Abstract
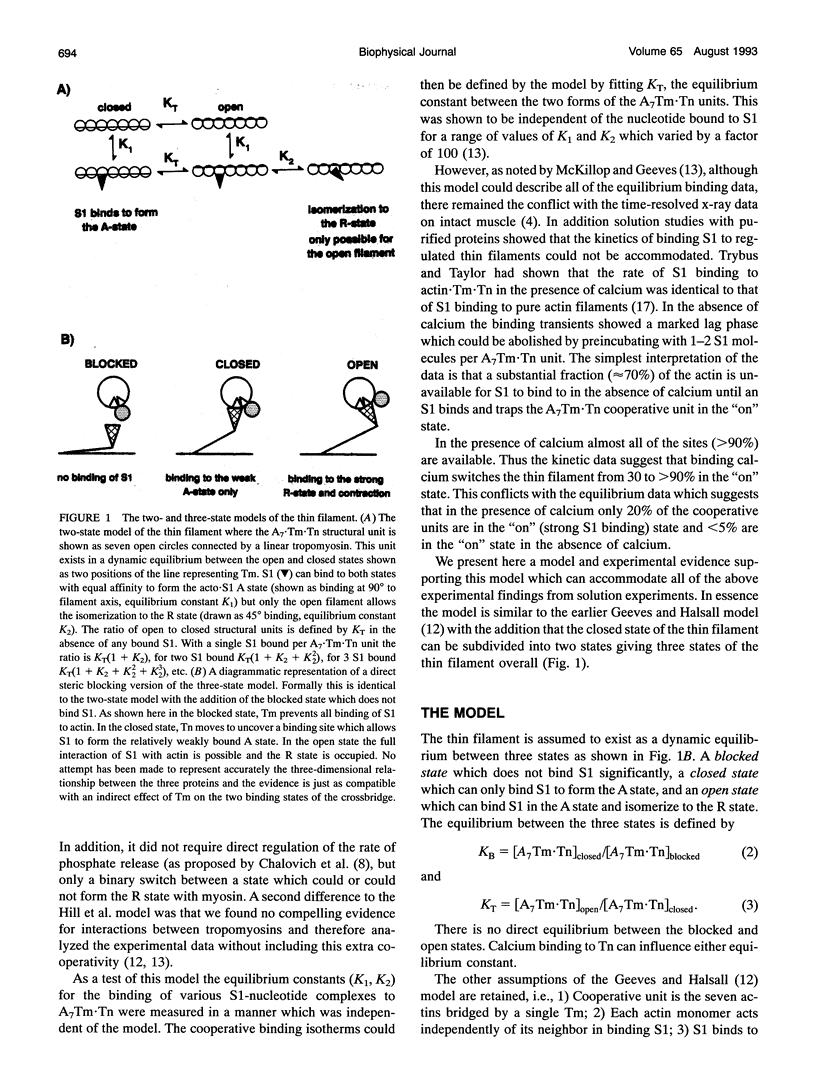
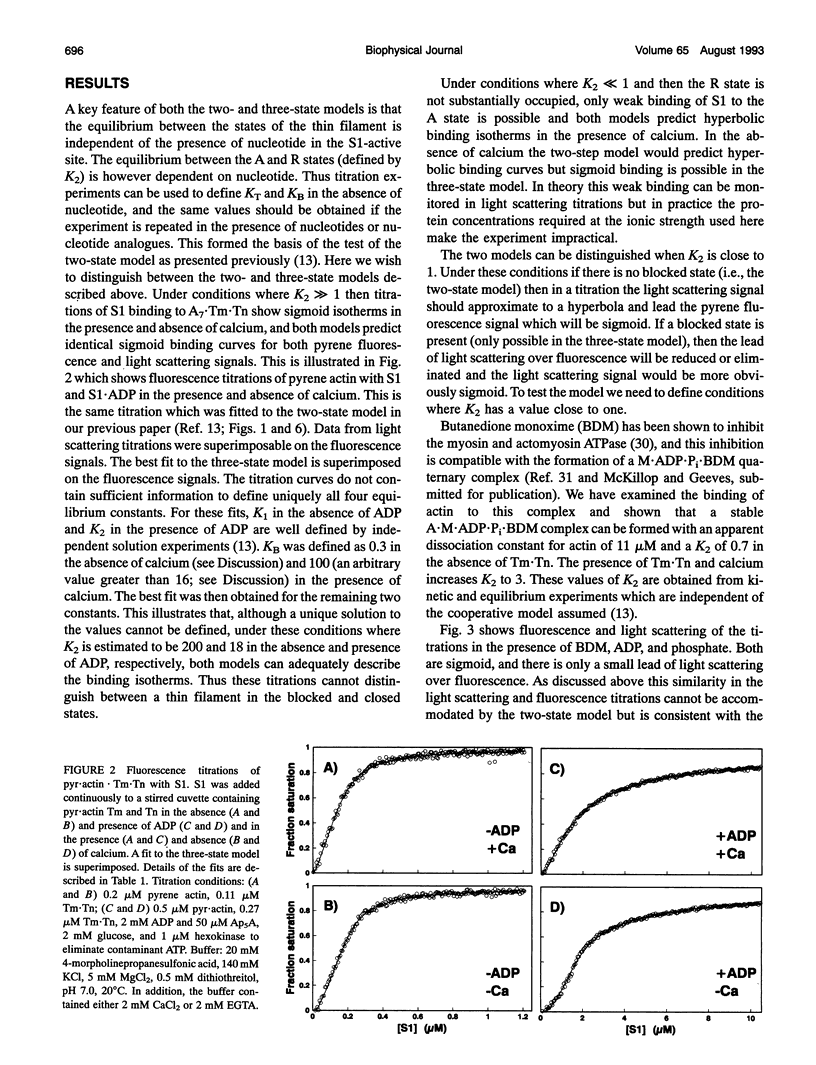
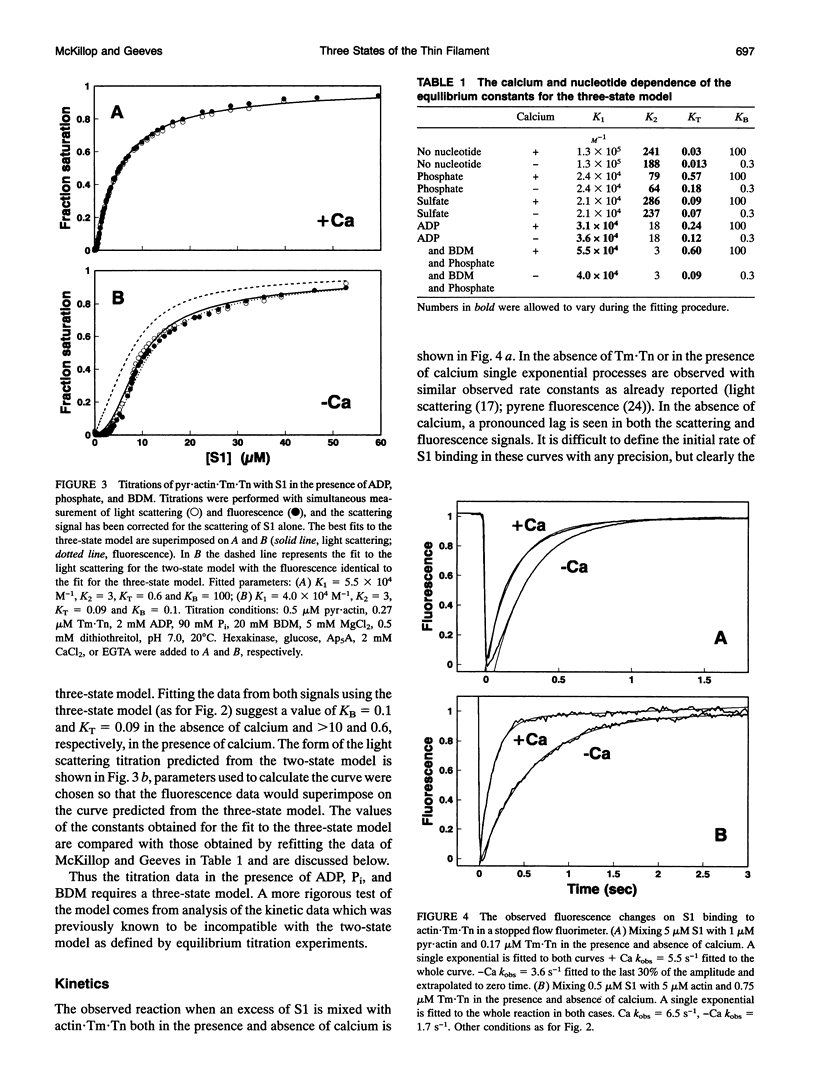
Equilibrium titrations and kinetic experiments were used to define the cooperative binding of myosin subfragment 1 (S1) to actin-troponin-tropomyosin. Both types of experiment require an equilibrium between two states of the thin filament in which one state (the off state) binds S1 less readily than the other. Equilibrium titrations are compatible with > 95% of the actin7.Tn.Tm units being in the off state in the absence of calcium and 80% in the off state in the presence of calcium. Kinetic binding data suggest that the presence of calcium switches the thin filament from 70% in the off state to < 5%. The two experiments, therefore, define quite different populations of the off states. We propose a three-state model of the thin filament. A "blocked state" which is unable to bind S1, a "closed state" which can only bind S1 relatively weakly and an "open state" in which the S1 can both bind and undergo an isomerization to a more strongly bound rigor-like conformation. The equilibrium between the three states is calcium-dependent; KB = [closed]/[blocked] = 0.3 and > or = 16 and KT = [open]/[closed] = 0.09 and 0.25 in the absence and presence of calcium, respectively. This model can account for both types of experimental data.
Full text
PDF




Images in this article
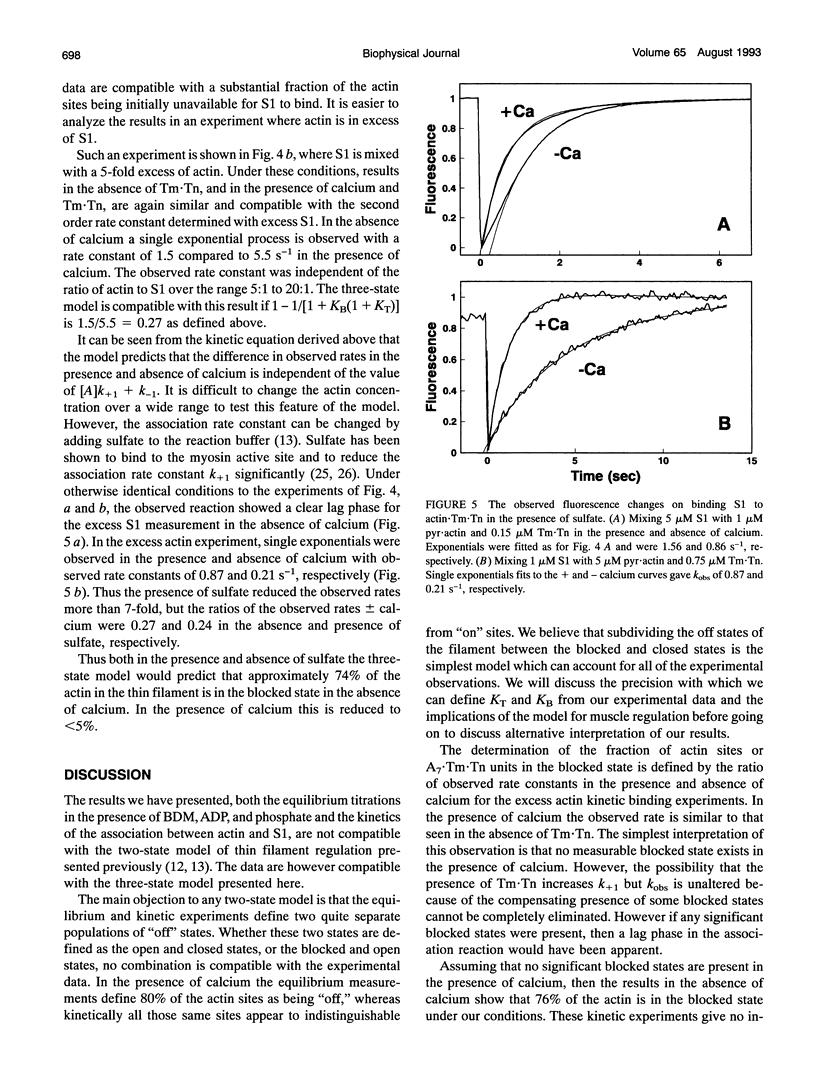
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babu A., Gulati J. Proposed mechanism for dual regulation of cross-bridge turn-over in vertebrate muscle. Adv Exp Med Biol. 1988;226:101–112. [PubMed] [Google Scholar]
