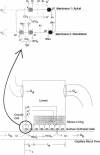
Abstract
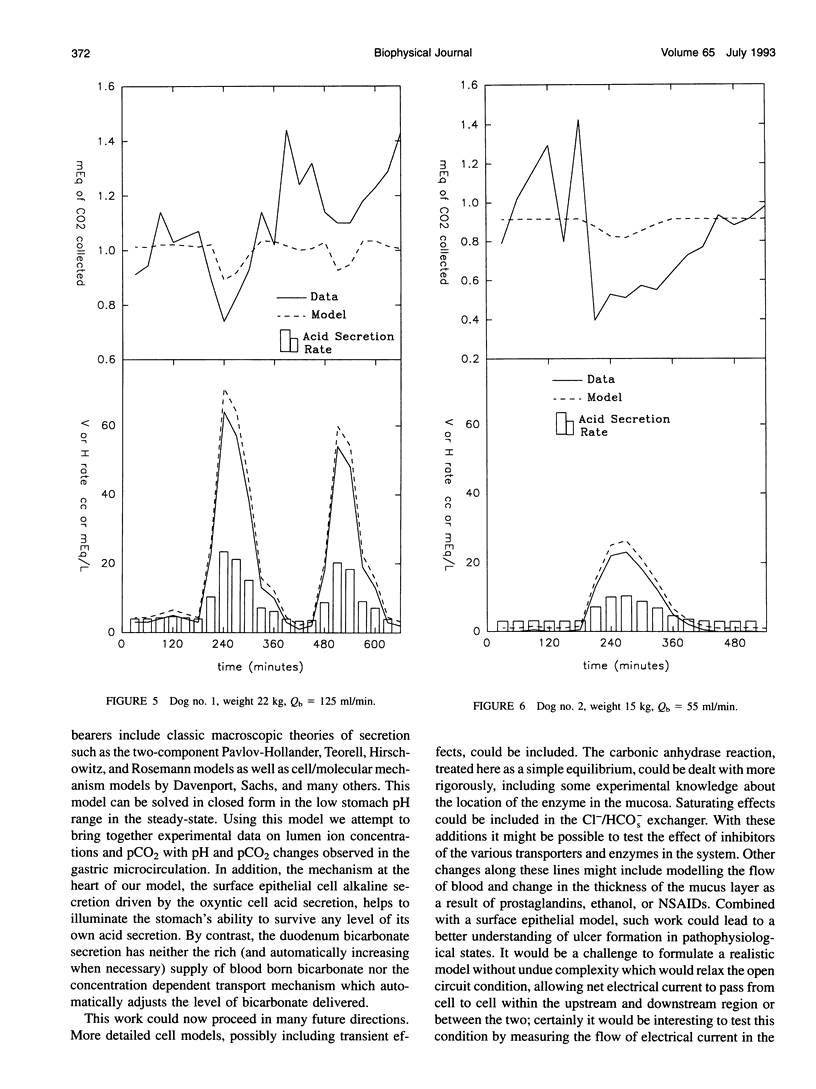
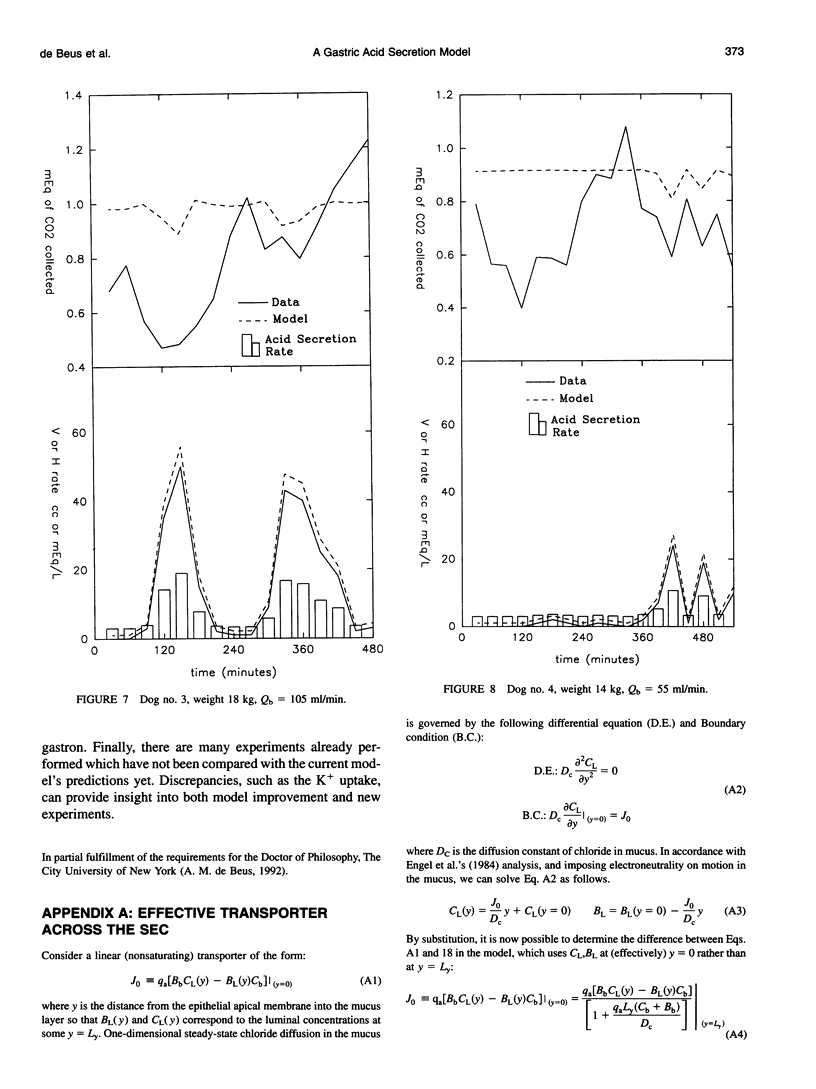
A theory of gastric acid production and self-protection is formulated mathematically and examined for clinical and experimental correlations, implications, and predictions using analytic and numerical techniques. In our model, gastric acid secretion in the stomach, as represented by an archetypal gastron, consists of two chambers, circulatory and luminal, connected by two different regions of ion exchange. The capillary circulation of the gastric mucosa is arranged in arterial-venous arcades which pass from the gastric glands up to the surface epithelial lining of the lumen; therefore the upstream region of the capillary chamber communicates with oxyntic cells, while the downstream region communicates with epithelial cells. Both cell types abut the gastric lumen. Ion currents across the upstream region are calculated from a steady-state oxyntic cell model with active ion transport, while the downstream ion fluxes are (facilitated) diffusion driven or secondarily active. Water transport is considered iso-osmotic. The steady-state model is solved in closed form for low gastric lumen pH. A wide variety of previously performed static and dynamic experiments on ion and CO2 transport in the gastric lumen and gastric blood supply are for the first time correlated with each other for an (at least) semiquantitative test of current concepts of gastric acid secretion and for the purpose of model verification. Agreement with the data is reported with a few outstanding and instructive exceptions. Model predictions and implications are also discussed.
Full text
PDF



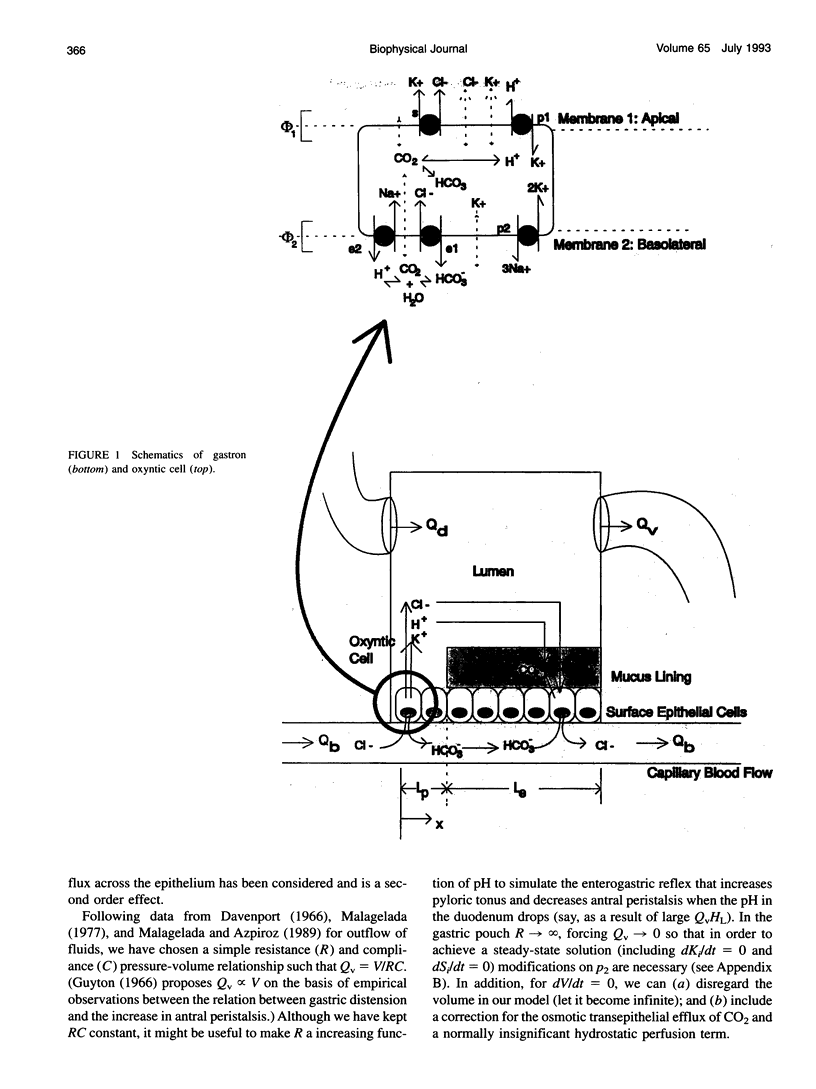



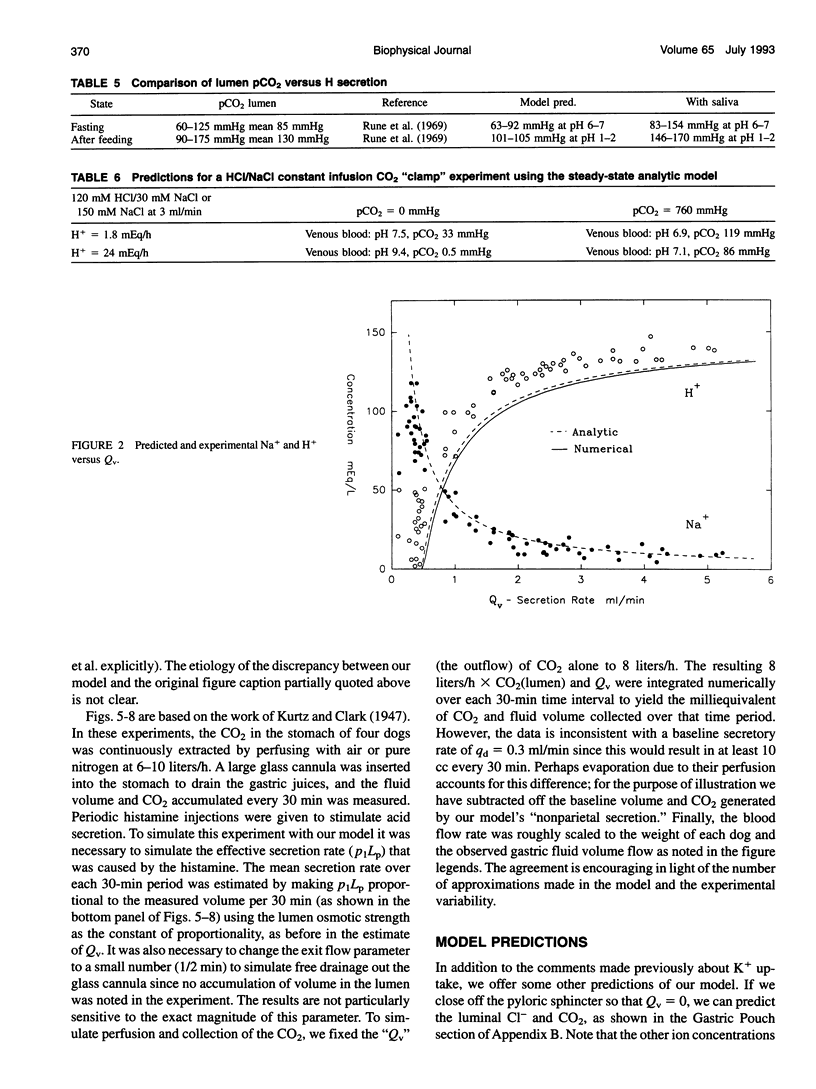
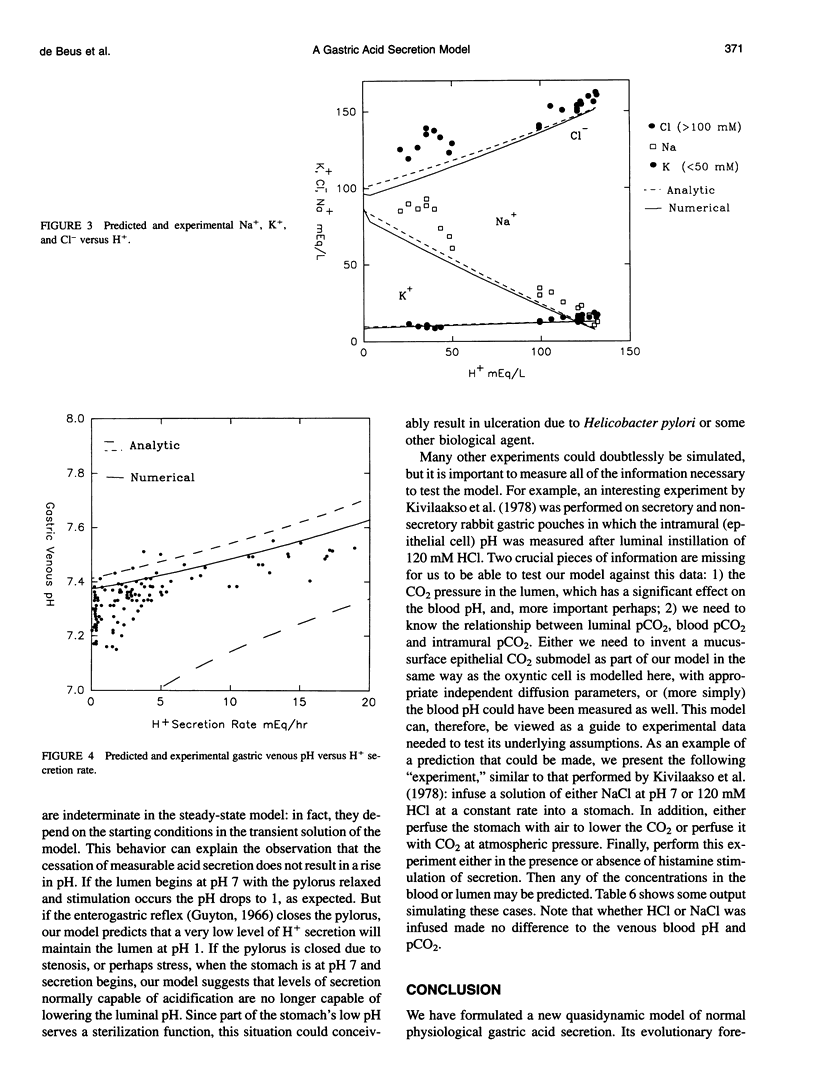





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apell H. J. Electrogenic properties of the Na,K pump. J Membr Biol. 1989 Sep;110(2):103–114. doi: 10.1007/BF01869466. [DOI] [PubMed] [Google Scholar]
