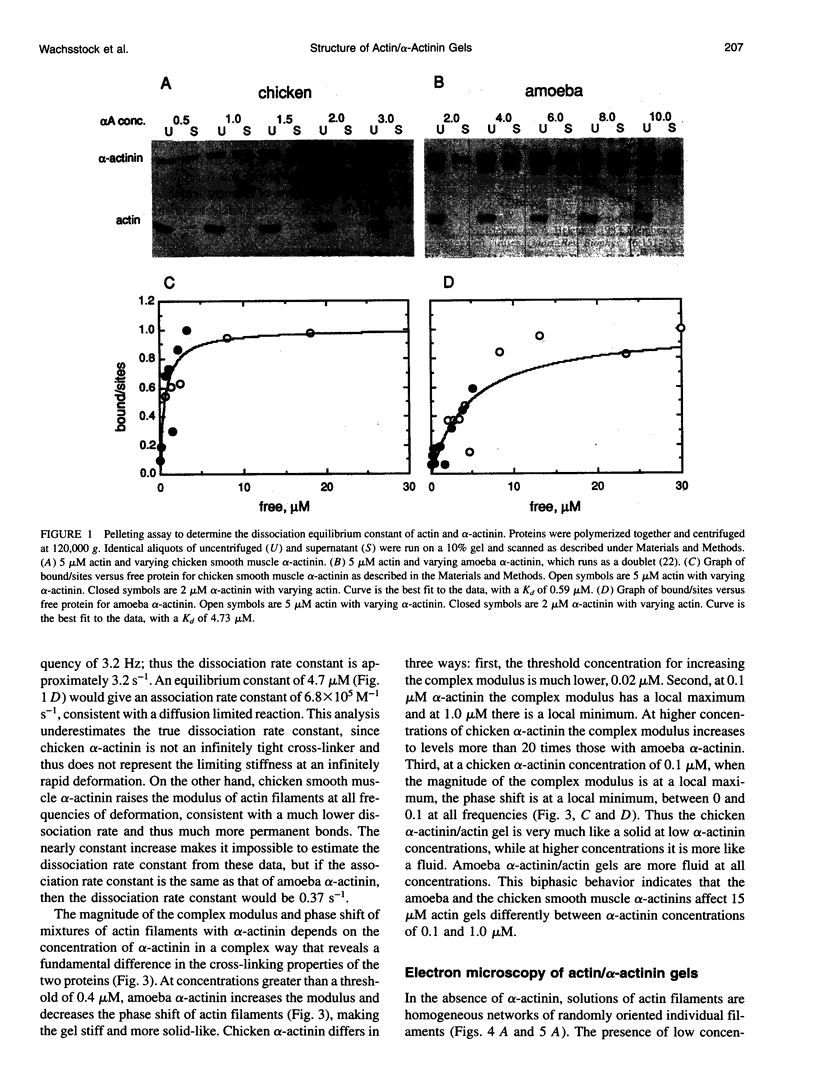
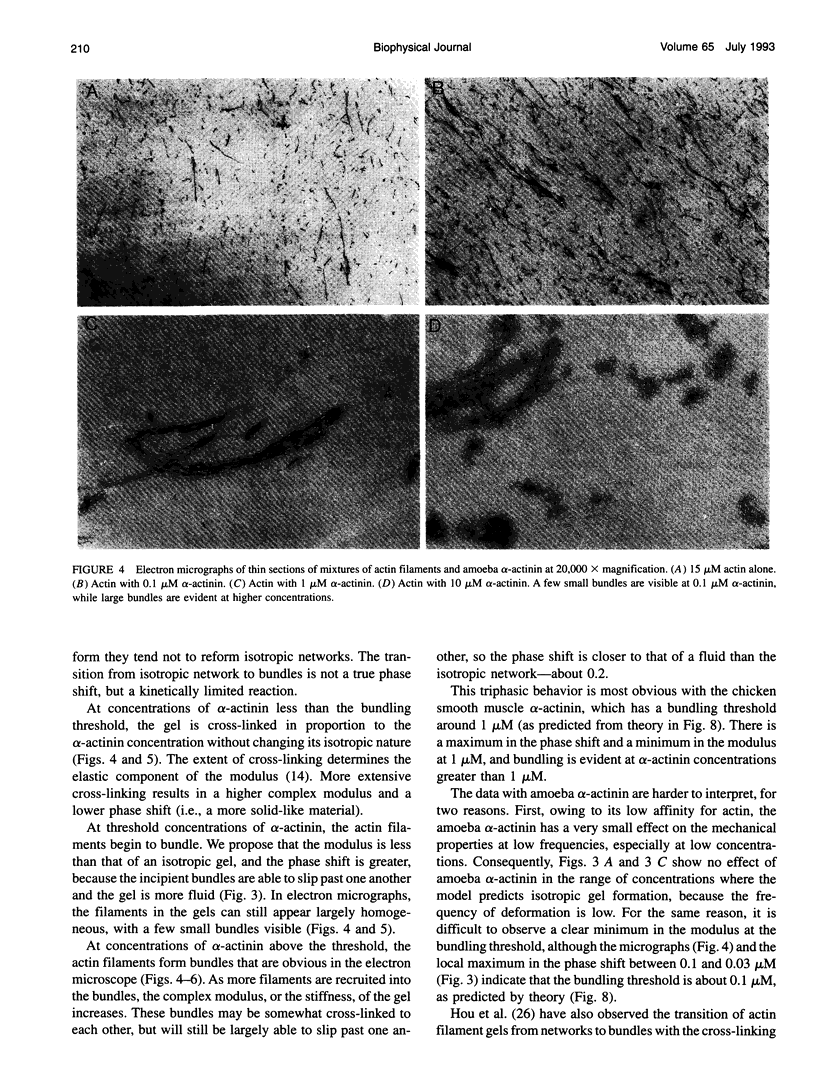
Proteins that cross-link actin filaments can either form bundles of parallel filaments or isotropic networks of individual filaments. We have found that mixtures of actin filaments with alpha-actinin purified from either Acanthamoeba castellanii or chicken smooth muscle can form bundles or isotropic networks depending on their concentration. Low concentrations of alpha-actinin and actin filaments form networks indistinguishable in electron micrographs from gels of actin alone. Higher concentrations of alpha-actinin and actin filaments form bundles. The threshold for bundling depends on the affinity of the alpha-actinin for actin. The complex of Acanthamoeba alpha-actinin with actin filaments has a Kd of 4.7 microM and a bundling threshold of 0.1 microM; chicken smooth muscle has a Kd of 0.6 microM and a bundling threshold of 1 microM. The physical properties of isotropic networks of cross-linked actin filaments are very different from a gel of bundles: the network behaves like a solid because each actin filament is part of a single structure that encompasses all the filaments. Bundles of filaments behave more like a very viscous fluid because each bundle, while very long and stiff, can slip past other bundles. We have developed a computer model that predicts the bundling threshold based on four variables: the length of the actin filaments, the affinity of the alpha-actinin for actin, and the concentrations of actin and alpha-actinin.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanchard A., Ohanian V., Critchley D. The structure and function of alpha-actinin. J Muscle Res Cell Motil. 1989 Aug;10(4):280–289. doi: 10.1007/BF01758424. [DOI] [PubMed] [Google Scholar]
- Bremer A., Millonig R. C., Sütterlin R., Engel A., Pollard T. D., Aebi U. The structural basis for the intrinsic disorder of the actin filament: the "lateral slipping" model. J Cell Biol. 1991 Nov;115(3):689–703. doi: 10.1083/jcb.115.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. Rapid dissociation and reassociation of actomyosin cross-bridges during force generation: a newly observed facet of cross-bridge action in muscle. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10490–10494. doi: 10.1073/pnas.88.23.10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnick A. R., Warren V., Condeelis J. Identification of a short sequence essential for actin binding by Dictyostelium ABP-120. J Biol Chem. 1990 Jun 5;265(16):9236–9240. [PubMed] [Google Scholar]
