Abstract
Loss of expression of the Fhit protein is often associated with the development of many human epithelial cancers, including lung and cervical carcinomas. Restoration of Fhit expression in cell lines derived from these tumors has however yielded conflicting results, prompting the need for careful evaluation of the oncosuppressive potential of FHIT. In the present study, we have investigated the effect of Fhit reintroduction in seven lung cancer and three cervical cancer cell lines. To achieve efficient gene transfer and high levels of transgene expression, we have used an adenoviral vector to transduce the FHIT gene. The induction of apoptosis was evaluated by using the terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling assay and propidium iodide staining. Activation of caspases was detected by using Western blot analysis, and tumorigenic potential of transduced cells in the nude mouse was also assessed. Restoration of Fhit expression induced apoptosis in all Fhit-negative cell lines, with Calu-1, H460, and A549 being the most susceptible among the lung cancer cell lines and SiHa cells among cervical carcinomas. Activation of caspase-8 was always associated with Fhit-mediated apoptosis, and in vivo tumorigenicity was either abolished by FHIT gene transfer (in H460 and SK-Mes cells) or strongly suppressed (in A549 and SiHa cells). Our data demonstrate oncosuppressive properties and strong proapoptotic activity of the Fhit protein in lung and cervical cancer cell lines and strengthens the hypothesis of its possible use as a therapeutic tool.
Keywords: tumor suppressor gene‖gene therapy
The analysis of the chromosomal regions involved in a translocation, t(3;8) (p14.2-q24), segregating in a family with susceptibility to clear cell renal cancer, led in 1996 to the identification in 3p14.2 of the fragile histidine triad (FHIT) gene (1). The gene spans almost 2 Mb of genomic DNA, including FRA3B, the most active among the aphidicolin-inducible common fragile sites. Genomic rearrangements, altered mRNA transcripts, and absence or reduction of the Fhit protein have been reported in numerous epithelial cancers, including lung, bladder, breast, and cervical carcinomas (2–6). Fhit alterations are detected very early in lung and esophageal carcinogenesis (2, 7), and it has recently been suggested that they might also have a prognostic value in the prediction of the malignant potential of high-grade squamous intraepithelial lesions of the uterine cervix (6). The detection of FHIT mRNA alterations also in normal tissues (8) and the location of the gene in a region prone to stress-induced damage, however, created concern that some of the genetic changes observed might reflect just an intrinsic instability, exacerbated during the rapid growth of cancer cells, rather than being causally related to the development of neoplasia. Studies designed to ascertain the tumor suppressor activity of FHIT included its transfer into cancer cells by using plasmids (9–12) and retroviral (13) and adenoviral vectors (14–16). The results obtained have been somewhat conflicting, with some reports showing efficient suppression of the tumorigenic phenotype at least in some cell types (9, 11, 12, 14–16) and others failing to detect any difference between Fhit reexpressing cells and the parental cell lines (10, 13). Although some of these results could reflect differences among the cell types tested, it is also possible that they could be due to the efficacy of gene transfer and protein expression. To obtain efficient gene transfer and high levels of transgene expression, we therefore used an adenoviral vector to study the effects of FHIT reintroduction in a panel of cancer cell lines representing the major histological subtypes of lung cancer and in three cell lines established from cervical carcinomas. Our analysis of the effect of FHIT gene transfer on apoptosis, on cell growth kinetics, and on in vivo tumorigenicity demonstrates that Fhit has a broad oncosuppressive activity and strengthens the hypothesis of its possible use in diagnostic and therapeutic applications.
Materials and Methods
Cell Lines.
Lung cancer cell lines H460, A549, Calu-1, Calu-3 and SK-MES and cervical cancer cell lines SiHa, HeLa, and CaSki were purchased from the American Type Culture Collection. Lung cancer cell lines AFL and POVD were originally established at the Istituto Nazionale Tumori. Characteristics of the cell lines used in this study are summarized in Table 1.
Table 1.
Cancer cell lines characteristics
| Cell line | Histology* | Fhit status† | Apoptosis, %‡ |
|---|---|---|---|
| Lung | |||
| H460 | LCC | − | 53 |
| SK-MES | SQC | − | 39 |
| AFL | SCLC | − | 38 |
| POVD | SCLC | +/− | 10 |
| A549 | ADC | − | 46 |
| Calu-1 | SQC | − | 64 |
| Calu-3 | ADC | ++ | 6 |
| Cervical | |||
| SiHa | SQC | − | 24 |
| CaSki | SQC | ++ | 1 |
| Hela | ADC | − | 22 |
LCC, large cell carcinoma; SQC, squamous cell carcinoma; SCLC, small cell lung cancer; ADC, adenocarcinoma.
Endogenous Fhit expression by Western blot analysis: −, negative; +/−, weak positive; ++, strong positive.
