Abstract
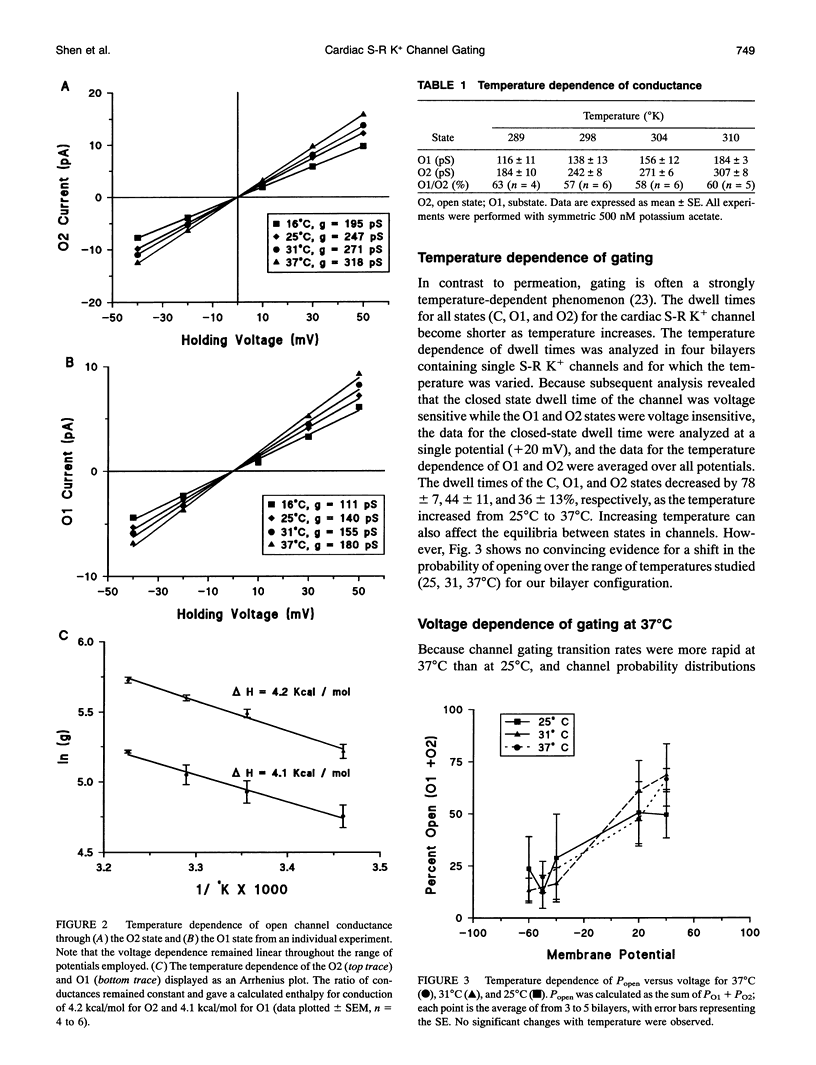
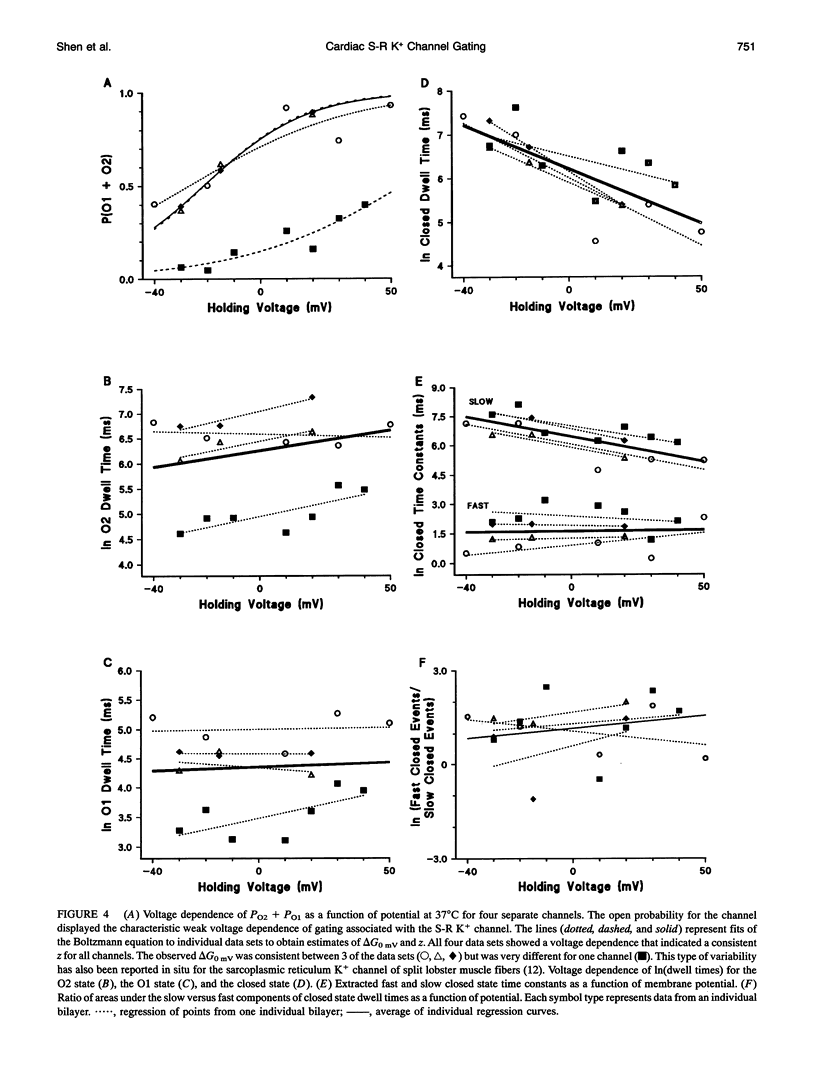
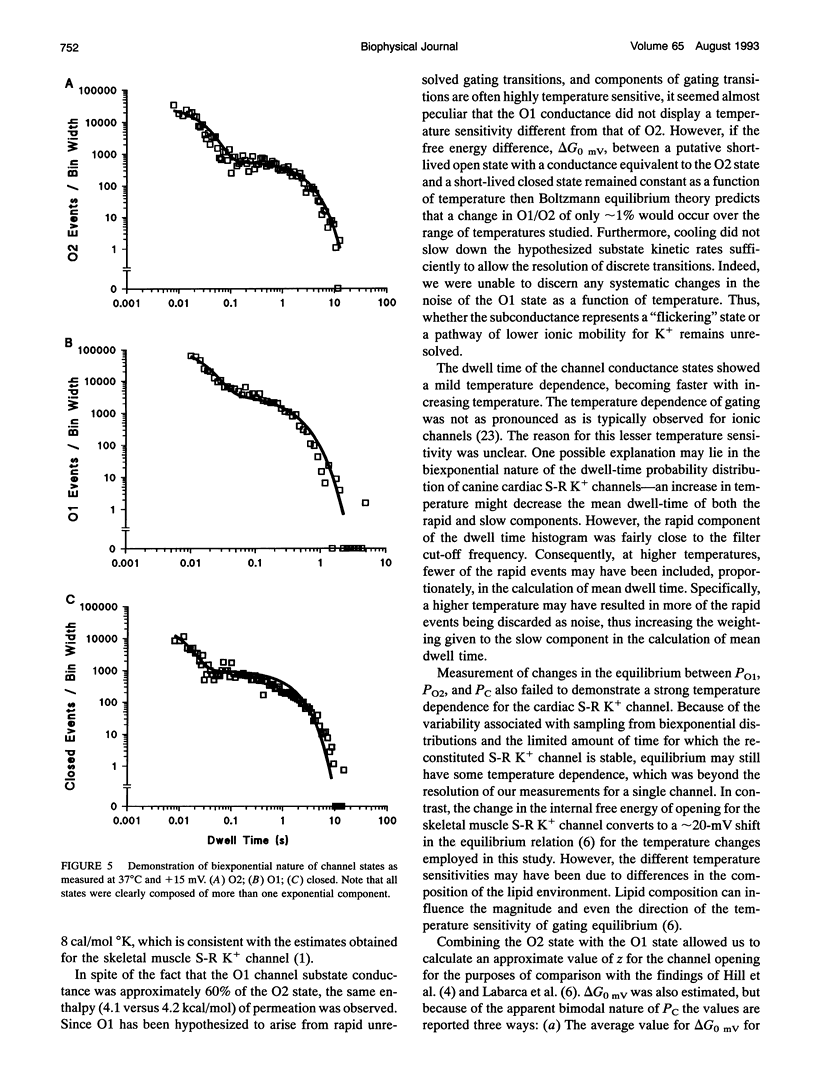
The temperature and voltage dependence of gating and conductance of sarcoplasmic reticulum K+ channels (S-R K+) isolated from adult canine hearts were studied using the reconstituted bilayer technique. Fusion of vesicles from this preparation frequently resulted in the incorporation of a single channel. Only bilayers into which a single S-R K+ channel had fused were studied. The three conductance states of the channel, fully open (O2), substate conductance (O1), and closed (C) were studied as a function of voltage (-50 to +50 mV) and temperature (16 to 37 degrees C). Permeation through the O1 state showed the same temperature dependence as the O2 state corresponding to an enthalpy of permeation of 4.1-4.2 kcal/mol, which is similar to that for K+ diffusion through water. As expected, increased temperature increased the frequency of gating transitions and shortened the average dwell time spent in any conductance state. Over the range of 25 to 37 degrees C, the average dwell time spent in the O1, O2, and C states decreased by 44 +/- 11, 36 +/- 13, and 78 +/- 7% (n = 3 to 4 channels), respectively. The ratio of probabilities between the various conductance states was not strongly temperature sensitive. Analysis of the voltage dependence of this channel was carried out at 37 degrees C and revealed that the dwell times of the O1 and O2 states were voltage insensitive and the probability ratio (PO2:PO1) was approximately 7 and was voltage insensitive. Nonlinear least-squares analysis of dwell times revealed that the closed state was biexponential and was thus composed of a fast (Cf) and a slow (C8) component.Tcf was voltage insensitive with an average value of 5.9 ms, whereas tau c was approximately two orders of magnitude slower and was voltage dependent. The voltage dependence of Cs was described by Tau c (ms) = exp(-0.025-(Vm (mV) - 250)).