- Brown S. S. A Ca2+ insensitive actin-crosslinking protein from Dicytostelium discoideum. Cell Motil. 1985;5(6):529–543. doi: 10.1002/cm.970050608. [DOI] [PubMed] [Google Scholar]
- Burlacu S., Janmey P. A., Borejdo J. Distribution of actin filament lengths measured by fluorescence microscopy. Am J Physiol. 1992 Mar;262(3 Pt 1):C569–C577. doi: 10.1152/ajpcell.1992.262.3.C569. [DOI] [PubMed] [Google Scholar]
- Burridge K., Feramisco J. R. Non-muscle alpha actinins are calcium-sensitive actin-binding proteins. Nature. 1981 Dec 10;294(5841):565–567. doi: 10.1038/294565a0. [DOI] [PubMed] [Google Scholar]
- Cano M. L., Lauffenburger D. A., Zigmond S. H. Kinetic analysis of F-actin depolymerization in polymorphonuclear leukocyte lysates indicates that chemoattractant stimulation increases actin filament number without altering the filament length distribution. J Cell Biol. 1991 Nov;115(3):677–687. doi: 10.1083/jcb.115.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppin C. M., Leavis P. C. Quantitation of liquid-crystalline ordering in F-actin solutions. Biophys J. 1992 Sep;63(3):794–807. doi: 10.1016/S0006-3495(92)81647-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese J. D., Frieden C. Effect of filamin and controlled linear shear on the microheterogeneity of F-actin/gelsolin gels. Cell Motil Cytoskeleton. 1990;17(3):236–249. doi: 10.1002/cm.970170310. [DOI] [PubMed] [Google Scholar]
- Craig S. W., Lancashire C. L., Cooper J. A. Preparation of smooth muscle alpha-actinin. Methods Enzymol. 1982;85(Pt B):316–321. doi: 10.1016/0076-6879(82)85031-3. [DOI] [PubMed] [Google Scholar]
- Detmers P., Weber A., Elzinga M., Stephens R. E. 7-Chloro-4-nitrobenzeno-2-oxa-1,3-diazole actin as a probe for actin polymerization. J Biol Chem. 1981 Jan 10;256(1):99–105. [PubMed] [Google Scholar]
- Fechheimer M., Taylor D. L. Isolation and characterization of a 30,000-dalton calcium-sensitive actin cross-linking protein from Dictyostelium discoideum. J Biol Chem. 1984 Apr 10;259(7):4514–4520. [PubMed] [Google Scholar]