- Ashley S. W., Soybel D. I., Cheung L. Y. Measurements of intracellular pH in Necturus antral mucosa by microelectrode technique. Am J Physiol. 1986 May;250(5 Pt 1):G625–G632. doi: 10.1152/ajpgi.1986.250.5.G625. [DOI] [PubMed] [Google Scholar]
- Ashley S. W., Soybel D. I., Moore C. D., Cheung L. Y. Intracellular pH (pHi) in gastric surface epithelium is more susceptible to serosal than mucosal acidification. Surgery. 1987 Aug;102(2):371–379. [PubMed] [Google Scholar]
- Berglindh T. The mammalian gastric parietal cell in vitro. Annu Rev Physiol. 1984;46:377–392. doi: 10.1146/annurev.ph.46.030184.002113. [DOI] [PubMed] [Google Scholar]
- Browne J. S., Vineberg A. M. The interdependence of gastric secretion and the CO(2) content of the blood. J Physiol. 1932 Jul 12;75(3):345–365. doi: 10.1113/jphysiol.1932.sp002896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung L. Y., Ashley S. W. Gastric blood flow and mucosal defense mechanisms. Clin Invest Med. 1987 May;10(3):201–208. [PubMed] [Google Scholar]
- Cheung L. Y. Enhancement of gastric mucosal tolerance to hydrogen ions (H+) by intraarterial infusion of sodium bicarbonate. Surg Forum. 1978;29:408–409. [PubMed] [Google Scholar]
- Cheung L. Y., Sonnenschein L. A. Measurement of regional gastric mucosal blood flow by hydrogen gas clearance. Am J Surg. 1984 Jan;147(1):32–37. doi: 10.1016/0002-9610(84)90030-8. [DOI] [PubMed] [Google Scholar]
- Cuppoletti J., Sachs G. Regulation of gastric acid secretion via modulation of a chloride conductance. J Biol Chem. 1984 Dec 10;259(23):14952–14959. [PubMed] [Google Scholar]
- Demarest J. R., Loo D. D., Sachs G. Activation of apical chloride channels in the gastric oxyntic cell. Science. 1989 Jul 28;245(4916):402–404. doi: 10.1126/science.2474200. [DOI] [PubMed] [Google Scholar]
- Eichenholz A., McQuarrie D., Blumentals A. S., Vennes J. A. Unique acid-base parameters of gastric venous blood during secretory activity. J Appl Physiol. 1967 Mar;22(3):580–583. doi: 10.1152/jappl.1967.22.3.580. [DOI] [PubMed] [Google Scholar]