- Balazs A. C., Epstein I. R. Kinetic model for the interaction of myosin subfragment 1 with regulated actin. Biophys J. 1983 Nov;44(2):145–151. doi: 10.1016/S0006-3495(83)84286-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalovich J. M., Eisenberg E. Inhibition of actomyosin ATPase activity by troponin-tropomyosin without blocking the binding of myosin to actin. J Biol Chem. 1982 Mar 10;257(5):2432–2437. [PMC free article] [PubMed] [Google Scholar]
- Chalovich J. M., Eisenberg E. Inhibition of actomyosin ATPase activity by troponin-tropomyosin without blocking the binding of myosin to actin. J Biol Chem. 1982 Mar 10;257(5):2432–2437. [PMC free article] [PubMed] [Google Scholar]
- Coates J. H., Criddle A. H., Geeves M. A. Pressure-relaxation studies of pyrene-labelled actin and myosin subfragment 1 from rabbit skeletal muscle. Evidence for two states of acto-subfragment 1. Biochem J. 1985 Dec 1;232(2):351–356. doi: 10.1042/bj2320351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criddle A. H., Geeves M. A., Jeffries T. The use of actin labelled with N-(1-pyrenyl)iodoacetamide to study the interaction of actin with myosin subfragments and troponin/tropomyosin. Biochem J. 1985 Dec 1;232(2):343–349. doi: 10.1042/bj2320343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EBASHI S., EBASHI F. A NEW PROTEIN COMPONENT PARTICIPATING IN THE SUPERPRECIPITATION OF MYOSIN B. J Biochem. 1964 Jun;55:604–613. doi: 10.1093/oxfordjournals.jbchem.a127933. [DOI] [PubMed] [Google Scholar]