- Gorlin J. B., Yamin R., Egan S., Stewart M., Stossel T. P., Kwiatkowski D. J., Hartwig J. H. Human endothelial actin-binding protein (ABP-280, nonmuscle filamin): a molecular leaf spring. J Cell Biol. 1990 Sep;111(3):1089–1105. doi: 10.1083/jcb.111.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grazi E., Trombetta G., Guidoboni M. Binding of alpha-actinin to F-actin or to tropomyosin F-actin is a function of both alpha-actinin concentration and gel structure. J Muscle Res Cell Motil. 1991 Dec;12(6):579–584. doi: 10.1007/BF01738446. [DOI] [PubMed] [Google Scholar]
- Hou L., Luby-Phelps K., Lanni F. Brownian motion of inert tracer macromolecules in polymerized and spontaneously bundled mixtures of actin and filamin. J Cell Biol. 1990 May;110(5):1645–1654. doi: 10.1083/jcb.110.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockusch B. M., Isenberg G. Interaction of alpha-actinin and vinculin with actin: opposite effects on filament network formation. Proc Natl Acad Sci U S A. 1981 May;78(5):3005–3009. doi: 10.1073/pnas.78.5.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana E., Gratzer W. B. Properties of the spectrin-like structural element of smooth-muscle alpha-actinin. Cell Motil Cytoskeleton. 1991;20(3):242–248. doi: 10.1002/cm.970200307. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanni F., Ware B. R. Detection and characterization of actin monomers, oligomers, and filaments in solution by measurement of fluorescence photobleaching recovery. Biophys J. 1984 Jul;46(1):97–110. doi: 10.1016/S0006-3495(84)84002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciver S. K., Wachsstock D. H., Schwarz W. H., Pollard T. D. The actin filament severing protein actophorin promotes the formation of rigid bundles of actin filaments crosslinked with alpha-actinin. J Cell Biol. 1991 Dec;115(6):1621–1628. doi: 10.1083/jcb.115.6.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Modular organization of actin crosslinking proteins. Trends Biochem Sci. 1991 Mar;16(3):87–92. doi: 10.1016/0968-0004(91)90039-x. [DOI] [PubMed] [Google Scholar]
- Meyer R. K., Aebi U. Bundling of actin filaments by alpha-actinin depends on its molecular length. J Cell Biol. 1990 Jun;110(6):2013–2024. doi: 10.1083/jcb.110.6.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura N., Asano A. Isolation and characterization of a conserved actin-binding domain from rat hepatic actinogelin, rat skeletal muscle, and chicken gizzard alpha-actinins. J Biol Chem. 1986 Aug 15;261(23):10680–10687. [PubMed] [Google Scholar]