- Engel E., Peskoff A., Kauffman G. L., Jr, Grossman M. I. Analysis of hydrogen ion concentration in the gastric gel mucus layer. Am J Physiol. 1984 Oct;247(4 Pt 1):G321–G338. doi: 10.1152/ajpgi.1984.247.4.G321. [DOI] [PubMed] [Google Scholar]
- Fendler K., van der Hijden H., Nagel G., de Pont J. J., Bamberg E. Pump currents generated by renal Na+K+-ATPase and gastric H+K+-ATPase on black lipid membranes. Prog Clin Biol Res. 1988;268A:501–510. [PubMed] [Google Scholar]
- Flemström G., Turnberg L. A. Gastroduodenal defence mechanisms. Clin Gastroenterol. 1984 May;13(2):327–354. [PubMed] [Google Scholar]
- Forte J. G., Machen T. E., Obrink K. J. Mechanisms of gastric H+ and Cl- transport. Annu Rev Physiol. 1980;42:111–126. doi: 10.1146/annurev.ph.42.030180.000551. [DOI] [PubMed] [Google Scholar]
- Forte T. M., Machen T. E., Forte J. G. Ultrastructural changes in oxyntic cells associated with secretory function: a membrane-recycling hypothesis. Gastroenterology. 1977 Oct;73(4 Pt 2):941–955. [PubMed] [Google Scholar]
- Fröhlich O., Gunn R. B. Erythrocyte anion transport: the kinetics of a single-site obligatory exchange system. Biochim Biophys Acta. 1986 Sep 22;864(2):169–194. doi: 10.1016/0304-4157(86)90010-9. [DOI] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn R. B., Fröhlich O. Asymmetry in the mechanism for anion exchange in human red blood cell membranes. Evidence for reciprocating sites that react with one transported anion at a time. J Gen Physiol. 1979 Sep;74(3):351–374. doi: 10.1085/jgp.74.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS J. B., EDELMAN I. S. CHEMICAL CONCENTRATION GRADIENTS AND ELECTRICAL PROPERTIES OF GASTRIC MUCOSA. Am J Physiol. 1964 Apr;206:769–782. doi: 10.1152/ajplegacy.1964.206.4.769. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersey S. J., Sachs G., Kasbekar D. K. Acid secretion by frog gastric mucosa is electroneutral. Am J Physiol. 1985 Feb;248(2 Pt 1):G246–G250. doi: 10.1152/ajpgi.1985.248.2.G246. [DOI] [PubMed] [Google Scholar]
