Abstract
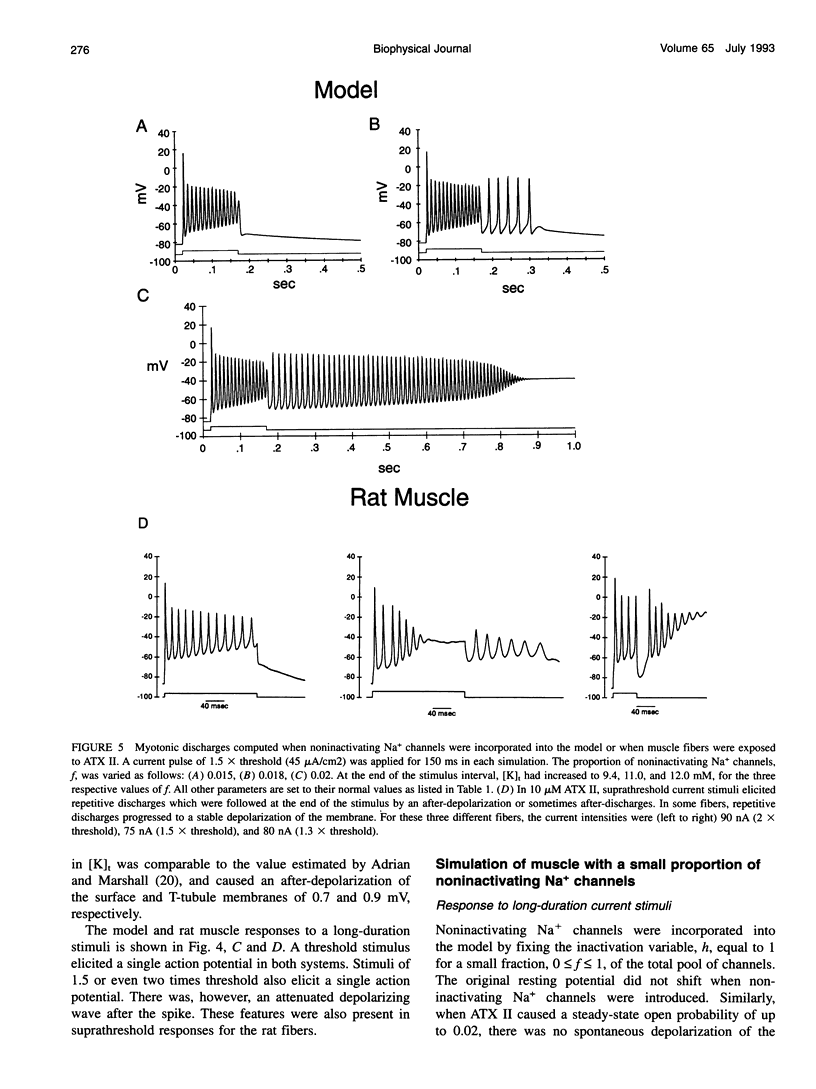
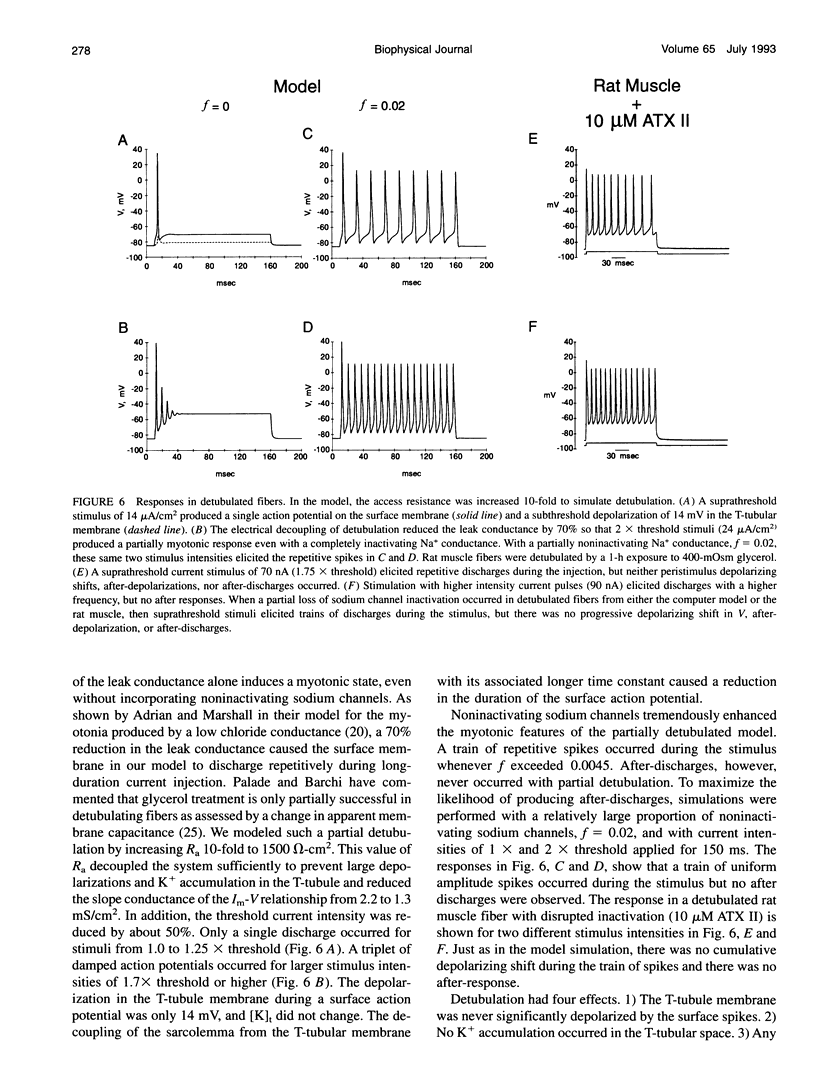
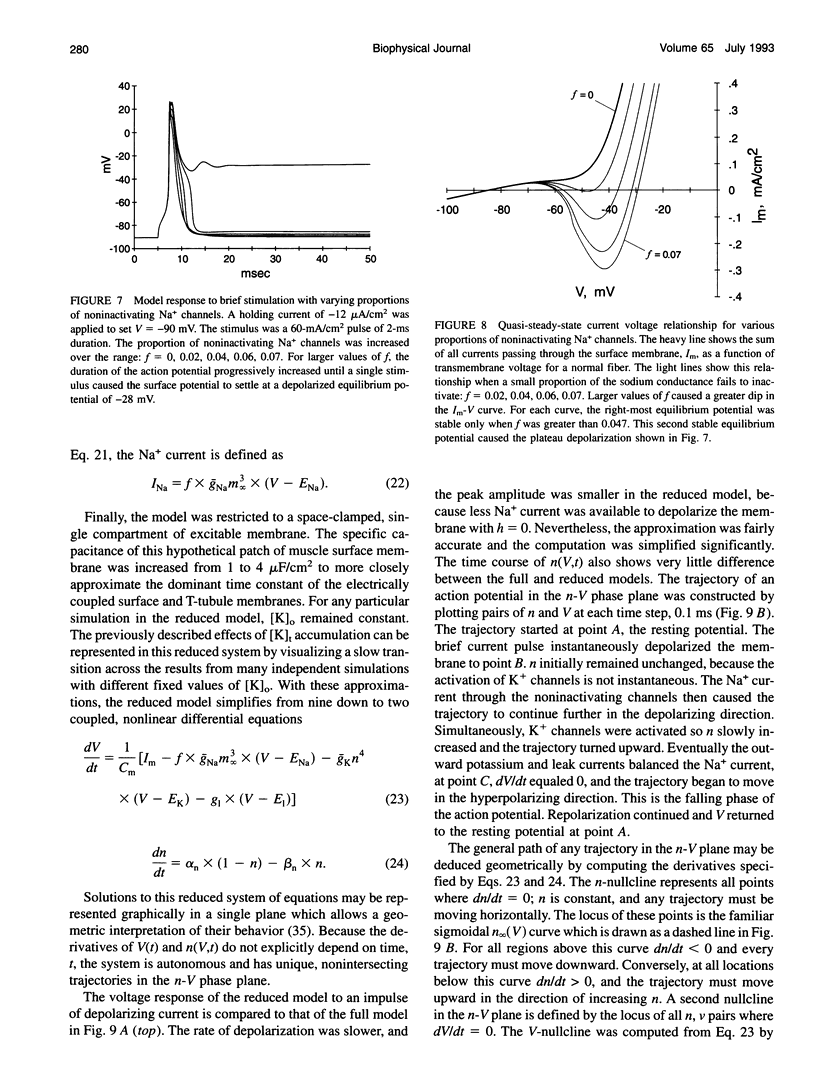
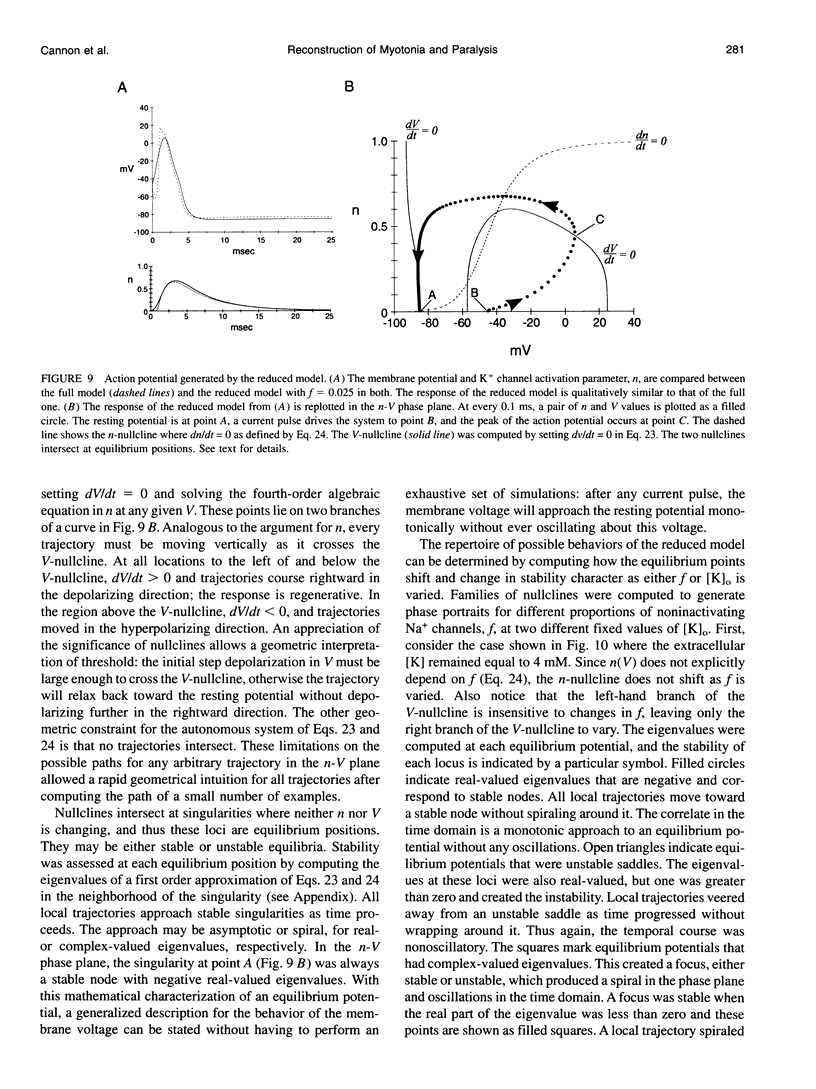
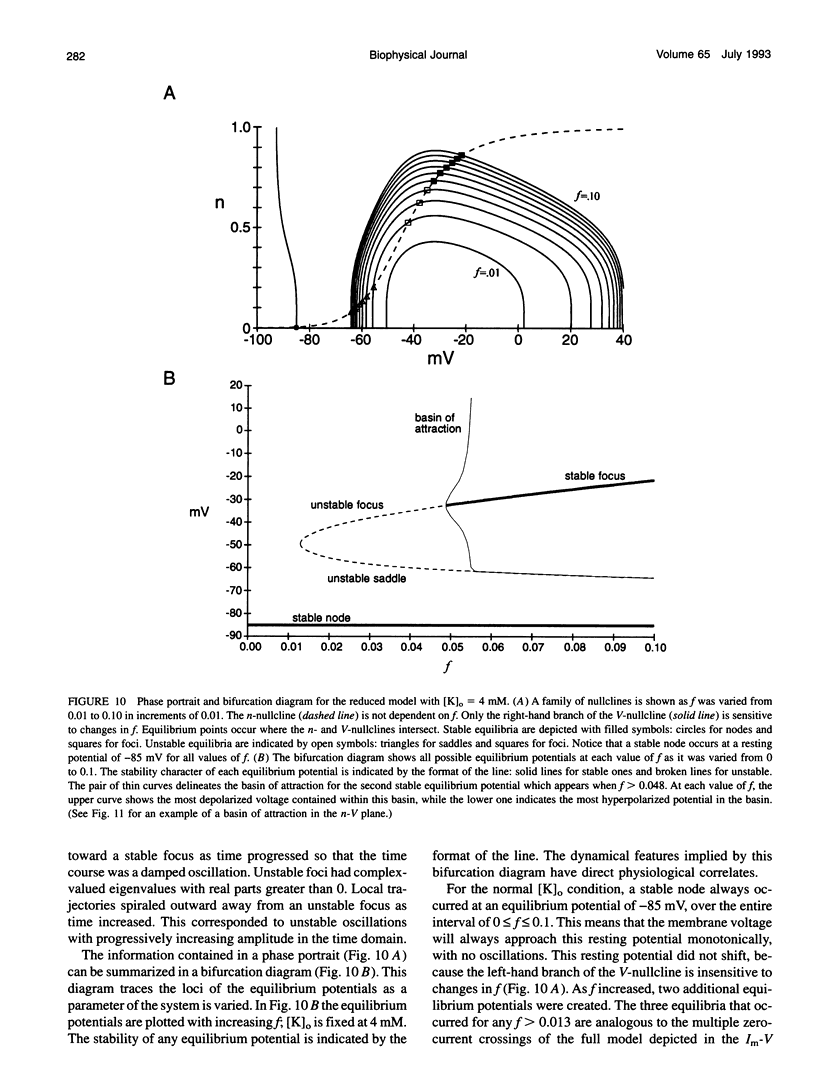
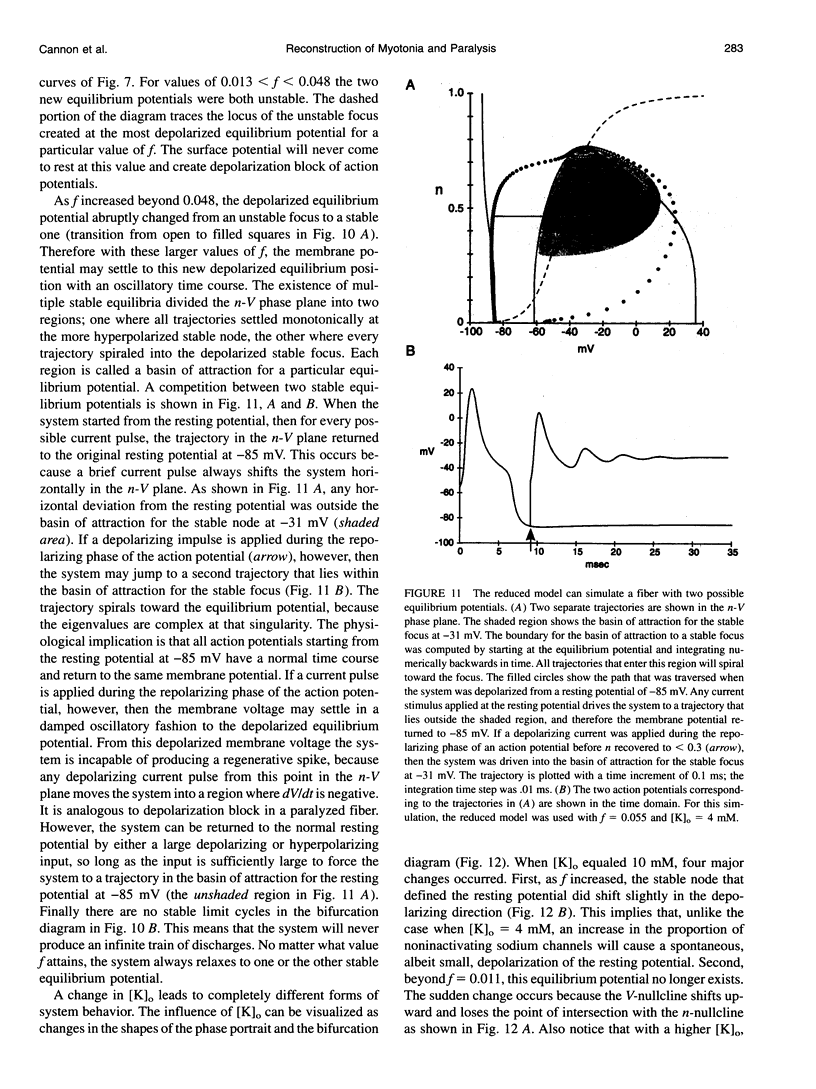
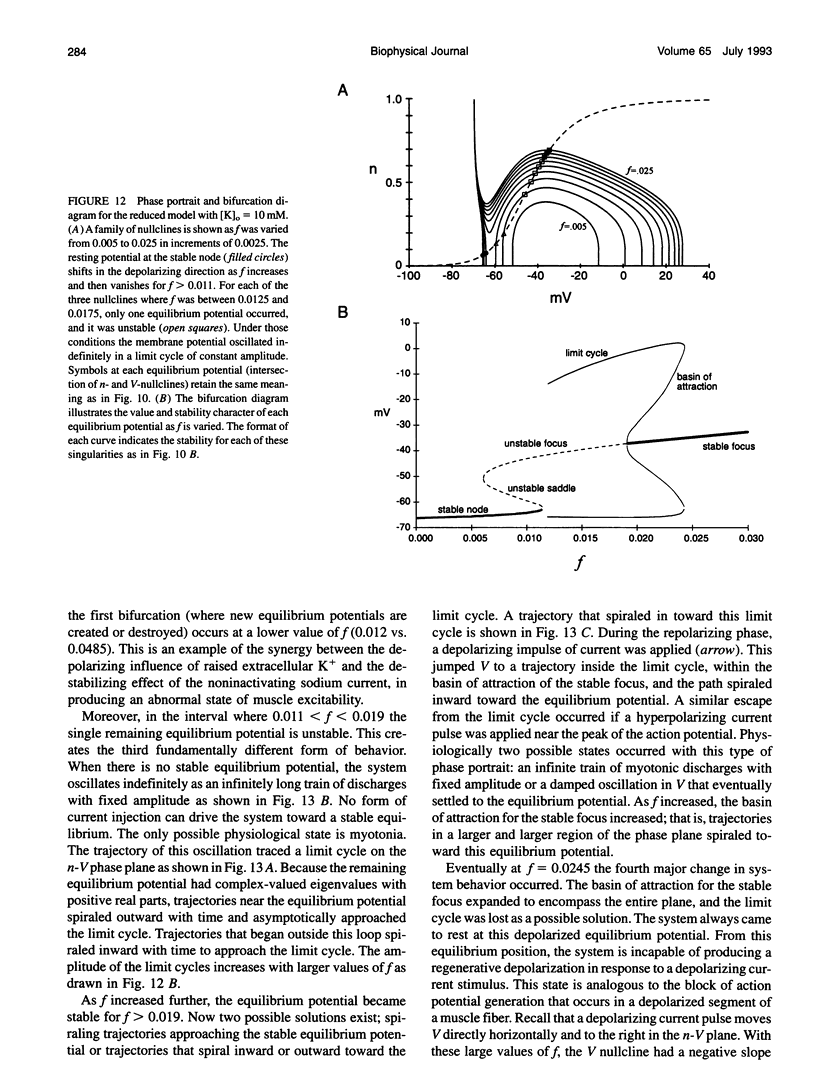
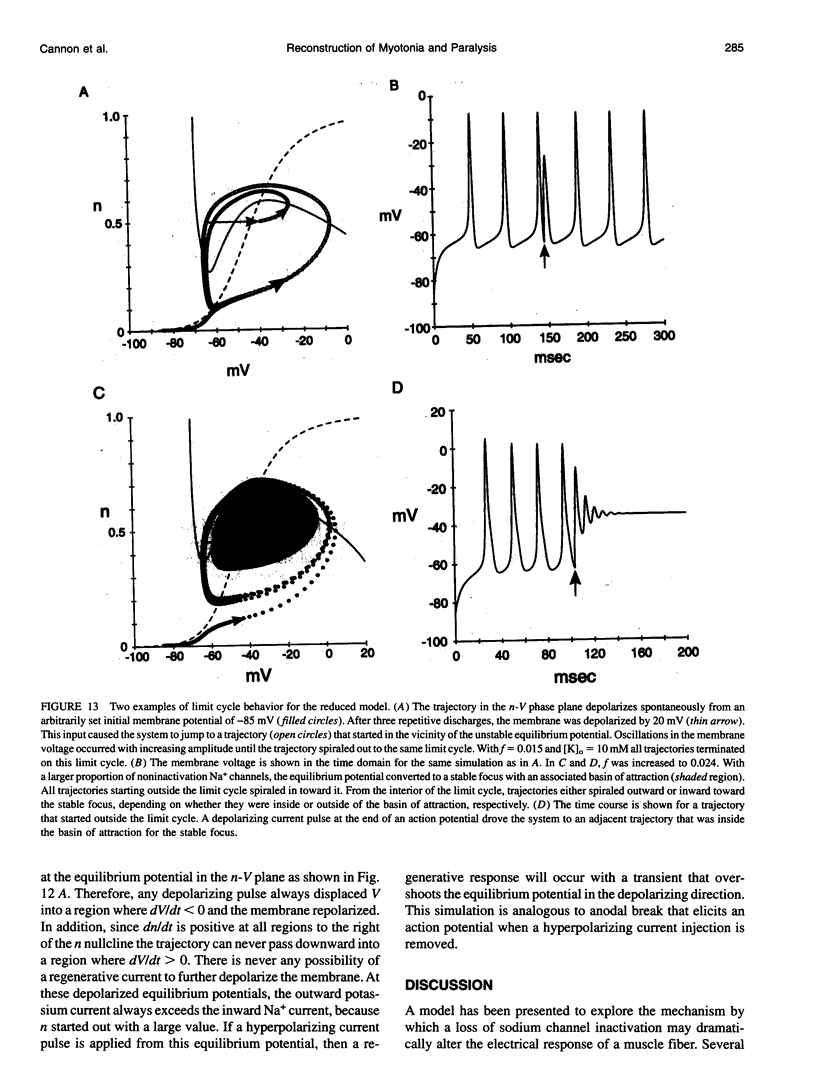
Muscle fibers from individuals with hyperkalemic periodic paralysis generate repetitive trains of action potentials (myotonia) or large depolarizations and block of spike production (paralysis) when the extracellular K+ is elevated. These pathologic features are thought to arise from mutations of the sodium channel alpha subunit which cause a partial loss of inactivation (steady-state Popen approximately 0.02, compared to < 0.001 in normal channels). We present a model that provides a possible mechanism for how this small persistent sodium current leads to repetitive firing, why the integrity of the T-tubule system is required to produce myotonia, and why paralysis will occur when a slightly larger proportion of channels fails to inactivate. The model consists of a two-compartment system to simulate the surface and T-tubule membranes. When the steady-state sodium channel open probability exceeds 0.0075, trains of repetitive discharges occur in response to constant current injection. At the end of the current injection, the membrane potential may either return to the normal resting value, continue to discharge repetitive spikes, or settle to a new depolarized equilibrium potential. This after-response depends on both the proportion of noninactivating sodium channels and the magnitude of the activity-driven K+ accumulation in the T-tubular space. A reduced form of model is presented in which a two-dimensional phase-plane analysis shows graphically how this diversity of after-responses arises as extracellular [K+] and the proportion of noninactivating sodium channels are varied.
