Abstract
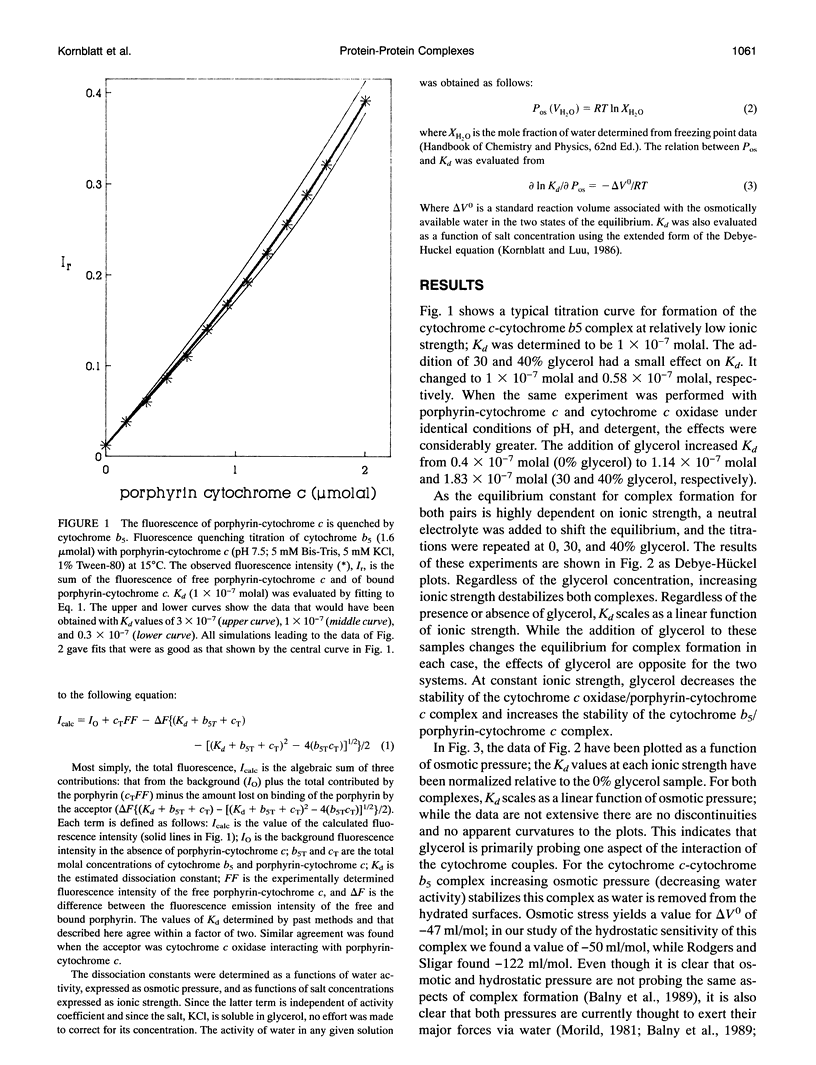
We have analyzed the stability of the cytochrome c-cytochrome b5 and cytochrome c-cytochrome c oxidase complexes as a function of solvent stress. High concentrations of glycerol were used to displace the two equilibria. Glycerol promotes complex formation between cytochrome c and cytochrome b5 but inhibits that between cytochrome c and cytochrome c oxidase. The results with cytochrome b5 and cytochrome c were expected; the association of this complex is largely entropy driven. Our interpretation is that the cytochrome c-cytochrome b5 complex excludes water. The results with the cytochrome c oxidase and cytochrome c couple were not expected. We interpret them to mean that either glycerol is binding to the oxidase, thereby displacing the cytochrome c, or that water is required at this protein-protein interface. A requirement for substantial quantities of water at the interface of some protein complexes is logical but has been reported only once.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. P., Feher G., Yeates T. O., Komiya H., Rees D. C. Structure of the reaction center from Rhodobacter sphaeroides R-26: the protein subunits. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6162–6166. doi: 10.1073/pnas.84.17.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amit A. G., Mariuzza R. A., Phillips S. E., Poljak R. J. Three-dimensional structure of an antigen-antibody complex at 2.8 A resolution. Science. 1986 Aug 15;233(4765):747–753. doi: 10.1126/science.2426778. [DOI] [PubMed] [Google Scholar]
- Arakawa T., Timasheff S. N. Stabilization of protein structure by sugars. Biochemistry. 1982 Dec 7;21(25):6536–6544. doi: 10.1021/bi00268a033. [DOI] [PubMed] [Google Scholar]
- Chothia C., Janin J. Principles of protein-protein recognition. Nature. 1975 Aug 28;256(5520):705–708. doi: 10.1038/256705a0. [DOI] [PubMed] [Google Scholar]
- Colombo M. F., Rau D. C., Parsegian V. A. Protein solvation in allosteric regulation: a water effect on hemoglobin. Science. 1992 May 1;256(5057):655–659. doi: 10.1126/science.1585178. [DOI] [PubMed] [Google Scholar]
- Daopin S., Piez K. A., Ogawa Y., Davies D. R. Crystal structure of transforming growth factor-beta 2: an unusual fold for the superfamily. Science. 1992 Jul 17;257(5068):369–373. doi: 10.1126/science.1631557. [DOI] [PubMed] [Google Scholar]
- Davies D. R., Sheriff S., Padlan E. A. Antibody-antigen complexes. J Biol Chem. 1988 Aug 5;263(22):10541–10544. [PubMed] [Google Scholar]
- Ferguson-Miller S., Brautigan D. L., Margoliash E. Definition of cytochrome c binding domains by chemical modification. III. Kinetics of reaction of carboxydinitrophenyl cytochromes c with cytochrome c oxidase. J Biol Chem. 1978 Jan 10;253(1):149–159. [PubMed] [Google Scholar]
- Greene R. F., Jr, Pace C. N. Urea and guanidine hydrochloride denaturation of ribonuclease, lysozyme, alpha-chymotrypsin, and beta-lactoglobulin. J Biol Chem. 1974 Sep 10;249(17):5388–5393. [PubMed] [Google Scholar]
- Kitchen D. B., Reed L. H., Levy R. M. Molecular dynamics simulation of solvated protein at high pressure. Biochemistry. 1992 Oct 20;31(41):10083–10093. doi: 10.1021/bi00156a031. [DOI] [PubMed] [Google Scholar]
- Kornblatt J. A., Baraff G. A., Williams G. R. The reaction of N-ethylmaleimide with different forms of cytochrome oxidase. Can J Biochem. 1973 Oct;51(10):1417–1427. doi: 10.1139/o73-186. [DOI] [PubMed] [Google Scholar]