Percentage of cells showing apoptosis by TUNEL analysis 5 days after Ad5-Fhit transduction (representative result of experiments performed at least twice, in which standard deviation was less than 15%).
Stable Fhit-transfectants of line H460, clones 2.I and 2.3, have been described (11); briefly, these clones were obtained by transfecting H460 cell line with vector pRc/CMV (Invitrogen) containing FHIT-Flag cDNA and selecting transfected cells with G418 at a concentration of 700 μg/ml. All lines were cultured in RPMI 1640 supplemented with 10% heat-inactivated FCS (Bio Whittaker).
Adenoviral Vectors Preparation.
The preparation of recombinant, E1 and E3 deleted, adenoviral vectors expressing the Fhit protein (Ad5-Fhit) or the control proteins lacZ (Ad5-lacZ) and green fluorescent protein (GFP) (Ad5-GFP) was performed by using standard techniques. For Ad5-Fhit, a 707-bp fragment of FHIT cDNA was amplified by reverse transcription–PCR from human placental cDNA and cloned into an adenoviral shuttle vector (pQBI-AdCMV5) purchased from Quantum Biotechnologies (Montreal). After sequence analysis, the recombinant shuttle vector containing wild-type FHIT was tested in transient transfection assays to confirm promoter activity. Recombinant adenoviruses were obtained in fetal kidney 293 cells (Microbix Biosystems, Toronto) cotransfected with the adenoviral shuttle vector and backbone viral DNA (Quantum Biotechnologies), according to the manufacturer's instructions. After calcium phosphate transfection, 293 cells were seeded in 96-well plates to get well-isolated plaques. After a 2- to 3-week incubation period, the cells were harvested, and viral particles were extracted by four freeze-and-thaw cycles. The viral supernatants were used for sequential infection of 293 cells in soft agar. Ten days postinfection, the resulting plaques were picked under microscope observation, and the presence of Ad5-Fhit recombinant virus in well-isolated plaques was confirmed by PCR analysis and Western blots on infected cells. The absence of replication-competent virus was also confirmed by PCR and plaque assays on nonpermissive cell lines. Recombinant Ad5-Fhit was subsequently expanded by sequential rounds of infection on 293 cells and purified by the CsCl gradient method. Titers of all of the recombinant adenoviral vectors were estimated by using two different methods (plaque assay and tissue culture infectious dose method), always run in duplicate, to assure that equal amounts of Ad5-Fhit and control vectors were used in all experiments.
Terminal Deoxynucleotidyltransferase-Mediated dUTP Nick End Labeling (TUNEL) Assay.
Analysis of apoptosis was performed by using TUNEL. Cells were plated at 2 × 106 cells/100-mm Petri dish, and the following day adenoviral vectors were added at a minimum multiplicity of infection (moi) of 5 in 4 ml of culture medium without serum. After 4 h, new medium was added and the cells were cultured 3–5 days before analysis. Preparation of cells for analysis of apoptosis was performed as follows: 2 × 106 cells per sample were fixed with 2% paraformaldehyde in PBS (10 min on ice), washed three times with TBS (50 mM Tris⋅HCl in saline solution, pH 7.5), permeabilized with ice-cold acetone (1 min on ice), and washed twice in TBS and once in distilled water. Staining was performed by incubating cells for 1 h at 37°C in a humidified atmosphere in the dark in 25 μl (final volume) of TUNEL reaction mixture (In Situ Cell Death Detection Kit, Fluorescein; Roche, Mannheim, Germany). Cells with fragmented DNA appeared positive at the analysis of green fluorescence on the FACSCalibur cytometer (Becton Dickinson). Apoptotic cells were defined on the basis of negative controls represented by cells mock infected, infected with a control adenovirus (lacZ), and by cells treated with TUNEL reaction mixture without the enzyme. Positive controls for enzyme activity were represented by γ-irradiated cells (20,000 rad).
Cell Cycle Analysis.
Distribution of the cells in the cell cycle was determined by propidium iodide staining and FACS analysis. Briefly, 2 × 105 cells were incubated overnight at 4°C in 0.2 ml of hypotonic fluorochrome solution, containing 50 μg/ml propidium iodide (Sigma), 0.1% sodium citrate (Sigma), and 0.1% Triton X-100 (Sigma). Analysis was performed with FACScan cytometer (Becton Dickinson). Cells with subdiploid DNA content were considered apoptotic cells. Cell cycle distributions were analyzed by the cell fit software package.
Cell Lysate Preparation and Western Blot Analysis.
