Abstract
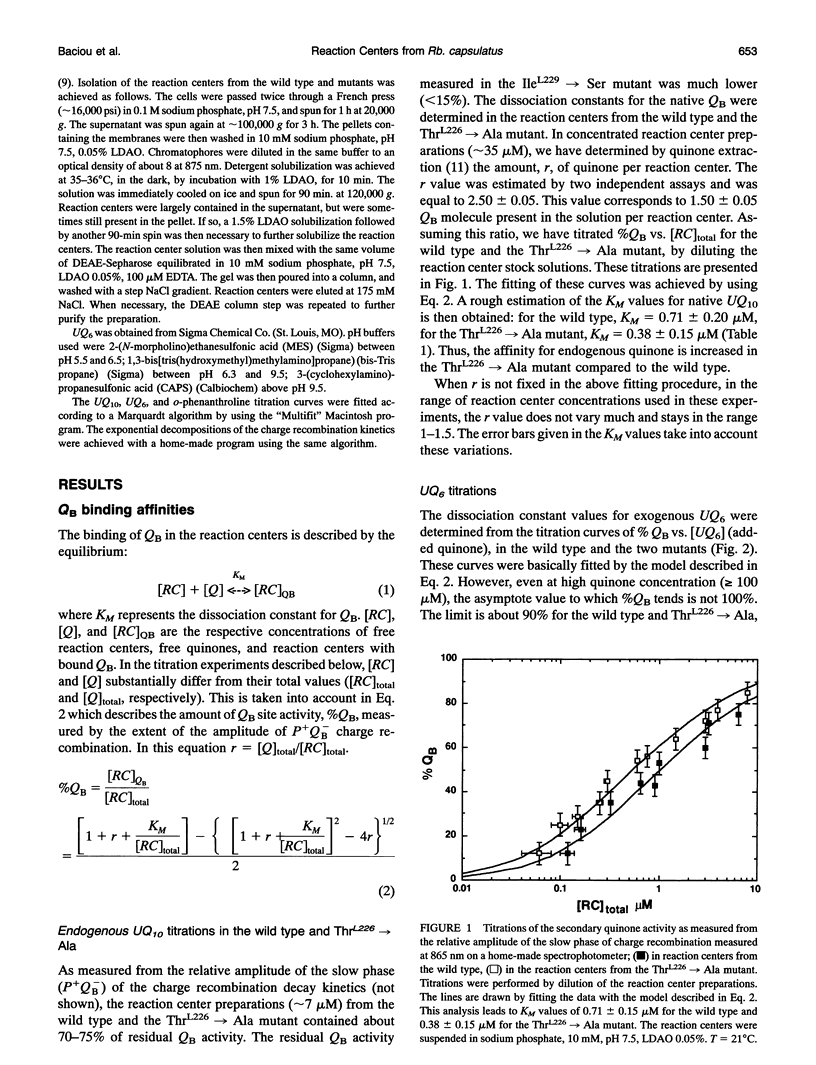
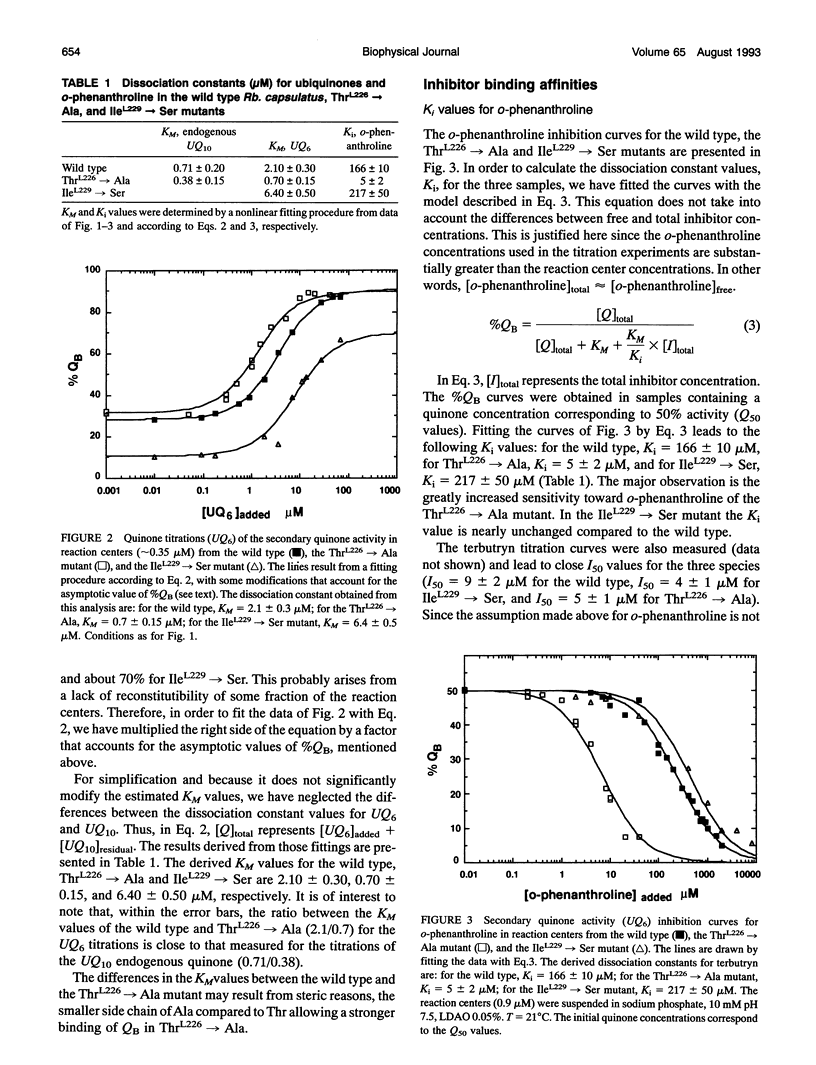
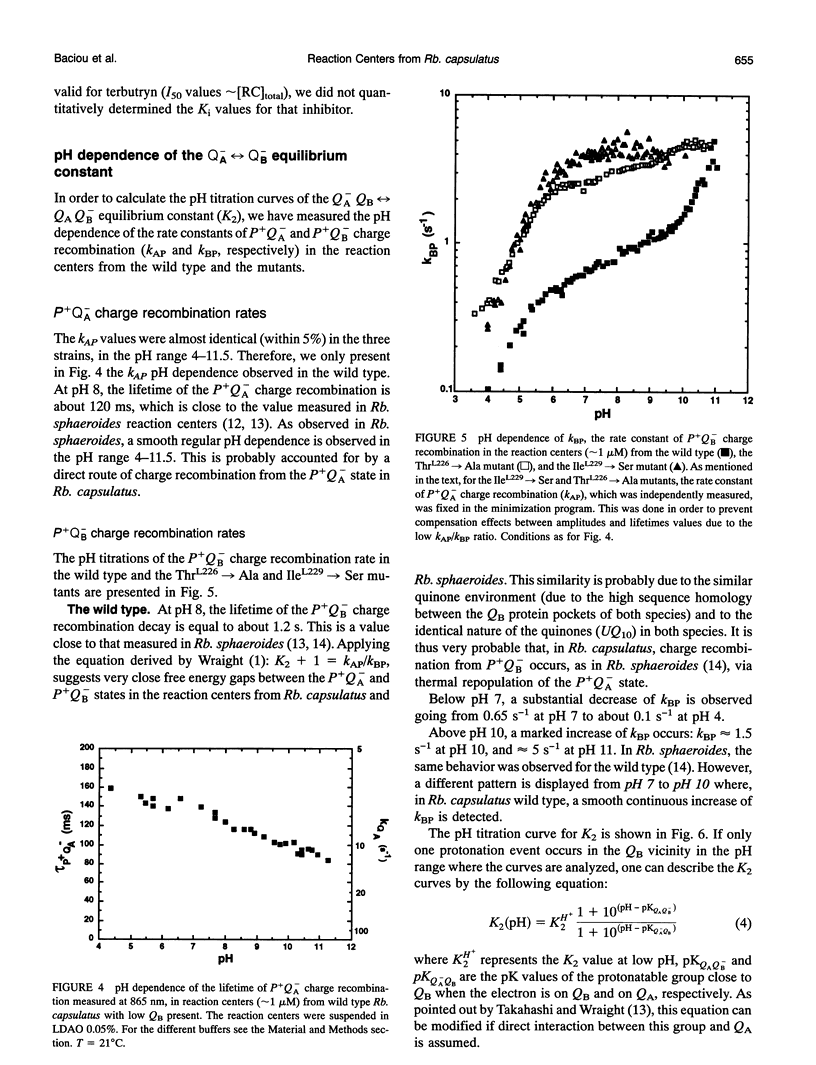
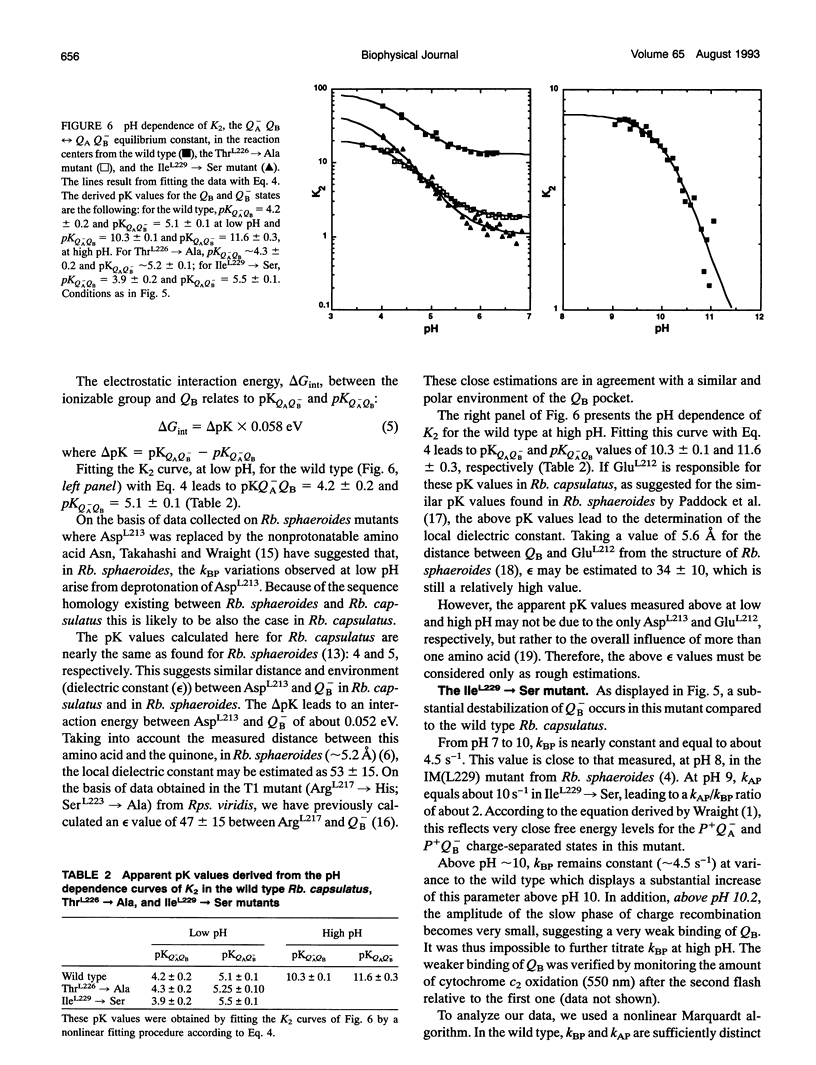
Reaction centers from the purple bacterium Rhodobacter (Rb.) capsulatus and from two mutants ThrL226-->Ala and IleL229-->Ser, modified in the binding protein pocket of the secondary quinone acceptor (QB), have been studied by flash-induced absorbance spectroscopy. In ThrL226-->Ala, the binding affinities for endogenous QB (ubiquinone 10) and UQ6 are found to be two to three times as high as the wild type. In contrast, in IleL229-->Ser, the binding affinity for UQ6 is decreased about three times compared to the wild type. In ThrL226-->Ala, a markedly increased sensitivity (approximately 30 times) to o-phenanthroline is observed. In Rhodopseudomonas viridis, where Ala is naturally in position L226, the sensitivity to o-phenanthroline is close to that observed in ThrL226-->Ala. We propose that the presence of Ala in position L226 is responsible for the high sensitivity to that inhibitor. The pH dependencies of the rate constants of P+QB- (kBP) charge recombination kinetics (P is a dimer of bacteriochlorophyll, and QB is the secondary quinone electron acceptor) show destabilization of QB- in ThrL226-->Ala and IleL229-->Ser, compared to the wild type. At low pH, similar apparent pK values of protonation of amino acids around QB- are measured in the wild type and the mutants. In contrast to Rb. sphaeroides, in the wild type Rb. capsulatus, kBP substantially increases in the pH range 7-10. This may