- Ito S., Schofield G. C. Studies on the depletion and accumulation of microvilli and changes in the tubulovesicular compartment of mouse parietal cells in relation to gastric acid secretion. J Cell Biol. 1974 Nov;63(2 Pt 1):364–382. doi: 10.1083/jcb.63.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURTZ L. D., CLARK B. B. The inverse relationship of the secretion of hydrochloric acid to the tension of carbon dioxide in the stomach. Gastroenterology. 1947 Nov;9(5):594–602. [PubMed] [Google Scholar]
- Kivilaakso E., Flemström G. HCO3- secretion and surface pH gradient in rat duodenum exposed to luminal acid. Scand J Gastroenterol Suppl. 1984;92:51–54. [PubMed] [Google Scholar]
- Kivilaakso E., Fromm D., Silen W. Effect of the acid secretory state on intramural pH of rabbit gastric mucosa. Gastroenterology. 1978 Oct;75(4):641–648. [PubMed] [Google Scholar]
- LEE P. R., CODE C. F., SCHOLER J. F. The influence of varying concentrations of sodium chloride on the rate of absorption of water from the stomach and small bowel of human beings. Gastroenterology. 1955 Dec;29(6):1008–1016. [PubMed] [Google Scholar]
- Larsen E. H., Rasmussen B. E. Chloride channels in toad skin. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 1;299(1097):413–434. doi: 10.1098/rstb.1982.0141. [DOI] [PubMed] [Google Scholar]
- Lew V. L., Bookchin R. M. Volume, pH, and ion-content regulation in human red cells: analysis of transient behavior with an integrated model. J Membr Biol. 1986;92(1):57–74. doi: 10.1007/BF01869016. [DOI] [PubMed] [Google Scholar]
- Lew V. L., Ferreira H. G., Moura T. The behaviour of transporting epithelial cells. I. Computer analysis of a basic model. Proc R Soc Lond B Biol Sci. 1979 Nov 30;206(1162):53–83. doi: 10.1098/rspb.1979.0091. [DOI] [PubMed] [Google Scholar]
- Lorentzon P., Sachs G., Wallmark B. Inhibitory effects of cations on the gastric H+, K+ -ATPase. A potential-sensitive step in the K+ limb of the pump cycle. J Biol Chem. 1988 Aug 5;263(22):10705–10710. [PubMed] [Google Scholar]
- Läuger P. Kinetic properties of ion carriers and channels. J Membr Biol. 1980 Dec 30;57(3):163–78(-RETURN-). doi: 10.1007/BF01869585. [DOI] [PubMed] [Google Scholar]