Cell pellets were washed twice with PBS, resuspended in solution A (125 mM Tris⋅HCl, pH6.8/5% SDS), incubated at 95°C for 2 min, and immediately stored on ice. An equal volume of solution B (125 mM Tris⋅HCl, pH6.8/5% SDS/1 mM EDTA/20 μg/ml each of aprotinin, leupeptin, and pepstatin/2 mM PMSF) was then added before sonication and centrifugation at 13,000 × g for 10 min. The protein concentration in the supernatant was quantified by using a bicinchoninic acid-based method (Micro BCA Protein Assay Reagent Kit; Pierce), and 30 μg of each sample was subjected to SDS/PAGE. The gel was blotted, and the membrane was blocked in 5% nonfat dry milk in PBS for 30 min at room temperature before overnight incubation at 4°C with the primary antibody. Antibodies used for the study were as follows: anti-Fhit 71-9000 (Zymed), anti-caspase-8 monoclonal antibody C-15 (gift from P. Krammer, Deutsches Krebsforschungszentrum, Heidelberg, Germany), anti-FADD A66-2 (PharMingen). As a control for uniformity of loading, we used an anti-actin antibody (Sigma). After incubation with the primary antibody, the membranes were washed, incubated for 1 h with peroxidase-conjugated secondary antibody, and then treated with the ECL Western blotting detection system (Amersham Pharmacia), according to the manufacturer's instructions.
CH-11 Treatment.
H460 cells, clones 2.I and 2.3 were incubated in the presence of CH-11 (agonistic anti-human Fas monoclonal antibody, Upstate Biotechnology, Lake Placid, NY) at a concentration of 0.3 μg/ml in culture medium. At different time points, cells were harvested, and lysates were prepared as described for Western blot analysis. Positive control for CH-11 activity was represented by the Jurkat cell line.
In Vivo Studies.
All animal studies were performed according to institutional guidelines. H460, A549, SK-MES, and SiHa cells were transduced in vitro at an moi of 10 with Ad5-Fhit, Ad5-lacZ, or Ad5-GFP. Two or 4 days after transduction, cells were counted, and 1 × 106 (H460, SK-MES), 4 × 106 (A549), and 5 × 106 (SiHa) viable cells were injected into the right flank of 6-week-old female nude mice, four to six mice per group. All experiments were repeated at least twice and gave similar results. Tumor size was measured with a linear caliper three times per week for up to 3 months, and volume was estimated by using the equation V = (a × b2)/2, where a is the larger dimension and b the perpendicular diameter.
Results
Proapoptotic Effect of Adenoviral-Mediated Expression of Fhit.
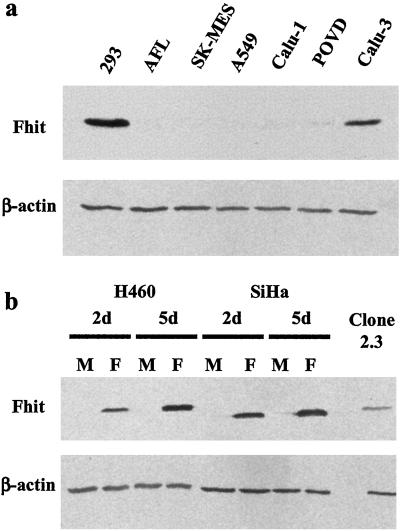
Most of the lines used in this study had low or undetectable levels of endogenous Fhit protein as measured by Western blot analysis (Fig. 1 and Table 1), with the exception of Calu-3 and CaSki cell lines, as reported (13,17). Transduction with the adenoviral vector containing FHIT cDNA (Ad5-Fhit) resulted in efficient expression of the transgene in all of the lines tested within 2 days (Fig. 1), and all of the experiments were performed 3–5 days after transduction. Preliminary experiments showed a higher level of Fhit-induced apoptosis in serum starvation conditions (data not shown), and these conditions were therefore used throughout the study.
Figure 1.
Endogenous Fhit expression in cancer cell lines and effect of adenoviral Fhit transfer. (a) Immunoblot analysis of Fhit expression in lung cancer cell lines. 293, Human embryonic kidney cells used as positive control. (b) Fhit expression in cell lines derived from lung (H460) and cervical (SiHa) carcinomas 2 (2d) and 5 (5d) days after treatment with Ad5-Fhit (lanes F) compared with endogenous Fhit levels in mock infected cells (lanes M). 2.3, Stable FHIT-transfectant of H460 cells used as positive control for high levels of Fhit expression.