- Kornblatt J. A., English A. M., Hui Bon Hoa G. The effects of pressure on yeast cytochrome c peroxidase. Eur J Biochem. 1986 Aug 15;159(1):39–43. doi: 10.1111/j.1432-1033.1986.tb09830.x. [DOI] [PubMed] [Google Scholar]
- Kornblatt J. A., Hoa G. H. A nontraditional role for water in the cytochrome c oxidase reaction. Biochemistry. 1990 Oct 9;29(40):9370–9376. doi: 10.1021/bi00492a010. [DOI] [PubMed] [Google Scholar]
- Kornblatt J. A., Hui Bon Hoa G., English A. M. Volume changes associated with cytochrome c oxidase-porphyrin cytochrome c equilibrium. Biochemistry. 1984 Dec 4;23(25):5906–5911. doi: 10.1021/bi00320a003. [DOI] [PubMed] [Google Scholar]
- Kornblatt J. A., Laberge M. Porphyrin cytochrome c. pH effects and interaction with cytochrome-c oxidase. Eur J Biochem. 1988 Aug 15;175(3):475–479. doi: 10.1111/j.1432-1033.1988.tb14219.x. [DOI] [PubMed] [Google Scholar]
- Kornblatt J. A., Luu H. A. The interactions of cytochrome c and porphyrin cytochrome c with cytochrome c oxidase. The resting, reduced and pulsed enzymes. Eur J Biochem. 1986 Sep 1;159(2):407–413. doi: 10.1111/j.1432-1033.1986.tb09883.x. [DOI] [PubMed] [Google Scholar]
- Kornblatt J. A., Theodorakis J., Hoa G. H., Margoliash E. Cytochrome c and cytochrome c oxidase interactions: the effects of ionic strength and hydrostatic pressure studied with site-specific modifications of cytochrome c. Biochem Cell Biol. 1992 Jul;70(7):539–547. doi: 10.1139/o92-084. [DOI] [PubMed] [Google Scholar]
- Kundrot C. E., Richards F. M. Effect of hydrostatic pressure on the solvent in crystals of hen egg-white lysozyme. J Mol Biol. 1988 Mar 20;200(2):401–410. doi: 10.1016/0022-2836(88)90249-5. [DOI] [PubMed] [Google Scholar]
- Matsumura M., Wozniak J. A., Sun D. P., Matthews B. W. Structural studies of mutants of T4 lysozyme that alter hydrophobic stabilization. J Biol Chem. 1989 Sep 25;264(27):16059–16066. [PubMed] [Google Scholar]
- Mauk M. R., Mauk A. G., Weber P. C., Matthew J. B. Electrostatic analysis of the interaction of cytochrome c with native and dimethyl ester heme substituted cytochrome b5. Biochemistry. 1986 Nov 4;25(22):7085–7091. doi: 10.1021/bi00370a049. [DOI] [PubMed] [Google Scholar]
- Mauk M. R., Reid L. S., Mauk A. G. Spectrophotometric analysis of the interaction between cytochrome b5 and cytochrome c. Biochemistry. 1982 Apr 13;21(8):1843–1846. doi: 10.1021/bi00537a021. [DOI] [PubMed] [Google Scholar]
- Miller S., Lesk A. M., Janin J., Chothia C. The accessible surface area and stability of oligomeric proteins. 1987 Aug 27-Sep 2Nature. 328(6133):834–836. doi: 10.1038/328834a0. [DOI] [PubMed] [Google Scholar]
- Morild E. The theory of pressure effects on enzymes. Adv Protein Chem. 1981;34:93–166. doi: 10.1016/s0065-3233(08)60519-7. [DOI] [PubMed] [Google Scholar]
- Northrup S. H., Boles J. O., Reynolds J. C. Brownian dynamics of cytochrome c and cytochrome c peroxidase association. Science. 1988 Jul 1;241(4861):67–70. doi: 10.1126/science.2838904. [DOI] [PubMed] [Google Scholar]
- Northrup S. H., Erickson H. P. Kinetics of protein-protein association explained by Brownian dynamics computer simulation. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3338–3342. doi: 10.1073/pnas.89.8.3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski Z., Schevitz R. W., Zhang R. G., Lawson C. L., Joachimiak A., Marmorstein R. Q., Luisi B. F., Sigler P. B. Crystal structure of trp repressor/operator complex at atomic resolution. Nature. 1988 Sep 22;335(6188):321–329. doi: 10.1038/335321a0. [DOI] [PubMed] [Google Scholar]