- Geeves M. A., Halsall D. J. The dynamics of the interaction between myosin subfragment 1 and pyrene-labelled thin filaments, from rabbit skeletal muscle. Proc R Soc Lond B Biol Sci. 1986 Oct 22;229(1254):85–95. doi: 10.1098/rspb.1986.0076. [DOI] [PubMed] [Google Scholar]
- Geeves M. A., Halsall D. J. Two-step ligand binding and cooperativity. A model to describe the cooperative binding of myosin subfragment 1 to regulated actin. Biophys J. 1987 Aug;52(2):215–220. doi: 10.1016/S0006-3495(87)83208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeves M. A., Jeffries T. E. The effect of nucleotide upon a specific isomerization of actomyosin subfragment 1. Biochem J. 1988 Nov 15;256(1):41–46. doi: 10.1042/bj2560041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeves M. A. The dynamics of actin and myosin association and the crossbridge model of muscle contraction. Biochem J. 1991 Feb 15;274(Pt 1):1–14. doi: 10.1042/bj2740001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. Cooperative binding of myosin subfragment-1 to the actin-troponin-tropomyosin complex. Proc Natl Acad Sci U S A. 1980 May;77(5):2616–2620. doi: 10.1073/pnas.77.5.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann C., Wray J., Travers F., Barman T. Effect of 2,3-butanedione monoxime on myosin and myofibrillar ATPases. An example of an uncompetitive inhibitor. Biochemistry. 1992 Dec 8;31(48):12227–12232. doi: 10.1021/bi00163a036. [DOI] [PubMed] [Google Scholar]
- Higuchi H., Takemori S. Butanedione monoxime suppresses contraction and ATPase activity of rabbit skeletal muscle. J Biochem. 1989 Apr;105(4):638–643. doi: 10.1093/oxfordjournals.jbchem.a122717. [DOI] [PubMed] [Google Scholar]