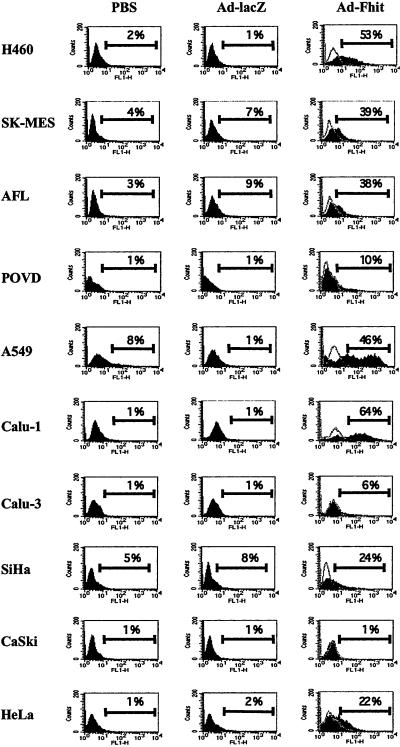
To analyze the proapoptotic effect of Fhit reintroduction, we performed TUNEL analysis of cells transduced with the Fhit vector, with an adenoviral vector containing lacZ as a control insert (Ad5-lacZ) or mock infected. The level of apoptosis, as measured by flow cytometry, was variable and ranged from 10% to 64% depending on the cell line; only Calu-3 and CaSki cells seemed to be resistant. Results are summarized in Table 1, and representative examples of the FACS analysis are shown in Fig. 2. The highest levels of Fhit-induced apoptosis were observed in Calu-1 (64%), H460 (53%), and A549 (46%). Consistently with these observations, Fhit-transduced cells from the susceptible cell lines showed reduced growth rate, with only few cells retaining viability 5 days posttransduction (data not shown). Overall, these results show a broad proapoptotic activity of the Fhit protein in several cancer cell lines.
Figure 2.
Flow cytometry evaluation of TUNEL analysis of Fhit-induced apoptosis. For each cell line, the fluorescence profile of mock infected cells (PBS) or cells treated with control adenovirus (Ad-lacZ) or with Fhit-expressing adenoviral vector (Ad-Fhit) is presented. Percentage of apoptotic cells (cells displaying increased fluorescence compared with controls) is indicated above the marker bar in each panel. In the profile of Ad5-Fhit-treated cells, the negative control represented by cells incubated in the absence of terminal transferase enzyme is also shown (open graph). Results shown are representative examples of experiments repeated at least twice, in which the SD was <15%.
Effects on the Cell Cycle.
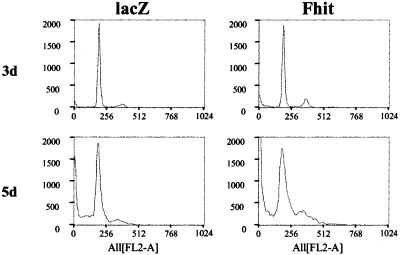
By using propidium iodide staining, distribution of Fhit-transduced cells in the cell cycle was compared with untransduced and lacZ-transduced cells. In the H460 cell line at 3 days postinfection, we observed an increase in the number of cells in the G2 phase or at the S-G2 boundary (13% vs. 6% of lacZ-transduced control, Fig. 3). This effect was visible before any evidence of apoptosis could be detected by TUNEL analysis. Prolonged observation of cells undergoing apoptosis (5 days postinfection) showed accumulation of cells in the G1 and S phases associated with massive cell death (appearance of a sub-G0 population). An early effect of Fhit reexpression could therefore be the induction of a G2 block, with subsequent activation of the apoptotic program.
Figure 3.
Effects of Fhit reexpression on the cell cycle. Distribution of H460 cells in the cell cycle was analyzed by propidium iodide staining. The percentage of cells in G0/G1, S, and G2 was, respectively, 81.6%, 12.4%, and 6% for Ad5-lacZ-infected cells (lacZ) and 80.2%, 6.6%, and 13.2% for Ad5-Fhit-infected cells (Fhit) 3 days postinfection (3d) and 84.4%, 10.1%, and 5.5% (lacZ) and 71.5%, 27.5%, and 1% (Fhit) 5 days postinfection (5d).
Analysis of the Apoptotic Pathway in FHIT-Reexpressing Cells.