- Läuger P., Stephan W., Frehland E. Fluctuations of barrier structure in ionic channels. Biochim Biophys Acta. 1980 Oct 16;602(1):167–180. doi: 10.1016/0005-2736(80)90299-0. [DOI] [PubMed] [Google Scholar]
- Makhlouf G. M., McManus J. P., Card W. I. A quantitative statement of the two-component hypothesis of gastric secretion. Gastroenterology. 1966 Aug;51(2):149–171. [PubMed] [Google Scholar]
- Malagelada J. R. Quantificantion of gastric solid-liquid discrimination during digestion of ordinary meals. Gastroenterology. 1977 Jun;72(6):1264–1267. [PubMed] [Google Scholar]
- Malinowska D. H., Sachs G. Cellular mechanisms of acid secretion. Clin Gastroenterol. 1984 May;13(2):309–326. [PubMed] [Google Scholar]
- Milhorn H. T., Jr, Pulley P. E., Jr A theoretical study of pulmonary capillary gas exchange and venous admixture. Biophys J. 1968 Mar;8(3):337–357. doi: 10.1016/S0006-3495(68)86492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagi S., Shoemaker C. P., Jr, Powers S. R., Jr Carbon dioxide metabolism in the intact mammalian stomach. Surgery. 1966 Jun;59(6):1083–1091. [PubMed] [Google Scholar]
- Muallem S., Blissard D., Cragoe E. J., Jr, Sachs G. Activation of the Na+/H+ and Cl-/HCO3- exchange by stimulation of acid secretion in the parietal cell. J Biol Chem. 1988 Oct 15;263(29):14703–14711. [PubMed] [Google Scholar]
- Muallem S., Burnham C., Blissard D., Berglindh T., Sachs G. Electrolyte transport across the basolateral membrane of the parietal cells. J Biol Chem. 1985 Jun 10;260(11):6641–6653. [PubMed] [Google Scholar]
- Negulescu P. A., Harootunian A., Tsien R. Y., Machen T. E. Fluorescence measurements of cytosolic free Na concentration, influx and efflux in gastric cells. Cell Regul. 1990 Feb;1(3):259–268. doi: 10.1091/mbc.1.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso A. M., Tsien R. Y., Demarest J. R., Machen T. E. Na-H and Cl-HCO3 exchange in rabbit oxyntic cells using fluorescence microscopy. Am J Physiol. 1987 Jul;253(1 Pt 1):C30–C36. doi: 10.1152/ajpcell.1987.253.1.C30. [DOI] [PubMed] [Google Scholar]
