Abstract
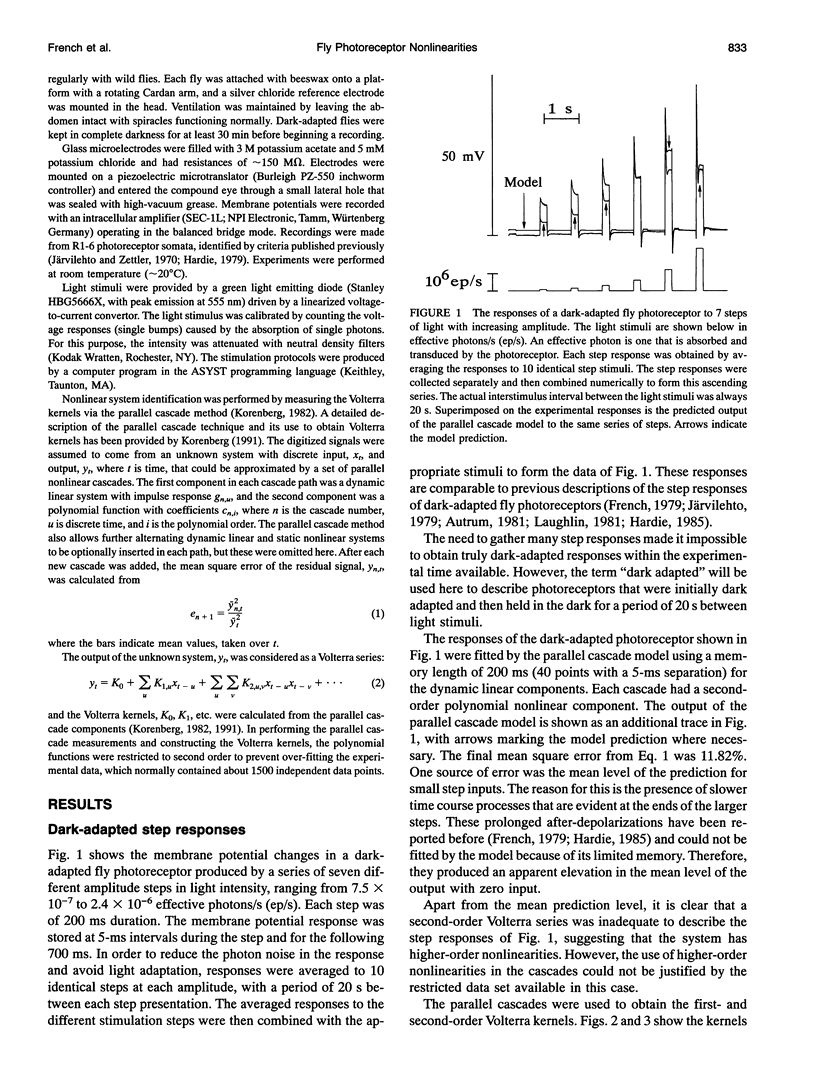
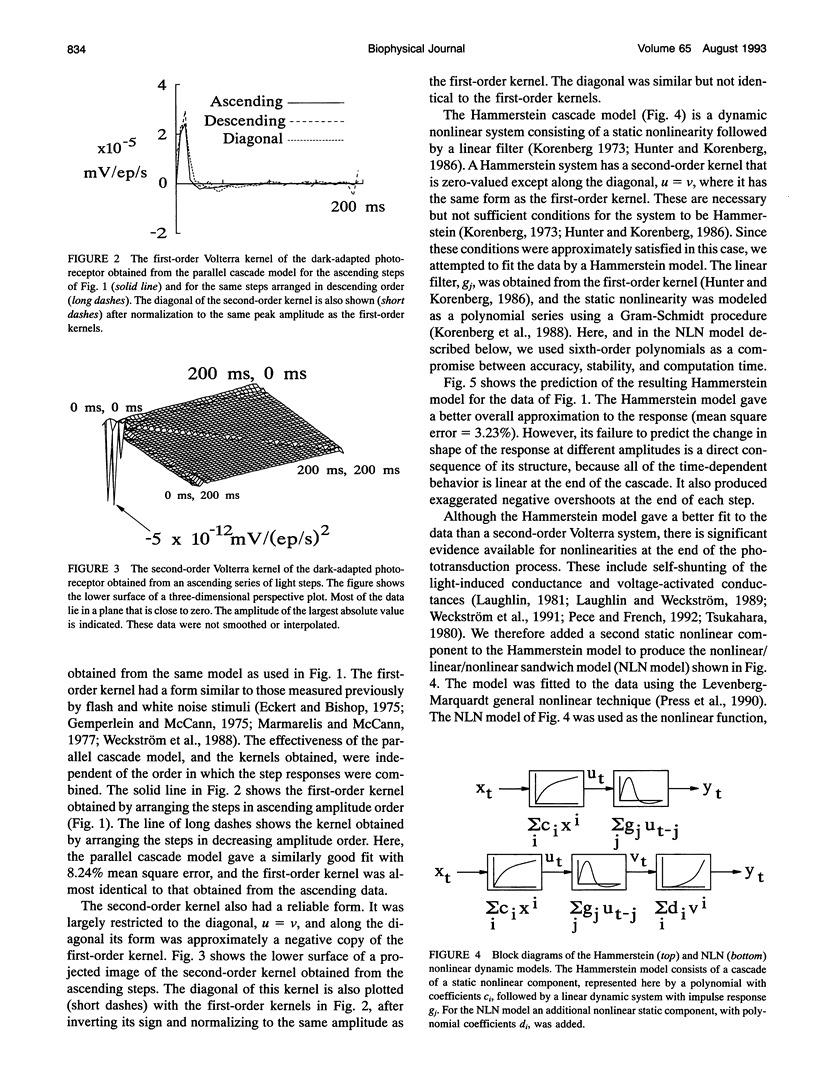
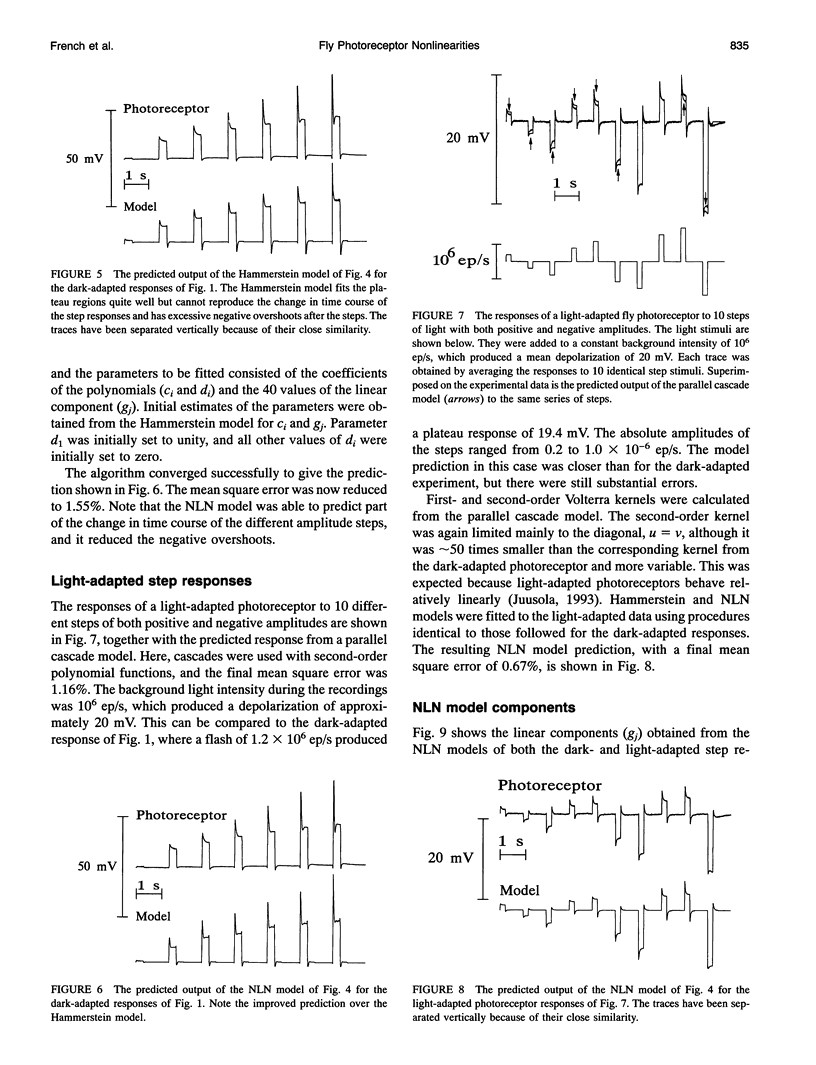
Fly photoreceptor cells were stimulated with steps of light over a wide intensity range. First- and second-order Volterra kernels were then computed from sequences of combined step responses. Diagonal values of the second-order Volterra kernels were much greater than the off-diagonal values, and the diagonal values were roughly proportional to the corresponding first-order kernels, suggesting that the response could be approximated by a static nonlinearity followed by a dynamic linear component (Hammerstein model). The amplitudes of the second-order kernels were much smaller in light-adapted than in dark-adapted photoreceptors. Hammerstein models constructed from the step input/output measurements gave reasonable approximations to the actual photoreceptor responses, with light-adapted responses being relatively better fitted. However, Hammerstein models could not account for several features of the photoreceptor behavior, including the dependence of the step response shape on step amplitude. A model containing an additional static nonlinearity after the dynamic linear component gave significantly better fits to the data. These results indicate that blowfly photoreceptors have a strong early gain control nonlinearity acting before the processes that create the characteristic time course of the response, in addition to the nonlinearities caused by membrane conductances.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Hodgkin A. L., Lamb T. D. The electrical response of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):685–727. doi: 10.1113/jphysiol.1974.sp010731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert H., Bishop L. G. Nonlinear dynamic transfer characteristics of cells in the peripheral visual pathway of flies. I. The retinula cells. Biol Cybern. 1975;17(1):1–6. doi: 10.1007/BF00326704. [DOI] [PubMed] [Google Scholar]