- Hill T. L., Eisenberg E., Greene L. Theoretical model for the cooperative equilibrium binding of myosin subfragment 1 to the actin-troponin-tropomyosin complex. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3186–3190. doi: 10.1073/pnas.77.6.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii Y., Lehrer S. S. Excimer fluorescence of pyrenyliodoacetamide-labeled tropomyosin: a probe of the state of tropomyosin in reconstituted muscle thin filaments. Biochemistry. 1990 Feb 6;29(5):1160–1166. doi: 10.1021/bi00457a010. [DOI] [PubMed] [Google Scholar]
- Kress M., Huxley H. E., Faruqi A. R., Hendrix J. Structural changes during activation of frog muscle studied by time-resolved X-ray diffraction. J Mol Biol. 1986 Apr 5;188(3):325–342. doi: 10.1016/0022-2836(86)90158-0. [DOI] [PubMed] [Google Scholar]
- Lehrer S. S., Kerwar G. Intrinsic fluorescence of actin. Biochemistry. 1972 Mar 28;11(7):1211–1217. doi: 10.1021/bi00757a015. [DOI] [PubMed] [Google Scholar]
- Lehrer S. S., Morris E. P. Dual effects of tropomyosin and troponin-tropomyosin on actomyosin subfragment 1 ATPase. J Biol Chem. 1982 Jul 25;257(14):8073–8080. [PubMed] [Google Scholar]
- McKillop D. F., Geeves M. A. Effect of phosphate and sulphate on the interaction of actin and myosin subfragment 1. Biochem Soc Trans. 1990 Aug;18(4):585–586. doi: 10.1042/bst0180585. [DOI] [PubMed] [Google Scholar]