- Niederman R., Amrein P. C., Hartwig J. Three-dimensional structure of actin filaments and of an actin gel made with actin-binding protein. J Cell Biol. 1983 May;96(5):1400–1413. doi: 10.1083/jcb.96.5.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal R. On the elasticity of cytoskeletal networks. Biophys J. 1988 Mar;53(3):349–359. doi: 10.1016/S0006-3495(88)83112-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolski J. L., Steck T. L. Length distribution of F-actin in Dictyostelium discoideum. J Biol Chem. 1990 Jan 25;265(3):1312–1318. [PubMed] [Google Scholar]
- Pollard T. D. Assembly and dynamics of the actin filament system in nonmuscle cells. J Cell Biochem. 1986;31(2):87–95. doi: 10.1002/jcb.240310202. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Tseng P. C., Rimm D. L., Bichell D. P., Williams R. C., Jr, Sinard J., Sato M. Characterization of alpha-actinin from Acanthamoeba. Cell Motil Cytoskeleton. 1986;6(6):649–661. doi: 10.1002/cm.970060613. [DOI] [PubMed] [Google Scholar]
- Sato M., Leimbach G., Schwarz W. H., Pollard T. D. Mechanical properties of actin. J Biol Chem. 1985 Jul 15;260(14):8585–8592. [PubMed] [Google Scholar]
- Sato M., Schwarz W. H., Pollard T. D. Dependence of the mechanical properties of actin/alpha-actinin gels on deformation rate. 1987 Feb 26-Mar 4Nature. 325(6107):828–830. doi: 10.1038/325828a0. [DOI] [PubMed] [Google Scholar]
- Simon J. R., Furukawa R. H., Ware B. R., Taylor D. L. The molecular mobility of alpha-actinin and actin in a reconstituted model of gelation. Cell Motil Cytoskeleton. 1988;11(1):64–82. doi: 10.1002/cm.970110107. [DOI] [PubMed] [Google Scholar]
- Small J. V. Organization of actin in the leading edge of cultured cells: influence of osmium tetroxide and dehydration on the ultrastructure of actin meshworks. J Cell Biol. 1981 Dec;91(3 Pt 1):695–705. doi: 10.1083/jcb.91.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Goll D. E., Stromer M. H., Temple J. -actinin from red and white porcine muscle. Biochim Biophys Acta. 1973 Jan 25;295(1):188–207. doi: 10.1016/0005-2795(73)90087-1. [DOI] [PubMed] [Google Scholar]