- FUORTES M. G., HODGKIN A. L. CHANGES IN TIME SCALE AND SENSITIVITY IN THE OMMATIDIA OF LIMULUS. J Physiol. 1964 Aug;172:239–263. doi: 10.1113/jphysiol.1964.sp007415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French A. S., Järvilehto M. The dynamic behaviour of photoreceptor cells in the fly in response to random (white noise) stimulation at a range of temperatures. J Physiol. 1978 Jan;274:311–322. doi: 10.1113/jphysiol.1978.sp012149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French A. S. The linear dynamic properties of phototransduction in the fly compound eye. J Physiol. 1980 Nov;308:385–401. doi: 10.1113/jphysiol.1980.sp013477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemperlein R., McCann G. D. A study of the response properties of retinula cells of flies using nonlinear identification theory. Biol Cybern. 1975 Sep 1;19(3):147–158. doi: 10.1007/BF00337254. [DOI] [PubMed] [Google Scholar]
- Grzywacz N. M., Hillman P. Statistical test of linearity of photoreceptor transduction process: Limulus passes, others fail. Proc Natl Acad Sci U S A. 1985 Jan;82(1):232–235. doi: 10.1073/pnas.82.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie R. C., Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992 Apr;8(4):643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- Hardie R. C., Voss D., Pongs O., Laughlin S. B. Novel potassium channels encoded by the Shaker locus in Drosophila photoreceptors. Neuron. 1991 Mar;6(3):477–486. doi: 10.1016/0896-6273(91)90255-x. [DOI] [PubMed] [Google Scholar]
- Howard J., Blakeslee B., Laughlin S. B. The intracellular pupil mechanism and photoreceptor signal: noise ratios in the fly Lucilia cuprina. Proc R Soc Lond B Biol Sci. 1987 Sep 22;231(1265):415–435. doi: 10.1098/rspb.1987.0053. [DOI] [PubMed] [Google Scholar]
- Hunter I. W., Korenberg M. J. The identification of nonlinear biological systems: Wiener and Hammerstein cascade models. Biol Cybern. 1986;55(2-3):135–144. doi: 10.1007/BF00341929. [DOI] [PubMed] [Google Scholar]
- Korenberg M. J., French A. S., Voo S. K. White-noise analysis of nonlinear behavior in an insect sensory neuron: kernel and cascade approaches. Biol Cybern. 1988;58(5):313–320. doi: 10.1007/BF00363940. [DOI] [PubMed] [Google Scholar]