- Parsegian V. A., Rand R. P., Fuller N. L., Rau D. C. Osmotic stress for the direct measurement of intermolecular forces. Methods Enzymol. 1986;127:400–416. doi: 10.1016/0076-6879(86)27032-9. [DOI] [PubMed] [Google Scholar]
- Pelletier H., Kraut J. Crystal structure of a complex between electron transfer partners, cytochrome c peroxidase and cytochrome c. Science. 1992 Dec 11;258(5089):1748–1755. doi: 10.1126/science.1334573. [DOI] [PubMed] [Google Scholar]
- Poulos T. L., Kraut J. A hypothetical model of the cytochrome c peroxidase . cytochrome c electron transfer complex. J Biol Chem. 1980 Nov 10;255(21):10322–10330. [PubMed] [Google Scholar]
- Poulos T. L., Mauk A. G. Models for the complexes formed between cytochrome b5 and the subunits of methemoglobin. J Biol Chem. 1983 Jun 25;258(12):7369–7373. [PubMed] [Google Scholar]
- Prouty M. S., Schechter A. N., Parsegian V. A. Chemical potential measurements of deoxyhemoglobin S polymerization. Determination of the phase diagram of an assembling protein. J Mol Biol. 1985 Aug 5;184(3):517–528. doi: 10.1016/0022-2836(85)90298-0. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Fuller N. L., Butko P., Francis G., Nicholls P. Measured change in protein solvation with substrate binding and turnover. Biochemistry. 1993 Jun 15;32(23):5925–5929. doi: 10.1021/bi00074a001. [DOI] [PubMed] [Google Scholar]
- Rodgers K. K., Pochapsky T. C., Sligar S. G. Probing the mechanisms of macromolecular recognition: the cytochrome b5-cytochrome c complex. Science. 1988 Jun 17;240(4859):1657–1659. doi: 10.1126/science.2837825. [DOI] [PubMed] [Google Scholar]
- Rodgers K. K., Sligar S. G. Mapping electrostatic interactions in macromolecular associations. J Mol Biol. 1991 Oct 20;221(4):1453–1460. doi: 10.1016/0022-2836(91)90945-3. [DOI] [PubMed] [Google Scholar]
- Salemme F. R. An hypothetical structure for an intermolecular electron transfer complex of cytochromes c and b5. J Mol Biol. 1976 Apr 15;102(3):563–568. doi: 10.1016/0022-2836(76)90334-x. [DOI] [PubMed] [Google Scholar]
- Schlunegger M. P., Grütter M. G. An unusual feature revealed by the crystal structure at 2.2 A resolution of human transforming growth factor-beta 2. Nature. 1992 Jul 30;358(6385):430–434. doi: 10.1038/358430a0. [DOI] [PubMed] [Google Scholar]
- Timasheff S. N. Water as ligand: preferential binding and exclusion of denaturants in protein unfolding. Biochemistry. 1992 Oct 20;31(41):9857–9864. doi: 10.1021/bi00156a001. [DOI] [PubMed] [Google Scholar]
- WYMAN J., Jr LINKED FUNCTIONS AND RECIPROCAL EFFECTS IN HEMOGLOBIN: A SECOND LOOK. Adv Protein Chem. 1964;19:223–286. doi: 10.1016/s0065-3233(08)60190-4. [DOI] [PubMed] [Google Scholar]
- Wendoloski J. J., Matthew J. B., Weber P. C., Salemme F. R. Molecular dynamics of a cytochrome c-cytochrome b5 electron transfer complex. Science. 1987 Nov 6;238(4828):794–797. doi: 10.1126/science.2823387. [DOI] [PubMed] [Google Scholar]
- de Vlieg J., Berendsen H. J., van Gunsteren W. F. An NMR-based molecular dynamics simulation of the interaction of the lac repressor headpiece and its operator in aqueous solution. Proteins. 1989;6(2):104–127. doi: 10.1002/prot.340060203. [DOI] [PubMed] [Google Scholar]