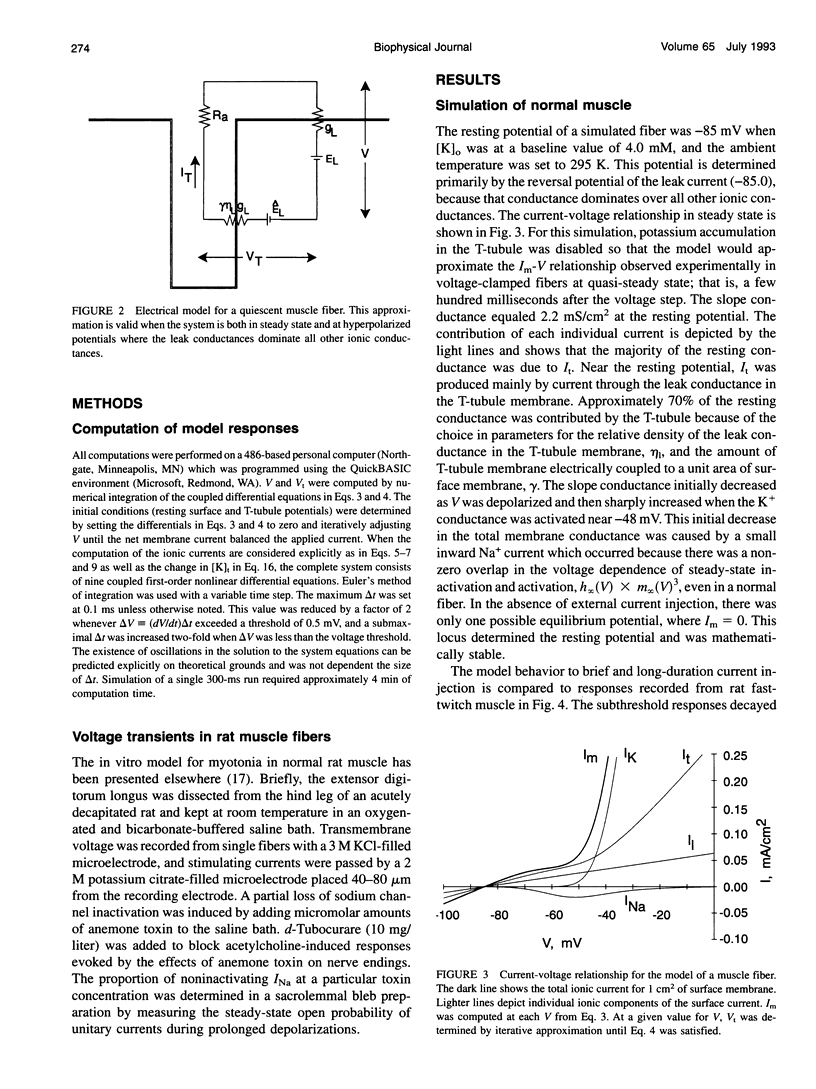
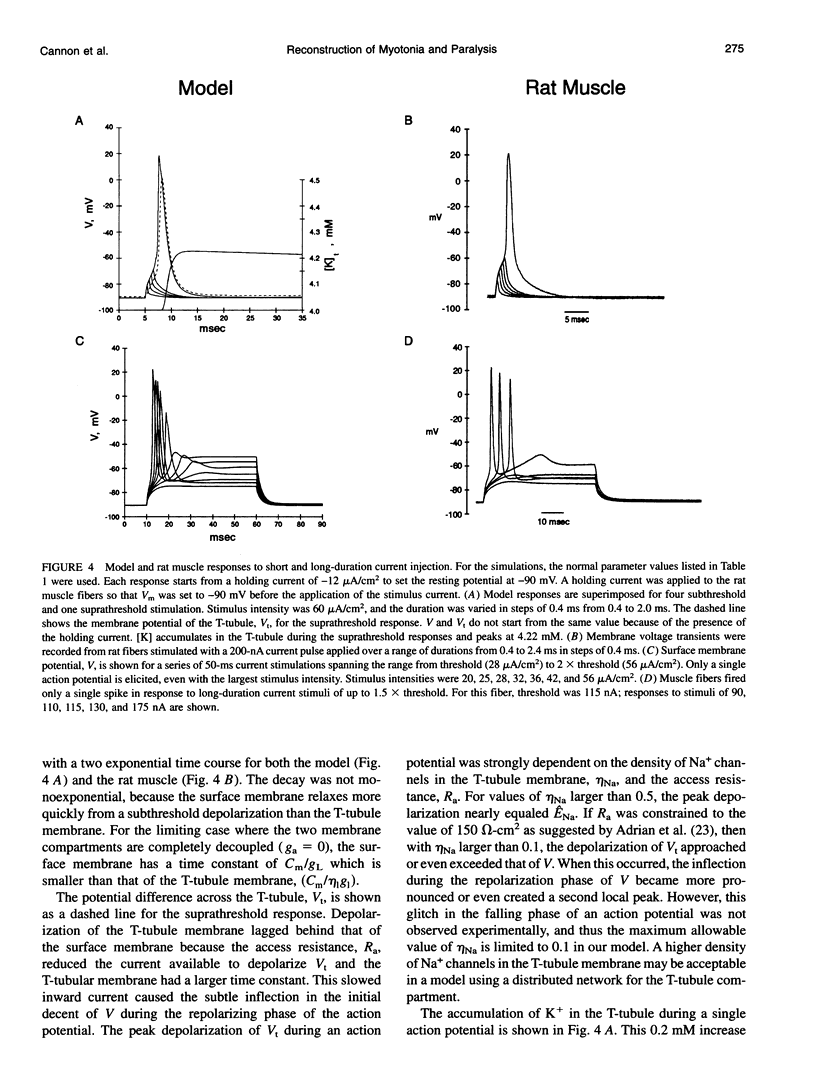
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Bryant S. H. On the repetitive discharge in myotonic muscle fibres. J Physiol. 1974 Jul;240(2):505–515. doi: 10.1113/jphysiol.1974.sp010620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Costantin L. L., Peachey L. D. Radial spread of contraction in frog muscle fibres. J Physiol. 1969 Sep;204(1):231–257. doi: 10.1113/jphysiol.1969.sp008910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Marshall M. W. Action potentials reconstructed in normal and myotonic muscle fibres. J Physiol. 1976 Jun;258(1):125–143. doi: 10.1113/jphysiol.1976.sp011410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Marshall M. W. Sodium currents in mammalian muscle. J Physiol. 1977 Jun;268(1):223–250. doi: 10.1113/jphysiol.1977.sp011855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Peachey L. D. Reconstruction of the action potential of frog sartorius muscle. J Physiol. 1973 Nov;235(1):103–131. doi: 10.1113/jphysiol.1973.sp010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W. Potassium conductance changes in skeletal muscle and the potassium concentration in the transverse tubules. J Physiol. 1972 Aug;225(1):33–56. doi: 10.1113/jphysiol.1972.sp009928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., Roberts W. M., Ruff R. L. Voltage clamp of rat and human skeletal muscle: measurements with an improved loose-patch technique. J Physiol. 1984 Feb;347:751–768. doi: 10.1113/jphysiol.1984.sp015094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam K. G., Donaldson P. L. A quantitative study of potassium channel kinetics in rat skeletal muscle from 1 to 37 degrees C. J Gen Physiol. 1983 Apr;81(4):485–512. doi: 10.1085/jgp.81.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillé J., Ildefonse M., Rougier O. Existence of a sodium current in the tubular membrane of frog twitch muscle fibre; its possible role in the activation of contraction. Pflugers Arch. 1978 May 18;374(2):167–177. doi: 10.1007/BF00581298. [DOI] [PubMed] [Google Scholar]
- Caldwell J. H., Schaller K. L. Opening the gates on ion channel diseases. Nat Genet. 1992 Oct;2(2):87–89. doi: 10.1038/ng1092-87. [DOI] [PubMed] [Google Scholar]
- Cannon S. C., Brown R. H., Jr, Corey D. P. A sodium channel defect in hyperkalemic periodic paralysis: potassium-induced failure of inactivation. Neuron. 1991 Apr;6(4):619–626. doi: 10.1016/0896-6273(91)90064-7. [DOI] [PubMed] [Google Scholar]
- Cannon S. C., Corey D. P. Loss of Na+ channel inactivation by anemone toxin (ATX II) mimics the myotonic state in hyperkalaemic periodic paralysis. J Physiol. 1993 Jul;466:501–520. [PMC free article] [PubMed] [Google Scholar]
- Cannon S. C., Strittmatter S. M. Functional expression of sodium channel mutations identified in families with periodic paralysis. Neuron. 1993 Feb;10(2):317–326. doi: 10.1016/0896-6273(93)90321-h. [DOI] [PubMed] [Google Scholar]