reflect some differences in the respective structures of both strains or, alternatively, may be due to deprotonation of TyrL 215 in Rb. capsulatus. At pH 7, measurements of the rate constant of QA to QB electron transfer reveal a threefold greater rate in the reaction centers from wild type Rb. capsulatus (65 +/- 1 0 ps)-1 compared to Rb. sphaeroides.We suggest that this may arise from a 0.7-A smaller distance between the quinones in the former strain. Our spectroscopic data on the wild type Rb. capsulatus reaction center suggest the existence of notable differences with the Rb. sphaeroides reaction center structure.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. P., Feher G., Yeates T. O., Komiya H., Rees D. C. Structure of the reaction center from Rhodobacter sphaeroides R-26: protein-cofactor (quinones and Fe2+) interactions. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8487–8491. doi: 10.1073/pnas.85.22.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baciou L., Sinning I., Sebban P. Study of QB- stabilization in herbicide-resistant mutants from the purple bacterium Rhodopseudomonas viridis. Biochemistry. 1991 Sep 17;30(37):9110–9116. doi: 10.1021/bi00101a029. [DOI] [PubMed] [Google Scholar]
- Chang C. H., el-Kabbani O., Tiede D., Norris J., Schiffer M. Structure of the membrane-bound protein photosynthetic reaction center from Rhodobacter sphaeroides. Biochemistry. 1991 Jun 4;30(22):5352–5360. doi: 10.1021/bi00236a005. [DOI] [PubMed] [Google Scholar]