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bezprozvanny I. B., Benevolensky D. S., Naumov A. P. Potassium channels in aortic microsomes: conductance, selectivity, barium-induced blockage and subconductance states. Biochim Biophys Acta. 1991 Apr 26;1064(1):75–80. doi: 10.1016/0005-2736(91)90413-3. [DOI] [PubMed] [Google Scholar]
- Caillé J., Ildefonse M., Rougier O. Excitation-contraction coupling in skeletal muscle. Prog Biophys Mol Biol. 1985;46(3):185–239. doi: 10.1016/0079-6107(85)90009-4. [DOI] [PubMed] [Google Scholar]
- Campos-de-Carvalho A. C. Potassium concentration influences the gating of K+ channels from sarcoplasmic reticulum. Braz J Med Biol Res. 1989;22(1):107–109. [PubMed] [Google Scholar]
- Coronado R., Rosenberg R. L., Miller C. Ionic selectivity, saturation, and block in a K+-selective channel from sarcoplasmic reticulum. J Gen Physiol. 1980 Oct;76(4):425–446. doi: 10.1085/jgp.76.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman S., Yellen G., Miller C. The K+ channel of sarcoplasmic reticulum. A new look at Cs+ block. Biophys J. 1985 Sep;48(3):477–484. doi: 10.1016/S0006-3495(85)83803-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. A. Ion channel subconductance states. J Membr Biol. 1987;97(1):1–8. doi: 10.1007/BF01869609. [DOI] [PubMed] [Google Scholar]
- Hill J. A., Jr, Coronado R., Strauss H. C. Open-channel subconductance state of K+ channel from cardiac sarcoplasmic reticulum. Am J Physiol. 1990 Jan;258(1 Pt 2):H159–H164. doi: 10.1152/ajpheart.1990.258.1.H159. [DOI] [PubMed] [Google Scholar]