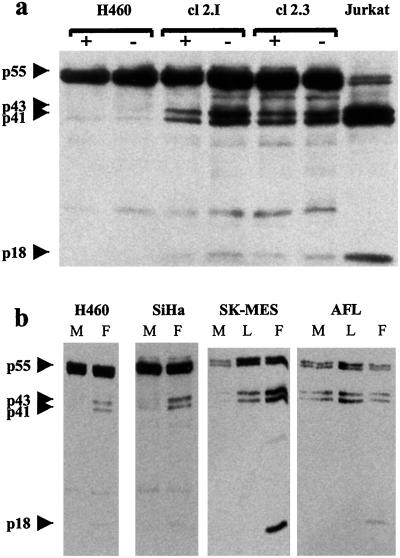
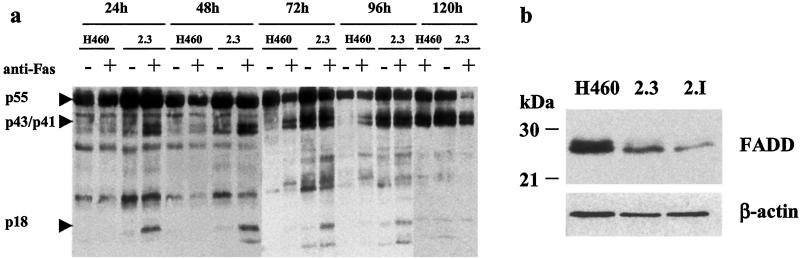
We previously reported that clones of the H460 cell line in which the expression of the Fhit protein was restored by a plasmid construct showed reduced growth in vitro and in vivo, high apoptotic rate, and altered cell cycle kinetics, as compared with the parental cell line (11). To investigate the molecular mechanism of the oncosuppressive activity of FHIT, we studied in two clones (2.3 and 2.I) the expression of molecules involved in the apoptotic cascade. Western blot analysis revealed cleavage of caspase substrates such as poly(ADP-ribose) polymerase (PARP) and β-catenin, especially in serum starvation conditions (data not shown). The activated forms of caspase-8 were readily detectable in the lysates of Fhit-reexpressing cells (Fig. 4). On the contrary, analysis of mitochondrial mediators of apoptosis, such as Bcl-2 and Bcl-XL, had failed to detect any difference between clones and parental cell line, suggesting that the proapoptotic effect of Fhit could be mainly mediated at the cytoplasmic level (11). To test this hypothesis, we treated H460 cells and clones 2.3 and 2.I with a Fas agonistic antibody (CH-11) to evaluate the response to external apoptotic stimuli transduced through the caspase-8 pathway. Although expression of the Fas receptor was comparable in all of the cells, clones 2.3 and 2.I displayed a very high sensitivity to CH-11 treatment, showing cleavage of the 55-kDa pro-caspase-8 into the active p43/p41 and p18 subunits as early as 24 h after CH-11 addition (Fig. 5). Morphological examination of the cultured cells confirmed the presence of cells displaying clear apoptotic features. Complete apoptosis in Fhit-reexpressing clones was achieved in 5 days whereas the control parental cells exhibited only mild toxicity.
Figure 4.
Restored Fhit expression induces caspase-8 activation. (a) H460 cells and its stable Fhit transfectants clone 2.I and 2.3 were kept in normal culture conditions (10% FCS, +) or serum starved (−) and then analyzed by Western blot by using a caspase-8-specific monoclonal antibody. Presence of cleavage forms of pro-caspase (p43/p41 and the active p18 subunit) indicates activation. (b) Indicated cell lines were infected with adenoviral vectors expressing Fhit (F), lacZ (L), or mock-infected (M) and analyzed after 5 days.
Figure 5.
(a) Fhit-expression modulates apoptotic response to Fas treatment. H460 cell line and its stable Fhit-transfectant (clone 2.3) were cultured in the presence (+) or absence (−) of an agonistic anti-Fas antibody (CH-11). At the indicated time points, cells were harvested and processed for immunoblotting with the anti-caspase-8 monoclonal antibody C-15. Cleavage bands (p43/41 and p18), indicating caspase-8 activation, are already detected in Fhit-expressing cells after 24 h, and, after 120 h, the inactive form of caspase-8 is almost entirely converted into the active subunits. Toxic effects of the CH-11 antibody are also visible in H460 cells after prolonged treatment, confirming that the Fas pathway is functional in these cells and suggesting that the higher sensitivity of the clones to Fas-induced apoptosis could be due to a direct effect of Fhit on the apoptotic signal transduction or on the apoptotic threshold. (b) Down-regulation of FADD in stable Fhit-transfectants. Western blot showing reduced expression of FADD in stable Fhit transfectants (clones 2.3 and 2.I) compared with H460 parental cell line. β-actin, same blot hybridized with anti-actin antibody.
To clarify the connection between Fhit and the Fas-activated apoptotic pathway, we investigated the status of the adapter molecule Fas-associated death domain protein (FADD) in clones 2.I and 2.3 because FADD recruitment to the death inducing signaling complex (DISC) is required for transduction of Fas death signal (18,19). Western blot analysis with anti-FADD-specific antibody revealed down-regulation in both 2.I and 2.3 (Fig. 5). One possible explanation for this finding, taking into account the observation that Fhit-reexpressing clones are highly susceptible to apoptosis induction, is the establishment of a compensatory mechanism during stable transfectants isolation that would allow survival of endogenously Fhit-negative cells with high exogenous Fhit expression. In conclusion, the analysis of the apoptotic mechanism in Fhit stable transfectants could then indicate a possible synergy between FADD and Fhit in a caspase-8-mediated apoptotic pathway.
Ad5-Fhit-transduced cells were also analyzed by Western blot to investigate the molecular basis of the apoptotic induction. As expected in this assay, we could detect consistent activation of caspase-8 as indicated by the appearance of the active subunits (Fig. 4).
In Vivo Experiments.