- Hanson D. K., Baciou L., Tiede D. M., Nance S. L., Schiffer M., Sebban P. In bacterial reaction centers protons can diffuse to the secondary quinone by alternative pathways. Biochim Biophys Acta. 1992 Sep 25;1102(2):260–265. doi: 10.1016/0005-2728(92)90108-e. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D., Okamura M. Y., Feher G. Electron transfer in reaction centers of Rhodopseudomonas sphaeroides. I. Determination of the charge recombination pathway of D+QAQ(-)B and free energy and kinetic relations between Q(-)AQB and QAQ(-)B. Biochim Biophys Acta. 1984 Jul 27;766(1):126–140. doi: 10.1016/0005-2728(84)90224-x. [DOI] [PubMed] [Google Scholar]
- McComb J. C., Stein R. R., Wraight C. A. Investigations on the influence of headgroup substitution and isoprene side-chain length in the function of primary and secondary quinones of bacterial reaction centers. Biochim Biophys Acta. 1990 Jan 4;1015(1):156–171. doi: 10.1016/0005-2728(90)90227-u. [DOI] [PubMed] [Google Scholar]
- Michel H., Epp O., Deisenhofer J. Pigment-protein interactions in the photosynthetic reaction centre from Rhodopseudomonas viridis. EMBO J. 1986 Oct;5(10):2445–2451. doi: 10.1002/j.1460-2075.1986.tb04520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser C. C., Keske J. M., Warncke K., Farid R. S., Dutton P. L. Nature of biological electron transfer. Nature. 1992 Feb 27;355(6363):796–802. doi: 10.1038/355796a0. [DOI] [PubMed] [Google Scholar]
- Okamura M. Y., Feher G. Proton transfer in reaction centers from photosynthetic bacteria. Annu Rev Biochem. 1992;61:861–896. doi: 10.1146/annurev.bi.61.070192.004241. [DOI] [PubMed] [Google Scholar]