- Ebers G. C., George A. L., Barchi R. L., Ting-Passador S. S., Kallen R. G., Lathrop G. M., Beckmann J. S., Hahn A. F., Brown W. F., Campbell R. D. Paramyotonia congenita and hyperkalemic periodic paralysis are linked to the adult muscle sodium channel gene. Ann Neurol. 1991 Dec;30(6):810–816. doi: 10.1002/ana.410300610. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. S., Gage P. W. Ionic conductances of the surface and transverse tubular membranes of frog sartorius fibers. J Gen Physiol. 1969 Mar;53(3):279–297. doi: 10.1085/jgp.53.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREYGANG W. H., Jr, GOLDSTEIN D. A., HELLAM D. C. THE AFTER-POTENTIAL THAT FOLLOWS TRAINS OF IMPULSES IN FROG MUSCLE FIBERS. J Gen Physiol. 1964 May;47:929–952. doi: 10.1085/jgp.47.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine B., Khurana T. S., Hoffman E. P., Bruns G. A., Haines J. L., Trofatter J. A., Hanson M. P., Rich J., McFarlane H., Yasek D. M. Hyperkalemic periodic paralysis and the adult muscle sodium channel alpha-subunit gene. Science. 1990 Nov 16;250(4983):1000–1002. doi: 10.1126/science.2173143. [DOI] [PubMed] [Google Scholar]
- Franke C., Hatt H., Iaizzo P. A., Lehmann-Horn F. Characteristics of Na+ channels and Cl- conductance in resealed muscle fibre segments from patients with myotonic dystrophy. J Physiol. 1990 Jun;425:391–405. doi: 10.1113/jphysiol.1990.sp018110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke C., Iaizzo P. A., Hatt H., Spittelmeister W., Ricker K., Lehmann-Horn F. Altered Na+ channel activity and reduced Cl- conductance cause hyperexcitability in recessive generalized myotonia (Becker). Muscle Nerve. 1991 Aug;14(8):762–770. doi: 10.1002/mus.880140811. [DOI] [PubMed] [Google Scholar]
- Jaimovich E., Venosa R. A., Shrager P., Horowicz P. Density and distribution of tetrodotoxin receptors in normal and detubulated frog sartorius muscle. J Gen Physiol. 1976 Apr;67(4):399–416. doi: 10.1085/jgp.67.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann-Horn F., Iaizzo P. A., Franke C., Hatt H., Spaans F. Schwartz-Jampel syndrome: II. Na+ channel defect causes myotonia. Muscle Nerve. 1990 Jun;13(6):528–535. doi: 10.1002/mus.880130609. [DOI] [PubMed] [Google Scholar]
- Lehmann-Horn F., Küther G., Ricker K., Grafe P., Ballanyi K., Rüdel R. Adynamia episodica hereditaria with myotonia: a non-inactivating sodium current and the effect of extracellular pH. Muscle Nerve. 1987 May;10(4):363–374. doi: 10.1002/mus.880100414. [DOI] [PubMed] [Google Scholar]
- Lehmann-Horn F., Rüdel R., Dengler R., Lorković H., Haass A., Ricker K. Membrane defects in paramyotonia congenita with and without myotonia in a warm environment. Muscle Nerve. 1981 Sep-Oct;4(5):396–406. doi: 10.1002/mus.880040508. [DOI] [PubMed] [Google Scholar]