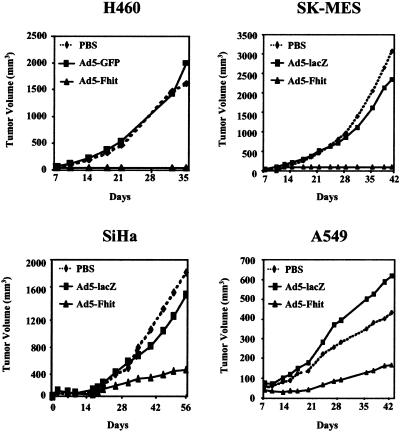
Inoculation in the nude mouse of in vitro transduced cells revealed a strong anti-tumorigenic effect of Fhit expression (Fig. 6). Whereas untransduced cells and cells infected with control adenoviruses always produced tumors of comparable size, Ad5-Fhit-transduced cells either lost their tumorigenic potential (H460, SK-MES) or produced tumors of considerably smaller size when compared with controls (SiHa, A549). In particular in four different experiments, more than 20 animals were injected with Fhit-reexpressing H460 cells, and all of the animals remained tumor free for more than 3 months. In two different experiments, eight animals were injected with Ad5-Fhit-transduced SK-MES cells, and no animal developed detectable tumor in over 4 months of observation.
Figure 6.
Adenoviral-mediated Fhit expression suppresses in vivo tumorigenicity in lung and cervical cancer cell lines. Cell lines were infected in vitro with Ad5-Fhit, control adenoviruses (Ad5-GFP or Ad5-lacZ), or mock infected (PBS) and then injected s.c. into nude mice (four to six animals per group). All experiments were repeated at least twice, and representative results are reported as the mean for each treatment group. Although variability in tumor sizes was observed in some treatment groups, Fhit expression completely suppressed tumorigenicity of H460 and SK-MES cell lines, and the differences between the tumor volumes in the groups injected with control vector-treated cells and mock-infected cells were not significant (Student's t test, two-tail; P = 0.39 for H460, P = 0.27 for SK-MES, P = 0.37 for SiHa, and P = 0.34 for A549 cells). A significant reduction (P < 0.05) of tumor size was observed in Ad5-Fhit-treated SiHa and A549 cells compared with untreated (P = 0.008 for SiHa and P = 0.025 for A549) or Ad5-lacZ-treated cells (P = 0.001 for SiHa and P = 0.022 for A549 cells).
Discussion
Although chromosomal deletions on the short arm of chromosome 3 are frequent in different types of human epithelial cancer (20) and the loss of expression of the Fhit protein seems to be one of the most common findings in lung cancer (2), only a few functional studies have addressed the issue of the effect of restored FHIT expression in cancer cells lacking the endogenous protein. Studies performed by using plasmids as gene transfer tools have resulted in different outcomes depending on the cell line transfected and the number of clones analyzed: suppression of growth potential, induction of apoptosis, effects on the cell cycle, and inhibition of tumorigenicity in nude mice have all been reported in Fhit-reexpressing cells from lung (11), renal (12), and stomach cancer (9), but other studies have reported lack of effect on cervical cancer cell lines (10). The use of a retroviral vector as a more efficient gene delivery system has also resulted in the questioning of the relevance of the FHIT gene as a tumor suppressor in lung cancer because of the observation that restored expression in the H460 cell line didn't correlate with a change in the tumorigenic phenotype (13). The use of adenoviral vectors on lung and head and neck cancer cell lines (14) and, more recently, also on esophageal (15) and pancreatic cancer cell lines (16) showed an antitumorigenic effect of the Fhit protein.
To clarify the potential of Fhit in gene therapy strategies, we analyzed the effect of adenoviral-mediated reintroduction of the FHIT gene expression in a panel of lung and cervical cancer cell lines. Our results clearly show that, when the expression of Fhit is fully restored, there is a sharp increase in the sensitivity of cancer cells to apoptotic stimuli, an alteration on the cycling properties, and a reduction of the tumorigenic potential in vivo. Although different cell lines displayed different sensitivity to Fhit-induced apoptosis, it is interesting to note that cell lines from all major different histological subtypes of lung cancer could be driven into apoptosis and lost their in vivo tumorigenicity. Some of the effects observed after FHIT gene transfer could be increased by putting the cells in stressful conditions, i.e., serum deprivation or external apoptotic stimuli, suggesting that Fhit might act as a mediator of the apoptotic response. Consistent with this hypothesis is the observation that clones from the H460 lung cancer cell line stably overexpressing Fhit are highly susceptible to Fas-mediated apoptosis compared with the parental cell line.
In our study, Fhit-induced apoptosis was always associated with activation of the caspase-8 pathway: caspase-8 cleavage and activation of the effector caspase-3, detected as cleavage of the poly(ADP-ribose) polymerase substrate, were detectable a few days after restoration of Fhit expression. The possibility that Fhit could increase the efficiency of the cytoplasmic caspase-8-dependent pathway of apoptosis seems to be substantiated also by two other observations: the decreased level of FADD observed in stable clones expressing high levels of Fhit, suggestive of a possible “adaptive” response to survive in the presence of a proapoptotic stimulus, and their very rapid apoptotic response to Fas treatment.