- Hill J. A., Jr, Coronado R., Strauss H. C. Potassium channel of cardiac sarcoplasmic reticulum is a multi-ion channel. Biophys J. 1989 Jan;55(1):35–45. doi: 10.1016/S0006-3495(89)82778-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirashima N., Ishibashi H., Kirino Y. Comparative electrophysiological study of reconstituted giant vesicle preparations of the rabbit skeletal muscle sarcoplasmic reticulum K+ channel. Biochim Biophys Acta. 1991 Aug 26;1067(2):235–240. doi: 10.1016/0005-2736(91)90049-e. [DOI] [PubMed] [Google Scholar]
- Ide T., Morita T., Kawasaki T., Taguchi T., Kasai M. Purification of a K(+)-channel protein of sarcoplasmic reticulum by assaying the channel activity in the planar lipid bilayer system. Biochim Biophys Acta. 1991 Aug 26;1067(2):213–220. doi: 10.1016/0005-2736(91)90046-b. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Structural elements involved in specific K+ channel functions. Annu Rev Physiol. 1992;54:537–555. doi: 10.1146/annurev.ph.54.030192.002541. [DOI] [PubMed] [Google Scholar]
- Jones L. R. Rapid preparation of canine cardiac sarcolemmal vesicles by sucrose flotation. Methods Enzymol. 1988;157:85–91. doi: 10.1016/0076-6879(88)57070-2. [DOI] [PubMed] [Google Scholar]
- Labarca P. P., Miller C. A K+-selective, three-state channel from fragmented sarcoplasmic reticulum of frog leg muscle. J Membr Biol. 1981;61(1):31–38. doi: 10.1007/BF01870750. [DOI] [PubMed] [Google Scholar]
- Labarca P., Coronado R., Miller C. Thermodynamic and kinetic studies of the gating behavior of a K+-selective channel from the sarcoplasmic reticulum membrane. J Gen Physiol. 1980 Oct;76(4):397–324. doi: 10.1085/jgp.76.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q. Y., Lai F. A., Shen W. K., Meissner G., Strauss H. C. Reconstitution of the solubilized cardiac sarcoplasmic reticulum potassium channel. Identification of a putative Mr approximately 80 kDa polypeptide constituent. FEBS Lett. 1991 Oct 7;291(1):13–16. doi: 10.1016/0014-5793(91)81092-m. [DOI] [PubMed] [Google Scholar]