- Lehmann-Horn F., Rüdel R., Ricker K., Lorković H., Dengler R., Hopf H. C. Two cases of adynamia episodica hereditaria: in vitro investigation of muscle cell membrane and contraction parameters. Muscle Nerve. 1983 Feb;6(2):113–121. doi: 10.1002/mus.880060206. [DOI] [PubMed] [Google Scholar]
- McClatchey A. I., Van den Bergh P., Pericak-Vance M. A., Raskind W., Verellen C., McKenna-Yasek D., Rao K., Haines J. L., Bird T., Brown R. H., Jr Temperature-sensitive mutations in the III-IV cytoplasmic loop region of the skeletal muscle sodium channel gene in paramyotonia congenita. Cell. 1992 Feb 21;68(4):769–774. doi: 10.1016/0092-8674(92)90151-2. [DOI] [PubMed] [Google Scholar]
- Palade P. T., Barchi R. L. Characteristics of the chloride conductance in muscle fibers of the rat diaphragm. J Gen Physiol. 1977 Mar;69(3):325–342. doi: 10.1085/jgp.69.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappone P. A. Voltage-clamp experiments in normal and denervated mammalian skeletal muscle fibres. J Physiol. 1980 Sep;306:377–410. doi: 10.1113/jphysiol.1980.sp013403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peachey L. D. The sarcoplasmic reticulum and transverse tubules of the frog's sartorius. J Cell Biol. 1965 Jun;25(3 Suppl):209–231. doi: 10.1083/jcb.25.3.209. [DOI] [PubMed] [Google Scholar]
- Ptacek L. J., Trimmer J. S., Agnew W. S., Roberts J. W., Petajan J. H., Leppert M. Paramyotonia congenita and hyperkalemic periodic paralysis map to the same sodium-channel gene locus. Am J Hum Genet. 1991 Oct;49(4):851–854. [PMC free article] [PubMed] [Google Scholar]
- Ptácek L. J., George A. L., Jr, Griggs R. C., Tawil R., Kallen R. G., Barchi R. L., Robertson M., Leppert M. F. Identification of a mutation in the gene causing hyperkalemic periodic paralysis. Cell. 1991 Nov 29;67(5):1021–1027. doi: 10.1016/0092-8674(91)90374-8. [DOI] [PubMed] [Google Scholar]
- Ricker K., Camacho L. M., Grafe P., Lehmann-Horn F., Rüdel R. Adynamia episodica hereditaria: what causes the weakness? Muscle Nerve. 1989 Nov;12(11):883–891. doi: 10.1002/mus.880121103. [DOI] [PubMed] [Google Scholar]
- Rojas C. V., Wang J. Z., Schwartz L. S., Hoffman E. P., Powell B. R., Brown R. H., Jr A Met-to-Val mutation in the skeletal muscle Na+ channel alpha-subunit in hyperkalaemic periodic paralysis. Nature. 1991 Dec 5;354(6352):387–389. doi: 10.1038/354387a0. [DOI] [PubMed] [Google Scholar]
- Rüdel R., Lehmann-Horn F. Membrane changes in cells from myotonia patients. Physiol Rev. 1985 Apr;65(2):310–356. doi: 10.1152/physrev.1985.65.2.310. [DOI] [PubMed] [Google Scholar]
- Steinberg I. Z. Computer simulations of the effect of non-inactivating sodium channels on the electric behavior of excitable cells. J Theor Biol. 1988 Jul 21;133(2):193–214. doi: 10.1016/s0022-5193(88)80005-5. [DOI] [PubMed] [Google Scholar]