Adenoviral-mediated FHIT gene transfer also had a major effect in decreasing the in vivo tumorigenic potential of different cell lines. The tumorigenicity of the lung cancer cell lines H460 (large cell carcinoma) and SK-MES (squamous cell carcinoma) was completely suppressed by Ad5-Fhit treatment, and a significant reduction in tumor size was observed in animals injected with A549 (lung adenocarcinoma) and SiHa (cervical cancer) cells. A previous study also found suppression of in vivo tumorigenicity of H1299 and A549 lung carcinoma cell lines transduced with an adenovirus expressing Fhit (14), whereas others have reported lack of effect of Fhit reintroduction in H460 cell line by using a retroviral vector (13). In our experiments, H460 cells were consistently suppressed after Fhit transduction, and no evidence of tumor growth was observed in more than 20 mice treated in four separate experiments. The major experimental difference in our study concerns the quantity of cells injected: Wu et al. (13) used 5 × 106 cells, whereas we used only 1 × 106. In our hands, this quantity is sufficient to yield rapid tumor growth in all of the control animal treated, and the use of a larger number of cells doesn't result in a proportionally bigger tumor (our unpublished observation). By using more cells than the quantity needed to establish the tumor, it is therefore possible that anti-tumor activity could be masked or underestimated by rapid outgrowth of unsuppressed cells (either untransduced or expressing a low level of the protein and therefore resistant to the proapoptotic effect of Fhit).
Even though multiple studies have reported lack of protein expression in a large percentage of cervical cancers, functional studies have failed to show oncosuppressive properties of the protein when reintroduced in HeLa and CH-3 cells (13). We found evidence of proapoptotic effect of Fhit in HeLa and SiHa cells, and the in vivo growth of Ad-Fhit-transduced SiHa cells was potently suppressed, confirming an important role of FHIT in cervical carcinogenesis.
One point that remains to be elucidated is the precise mechanism of action of Fhit, in the interest of identifying interacting proteins whose status could influence the efficacy of FHIT gene transfer. Our analysis of the apoptotic pathway induced by Fhit reexpression seems to indicate a role in the cytoplasmic pathway, but further studies are needed to confirm these data and to extend the observation in other cell types.
The potential of Fhit as a gene therapy tool was recently substantiated by experiments conducted in Fhit-deficient mice (21), where the high susceptibility to carcinogen-induced tumors could be reverted by treatment with Fhit-expressing adenoviral or adeno-associated vectors (22). It has also to be noted that cells expressing conspicuous levels of endogenous Fhit protein seem to be insensitive to exogenous Fhit overexpression (9, 14), suggesting that transgene-associated toxicity should be low in gene therapy applications.
In conclusion, our data present evidence of efficient induction of apoptosis and suppression of in vivo tumorigenicity of lung and cervical cancer cell lines by an adenoviral vector transducing the FHIT gene and suggest the possibility of its use in clinical applications against these malignancies.
Acknowledgments
We thank Drs. A. Gelman, G. Finocchiaro, P. Accornero, and M. P. Colombo for help with preliminary experiments and Prof. P. Krammer for providing the anti caspase-8 antibody. This work was supported by grants to G.S. from Associazione and Fondazione Italiana per la Ricerca sul Cancro (AIRC/FIRC) and from Ministero Italiano della Sanita'. L.R. is recipient of a fellowship from AIRC. This study was also partially supported by Program Project Grant CA77738 and Cancer Center Core Grant CA56036 from the U.S. Public Health Service.