- McKillop D. F., Geeves M. A. Regulation of the acto.myosin subfragment 1 interaction by troponin/tropomyosin. Evidence for control of a specific isomerization between two acto.myosin subfragment 1 states. Biochem J. 1991 Nov 1;279(Pt 3):711–718. doi: 10.1042/bj2790711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J. M. Hybridization and reconstitution of the thin filament. Methods Enzymol. 1982;85(Pt B):15–17. doi: 10.1016/0076-6879(82)85006-4. [DOI] [PubMed] [Google Scholar]
- Rosenfeld S. S., Taylor E. W. The mechanism of regulation of actomyosin subfragment 1 ATPase. J Biol Chem. 1987 Jul 25;262(21):9984–9993. [PubMed] [Google Scholar]
- Tesi C., Barman T., Travers F. Sulphate is a competitive inhibitor of the binding of nucleotide to myosin. A comparison with phosphate. FEBS Lett. 1988 Aug 15;236(1):256–260. doi: 10.1016/0014-5793(88)80326-0. [DOI] [PubMed] [Google Scholar]
- Trybus K. M., Taylor E. W. Kinetic studies of the cooperative binding of subfragment 1 to regulated actin. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7209–7213. doi: 10.1073/pnas.77.12.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner P. D., Giniger E. Calcium-sensitive binding of heavy meromyosin to regulated actin in the presence of ATP. J Biol Chem. 1981 Dec 25;256(24):12647–12650. [PubMed] [Google Scholar]
- Williams D. L., Jr, Greene L. E. Comparison of the effects of tropomyosin and troponin-tropomyosin on the binding of myosin subfragment 1 to actin. Biochemistry. 1983 May 24;22(11):2770–2774. doi: 10.1021/bi00280a027. [DOI] [PubMed] [Google Scholar]
- el-Saleh S. C., Potter J. D. Calcium-insensitive binding of heavy meromyosin to regulated actin at physiological ionic strength. J Biol Chem. 1985 Nov 25;260(27):14775–14779. [PubMed] [Google Scholar]