- Perez A., Blissard D., Sachs G., Hersey S. J. Evidence for a chloride conductance in secretory membrane of parietal cells. Am J Physiol. 1989 Feb;256(2 Pt 1):G299–G305. doi: 10.1152/ajpgi.1989.256.2.G299. [DOI] [PubMed] [Google Scholar]
- REHM W. S. Effect of electric current on gastric hydrogen ion and chloride ion secretion. Am J Physiol. 1956 May;185(2):325–331. doi: 10.1152/ajplegacy.1956.185.2.325. [DOI] [PubMed] [Google Scholar]
- REHM W. S. Electrical resistance of resting and secreting stomach. Am J Physiol. 1953 Mar;172(3):689–699. doi: 10.1152/ajplegacy.1953.172.3.689. [DOI] [PubMed] [Google Scholar]
- REHM W. S., LEFEVRE M. E. EFFECT OF DINITROPHENOL ON POTENTIAL, RESISTANCE, AND H+ RATE OF FROG STOMACH. Am J Physiol. 1965 May;208:922–930. doi: 10.1152/ajplegacy.1965.208.5.922. [DOI] [PubMed] [Google Scholar]
- Rehm W. S., Butler C. F., Spangler S. G., Sanders S. S. A model to explain uphill water transport in the mammalian stomach. J Theor Biol. 1970 Jun;27(3):433–453. doi: 10.1016/s0022-5193(70)80008-x. [DOI] [PubMed] [Google Scholar]
- Rehm W. S., Sanders S. S. Implications of the neutral carrier Cl-HCO3- exchange mechanism in gastric mucosa. Ann N Y Acad Sci. 1975 Dec 30;264:442–455. doi: 10.1111/j.1749-6632.1975.tb31502.x. [DOI] [PubMed] [Google Scholar]
- Rehm W. S. Some aspects of the problem of gastric hydrochloric acid secretion. Arch Intern Med. 1972 Feb;129(2):270–278. [PubMed] [Google Scholar]
- Rossi R. C., Garrahan P. J. A comparison between the empirical behaviour of the Na,K-ATPase and the predictions of the Albers-Post model. Prog Clin Biol Res. 1988;268A:349–354. [PubMed] [Google Scholar]
- Rune S. J. Comparison of the rates of gastric acid secretion in man after ingestion of food and after maximal stimulation with histamine. Gut. 1966 Aug;7(4):344–350. doi: 10.1136/gut.7.4.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rune S. J., Henriksen F. W. Carbon dioxide tensions in the proximal part of the canine gastrointestinal tract. Gastroenterology. 1969 Apr;56(4):758–762. [PubMed] [Google Scholar]