- Liu Q. Y., Strauss H. C. Blockade of cardiac sarcoplasmic reticulum K+ channel by Ca2+: two-binding-site model of blockade. Biophys J. 1991 Jul;60(1):198–203. doi: 10.1016/S0006-3495(91)82043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. 1990: annus mirabilis of potassium channels. Science. 1991 May 24;252(5009):1092–1096. doi: 10.1126/science.252.5009.1092. [DOI] [PubMed] [Google Scholar]
- Oetliker H. An appraisal of the evidence for a sarcoplasmic reticulum membrane potential and its relation to calcium release in skeletal muscle. J Muscle Res Cell Motil. 1982 Sep;3(3):247–272. doi: 10.1007/BF00713037. [DOI] [PubMed] [Google Scholar]
- Richard E. A., Miller C. Steady-state coupling of ion-channel conformations to a transmembrane ion gradient. Science. 1990 Mar 9;247(4947):1208–1210. doi: 10.1126/science.2156338. [DOI] [PubMed] [Google Scholar]
- Shen W. K., Hill J. A., Jr, Rasmusson R., Strauss H. C. Reconstitution of the K channel of cardiac sarcoplasmic reticulum. Prog Clin Biol Res. 1990;334:205–230. [PubMed] [Google Scholar]
- Starmer C. F., Dietz M. A., Grant A. O. Signal discretization: a source of error in histograms of ion channel events. IEEE Trans Biomed Eng. 1986 Jan;33(1):70–73. doi: 10.1109/tbme.1986.325856. [DOI] [PubMed] [Google Scholar]
- Tang J. M., Wang J., Eisenberg R. S. K+-selective channel from sarcoplasmic reticulum of split lobster muscle fibers. J Gen Physiol. 1989 Aug;94(2):261–278. doi: 10.1085/jgp.94.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlins B., Williams A. J. Solubilisation and reconstitution of the rabbit skeletal muscle sarcoplasmic reticulum K+ channel into liposomes suitable for patch clamp studies. Pflugers Arch. 1986 Sep;407(3):341–347. doi: 10.1007/BF00585312. [DOI] [PubMed] [Google Scholar]
- Uehara A., Yasukohchi M., Ogata S., Imanaga I. Activation by intracellular calcium of a potassium channel in cardiac sarcoplasmic reticulum. Pflugers Arch. 1991 Feb;417(6):651–653. doi: 10.1007/BF00372965. [DOI] [PubMed] [Google Scholar]