Abbreviations
- FHIT
fragile histidine triad
- TUNEL
terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling
- FADD
Fas-associated death domain protein
References
- 1.Ohta M, Inoue H, Cotticelli M G, Kastury K, Baffa R, Palazzo J, Siprashvili Z, Mori M, McCue P, Druck T, et al. Cell. 1996;84:587–597. doi: 10.1016/s0092-8674(00)81034-x. [DOI] [PubMed] [Google Scholar]
- 2.Sozzi G, Veronese M L, Negrini M, Baffa R, Cotticelli M G, Inoue H, Tornielli S, Pilotti S, DeGregario L, Pastorino V, Pierotti M A, et al. Cell. 1996;85:17–26. doi: 10.1016/s0092-8674(00)81078-8. [DOI] [PubMed] [Google Scholar]
- 3.Sozzi G, Pastorino U, Moiraghi L, Tagliabue E, Pezzella F, Ghirelli C, Tornielli S, Sard L, Huebner K, Pierotti M A, et al. Cancer Res. 1998;58:5032–5037. [PubMed] [Google Scholar]
- 4.Baffa R, Gomella L G, Vecchione A, Bassi P, Mimori K, Sedor J, Calviello C M, Gardiman M, Minimo C, Strup S E, et al. Am J Pathol. 2000;156:419–424. doi: 10.1016/S0002-9440(10)64745-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campiglio M, Pekarsky Y, Menard S, Tagliabue E, Pilotti S, Croce C M. Cancer Res. 1999;59:3866–3869. [PubMed] [Google Scholar]
- 6.Connolly D C, Greenspan D L, Wu R, Ren X, Dunn R L, Shah K V, Jones R W, Bosch F X, Munoz N, Cho K R. Clin Cancer Res. 2000;6:3505–3510. [PubMed] [Google Scholar]
- 7.Mori M, Mimori K, Shiraishi T, Alder H, Inoue H, Tanaka Y, Sugimachi K, Huebner K, Croce C M. Cancer Res. 2000;60:1177–1182. [PubMed] [Google Scholar]
- 8.Matthews C P, Shera K, Kiviat N, McDougall J K. Oncogene. 2001;20:4665–4675. doi: 10.1038/sj.onc.1204622. [DOI] [PubMed] [Google Scholar]
- 9.Siprashvili Z, Sozzi G, Barnes L D, McCue P, Robinson A K, Eryomin V, Sard L, Tagliabue E, Greco A, Fusetti L, et al. Proc Natl Acad Sci USA. 1997;94:13771–13776. doi: 10.1073/pnas.94.25.13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otterson G A, Xiao G H, Geradts J, Jin F, Chen W D, Niklinska W, Kaye F J, Yeung R S. J Natl Cancer Inst. 1998;90:426–432. doi: 10.1093/jnci/90.6.426. [DOI] [PubMed] [Google Scholar]
- 11.Sard L, Accornero P, Tornielli S, Delia D, Bunone G, Campiglio M, Colombo M P, Gramegna M, Croce C M, Pierotti M A, et al. Proc Natl Acad Sci USA. 1999;96:8489–8492. doi: 10.1073/pnas.96.15.8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Werner N S, Siprashvili Z, Fong L Y, Marquitan G, Schroder J K, Bardenheuer W, Seeber S, Huebner K, Schutte J, Opalka B. Cancer Res. 2000;60:2780–2785. [PubMed] [Google Scholar]
- 13.Wu R, Connolly D C, Dunn R L, Cho K R. J Natl Cancer Inst. 2000;92:338–344. doi: 10.1093/jnci/92.4.338. [DOI] [PubMed] [Google Scholar]
- 14.Ji L, Fang B, Yen N, Fong K, Minna J D, Roth J A. Cancer Res. 1999;59:3333–3339. [PubMed] [Google Scholar]
- 15.Ishii H, Dumon K R, Vecchione A, Trapasso F, Mimori K, Alder H, Mori M, Sozzi G, Baffa R, Huebner K, et al. Cancer Res. 2001;61:1578–1584. [PubMed] [Google Scholar]
- 16.Dumon K R, Ishii H, Vecchione A, Trapasso F, Baldassarre G, Chakrani F, Druck T, Rosato E F, Williams N N, Baffa R, et al. Cancer Res. 2001;61:4827–4836. [PubMed] [Google Scholar]
- 17.Sozzi G, Tornielli S, Tagliabue E, Sard L, Pezzella F, Pastorino U, Minoletti F, Pilotti S, Ratcliffe C, Veronese M L, et al. Cancer Res. 1997;57:5207–5212. [PubMed] [Google Scholar]
- 18.Kischkel F C, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer P H, Peter M E. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chinnaiyan A M, Tepper C G, Seldin M F, O'Rourke K, Kischkel F C, Hellbardt S, Krammer P H, Peter M E, Dixit V M. J Biol Chem. 1996;271:4961–4965. doi: 10.1074/jbc.271.9.4961. [DOI] [PubMed] [Google Scholar]
- 20.Braga E, Pugacheva E, Bazov I, Ermilova V, Kazubskaya T, Mazurenko N, Kisseljov F, Liu J, Garkavtseva R, Zabarovsky E, et al. FEBS Lett. 1999;454:215–219. doi: 10.1016/s0014-5793(99)00807-8. [DOI] [PubMed] [Google Scholar]
- 21.Fong L Y, Fidanza V, Zanesi N, Lock L F, Siracusa L D, Mancini R, Siprashvili Z, Ottey M, Martin S E, Druck T, et al. Proc Natl Acad Sci USA. 2000;97:4742–4747. doi: 10.1073/pnas.080063497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dumon K R, Ishii H, Fong L Y, Zanesi N, Fidanza V, Mancini R, Vecchione A, Baffa R, Trapasso F, During M J, et al. Proc Natl Acad Sci USA. 2001;98:3346–3351. doi: 10.1073/pnas.061020098. [DOI] [PMC free article] [PubMed] [Google Scholar]