- Sachs G., Faller L. D., Rabon E. Proton/hydroxyl transport in gastric and intestinal epithelia. J Membr Biol. 1982;64(3):123–135. doi: 10.1007/BF01870878. [DOI] [PubMed] [Google Scholar]
- Sachs G., Muallem S., Hersey S. J. Passive and active transport in the parietal cell. Comp Biochem Physiol A Comp Physiol. 1988;90(4):727–731. doi: 10.1016/0300-9629(88)90691-3. [DOI] [PubMed] [Google Scholar]
- Solomon A. K., Chasan B., Dix J. A., Lukacovic M. F., Toon M. R., Verkman A. S. The aqueous pore in the red cell membrane: band 3 as a channel for anions, cations, nonelectrolytes, and water. Ann N Y Acad Sci. 1983;414:97–124. doi: 10.1111/j.1749-6632.1983.tb31678.x. [DOI] [PubMed] [Google Scholar]
- Soybel D. I., Ashley S. W., Yan Z. Y., Swarm R. A., Cheung L. Y. Regional gastric mucosal blood flow after parietal cell vagotomy in dogs. Surgery. 1986 Aug;100(2):167–174. [PubMed] [Google Scholar]
- Starlinger M., Jakesz R., Matthews J. B., Yoon C., Schiessel R. The relative importance of HCO3- and blood flow in the protection of rat gastric mucosa during shock. Gastroenterology. 1981 Oct;81(4):732–735. [PubMed] [Google Scholar]
- Stevens M. H., Thirlby R. C., Feldman M. Mechanism for high PCO2 in gastric juice: roles of bicarbonate secretion and CO2 diffusion. Am J Physiol. 1987 Oct;253(4 Pt 1):G527–G530. doi: 10.1152/ajpgi.1987.253.4.G527. [DOI] [PubMed] [Google Scholar]
- Stewart B., Wallmark B., Sachs G. The interaction of H+ and K+ with the partial reactions of gastric (H+ + K+)-ATPase. J Biol Chem. 1981 Mar 25;256(6):2682–2690. [PubMed] [Google Scholar]
- THULL N. B., REHM W. S. Composition and osmolarity of gastric juice as a function of plasma osmolarity. Am J Physiol. 1956 May;185(2):317–324. doi: 10.1152/ajplegacy.1956.185.2.317. [DOI] [PubMed] [Google Scholar]
- TOSTESON D. C., HOFFMAN J. F. Regulation of cell volume by active cation transport in high and low potassium sheep red cells. J Gen Physiol. 1960 Sep;44:169–194. doi: 10.1085/jgp.44.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K., Magee D., Critchlow J., Matthews J., Silen W. Studies of the pH gradient and thickness of frog gastric mucus gel. Gastroenterology. 1983 Feb;84(2):331–340. [PubMed] [Google Scholar]