- Paddock M. L., Rongey S. H., Feher G., Okamura M. Y. Pathway of proton transfer in bacterial reaction centers: replacement of glutamic acid 212 in the L subunit by glutamine inhibits quinone (secondary acceptor) turnover. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6602–6606. doi: 10.1073/pnas.86.17.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinning I., Michel H., Mathis P., Rutherford A. W. Characterization of four herbicide-resistant mutants of Rhodopseudomonas viridis by genetic analysis, electron paramagnetic resonance, and optical spectroscopy. Biochemistry. 1989 Jun 27;28(13):5544–5553. doi: 10.1021/bi00439a031. [DOI] [PubMed] [Google Scholar]
- Takahashi E., Wraight C. A. Proton and electron transfer in the acceptor quinone complex of Rhodobacter sphaeroides reaction centers: characterization of site-directed mutants of the two ionizable residues, GluL212 and AspL213, in the QB binding site. Biochemistry. 1992 Jan 28;31(3):855–866. doi: 10.1021/bi00118a031. [DOI] [PubMed] [Google Scholar]
- Vermeglio A., Clayton R. K. Kinetics of electron transfer between the primary and the secondary electron acceptor in reaction centers from Rhodopseudomonas sphaeroides. Biochim Biophys Acta. 1977 Jul 7;461(1):159–165. doi: 10.1016/0005-2728(77)90078-0. [DOI] [PubMed] [Google Scholar]
- el-Kabbani O., Chang C. H., Tiede D., Norris J., Schiffer M. Comparison of reaction centers from Rhodobacter sphaeroides and Rhodopseudomonas viridis: overall architecture and protein-pigment interactions. Biochemistry. 1991 Jun 4;30(22):5361–5369. doi: 10.1021/bi00236a006. [DOI] [PubMed] [Google Scholar]