- Korenberg M. J. Identifying nonlinear difference equation and functional expansion representations: the fast orthogonal algorithm. Ann Biomed Eng. 1988;16(1):123–142. doi: 10.1007/BF02367385. [DOI] [PubMed] [Google Scholar]
- Korenberg M. J. Parallel cascade identification and kernel estimation for nonlinear systems. Ann Biomed Eng. 1991;19(4):429–455. doi: 10.1007/BF02584319. [DOI] [PubMed] [Google Scholar]
- Laughlin S. B. The role of sensory adaptation in the retina. J Exp Biol. 1989 Sep;146:39–62. doi: 10.1242/jeb.146.1.39. [DOI] [PubMed] [Google Scholar]
- Marmarelis V. Z., McCann G. D. A family of quasi-white random signals and its optimal use in biological system identification. Part II: application to the photoreceptor of Calliphora erythrocephala. Biol Cybern. 1977 Jul 8;27(1):57–62. doi: 10.1007/BF00357711. [DOI] [PubMed] [Google Scholar]
- Pece A. E., French A. S., Korenberg M. J., Kuster J. E. Nonlinear mechanisms for gain adaptation in locust photoreceptors. Biophys J. 1990 Apr;57(4):733–743. doi: 10.1016/S0006-3495(90)82594-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter R. B. Sinusoidal and delta function responses of visual cells of the Limulus eye. J Gen Physiol. 1966 Jan;49(3):565–593. doi: 10.1085/jgp.49.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H. M. White-noise analysis in neurophysiology. Physiol Rev. 1992 Apr;72(2):491–505. doi: 10.1152/physrev.1992.72.2.491. [DOI] [PubMed] [Google Scholar]
- Tsukahara Y. Effect of intracellular injection of EGTA and tetraethylammonium chloride on the receptor potential of locust photoreceptors. Photochem Photobiol. 1980 Oct;32(4):509–514. doi: 10.1111/j.1751-1097.1980.tb03796.x. [DOI] [PubMed] [Google Scholar]
- Weckström M., Hardie R. C., Laughlin S. B. Voltage-activated potassium channels in blowfly photoreceptors and their role in light adaptation. J Physiol. 1991;440:635–657. doi: 10.1113/jphysiol.1991.sp018729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckström M., Kouvalainen E., Järvilehto M. Non-linearities in response properties of insect visual cells: an analysis in time and frequency domain. Acta Physiol Scand. 1988 Jan;132(1):103–113. doi: 10.1111/j.1748-1716.1988.tb08303.x. [DOI] [PubMed] [Google Scholar]