- Ueda S., Loo D. D., Sachs G. Regulation of K+ channels in the basolateral membrane of Necturus oxyntic cells. J Membr Biol. 1987;97(1):31–41. doi: 10.1007/BF01869612. [DOI] [PubMed] [Google Scholar]
- VILLEGAS L. ACTION OF HISTAMINE ON THE PERMEABILITY OF THE FROG GASTRIC MUCOSA TO POTASSIUM AND WATER. Biochim Biophys Acta. 1963 Nov 29;75:377–386. doi: 10.1016/0006-3002(63)90625-5. [DOI] [PubMed] [Google Scholar]
- VILLEGAS L. Cellular location of the electrical potential difference in frog gastric mucosa. Biochim Biophys Acta. 1962 Oct 22;64:359–367. doi: 10.1016/0006-3002(62)90745-x. [DOI] [PubMed] [Google Scholar]
- Wenzl E., Machen T. E. Intracellular pH dependence of buffer capacity and anion exchange in the parietal cell. Am J Physiol. 1989 Nov;257(5 Pt 1):G741–G747. doi: 10.1152/ajpgi.1989.257.5.G741. [DOI] [PubMed] [Google Scholar]
- Wieth J. O., Andersen O. S., Brahm J., Bjerrum P. J., Borders C. L., Jr Chloride--bicarbonate exchange in red blood cells: physiology of transport and chemical modification of binding sites. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 1;299(1097):383–399. doi: 10.1098/rstb.1982.0139. [DOI] [PubMed] [Google Scholar]
- Wolosin J. M., Forte J. G. Kinetic properties of the KCl transport at the secreting apical membrane of the oxyntic cell. J Membr Biol. 1983;71(3):195–207. doi: 10.1007/BF01875461. [DOI] [PubMed] [Google Scholar]
- Wolosin J. M. Ion transport studies with H+-K+-ATPase-rich vesicles: implications for HCl secretion and parietal cell physiology. Am J Physiol. 1985 Jun;248(6 Pt 1):G595–G607. doi: 10.1152/ajpgi.1985.248.6.G595. [DOI] [PubMed] [Google Scholar]
- Wood J. G., Wicina G. M., Cheung L. Y. Effect of histamine and 1,4-methylhistamine on gastric vascular resistance in dogs. Am J Physiol. 1990 Mar;258(3 Pt 1):G440–G446. doi: 10.1152/ajpgi.1990.258.3.G440. [DOI] [PubMed] [Google Scholar]