Abstract
The strength of interactions between predators and their prey (interaction strength) varies enormously among species within ecological communities. Understanding the community-wide distribution of interaction strengths is vital, given that communities dominated by weak interactions may be more stable and resistant to invasion. In the oceans, previous studies have reported log-normal distributions of per capita interaction strength. We estimated the distribution of predator–prey interaction strengths within a subtidal speciose herbivore community (45 species). Laboratory experiments were used to determine maximum per capita interaction strengths for eight species of herbivores (including amphipods, isopods, gastropods, and sea urchins) that graze on giant kelp (Macrocystis pyrifera) microscopic stages. We found that maximum per capita interaction strength saturated as a function of individual herbivore biomass, likely caused by predator/prey size thresholds. Incorporating this nonlinearity, we predicted maximum per capita interaction strength for the remaining herbivore species. The resulting distribution of per capita interaction strengths was bimodal, in striking contrast to previous reports from other communities. Although small herbivores often had per capita interaction strengths similar to larger herbivores, their tendency to have greater densities in the field increased their potential impact as grazers. These results indicate that previous conclusions about the distributions of interaction strength in natural communities are not general, and that intermediate-sized predators can under realistic circumstances represent the most effective consumers in natural communities.
The strength of interactions among species within natural communities varies greatly (1–3). Understanding how these strengths are distributed in ecological communities is crucial if we are to understand the keystone role that many predators play in the structure and dynamics of these communities (4, 5). Theoretical work suggests that natural communities dominated by weak interactions among species are more stable and resistant to invasion than those dominated by strong interactions (6–8). Weak interactions may dampen oscillations between predators and prey, likely preventing chaotic dynamics, and therefore decreasing the probability of extinction and invasion. Natural communities are composed of hundreds or thousands of species, and empirical tests of this hypothesis are challenging due in part to difficulties in quantifying the distribution of strengths of interaction among species within communities. The strength of the interaction between predators and their prey is defined as the absolute, or more commonly, the proportional effect of one species on another as a function of time (1–3, 9–11), usually referred to as interaction strength. Community-wide distributions of interaction strength are vital to understanding the relative community role of interacting species and trophic groups and the consequences of variation in their abundance, and can be important management and conservation tools (6, 7). The few empirical studies that have addressed the distribution of interaction strength across multiple species have suggested that ecological communities may in fact be composed of only a few strong interactions embedded in a matrix of weak interactions (1, 2, 12, 13). Determining whether this dominance of weak interactions is general for entire communities has been hindered by the intensive experimental work needed to empirically estimate interaction strength. In this study we chose the best-studied subtidal community in the world to take advantage of decades of well-documented knowledge of natural history (14–18), and chose to focus on the consumption of key species of the community.
Efforts to determine patterns of interaction strength in ecological communities have encountered additional problems in defining interaction strength. Some studies have described the distribution of interaction strength by assigning species into two discrete groups, “strong” and “weak” interactors. A discrete distinction between strong and weak interactors, however, is not straightforward. Paine (1) suggested that a strong interactor is “a species capable of preventing the development of a monoculture, or destroying one already established,” whereas Berlow (11) defined weak effects as occurring “when the removal or addition of a species failed to cause a statistically discernible mean change in the abundance of a target species.” A problem with such definitions is that species shown to have the potential for strong effects (sensu Paine; ref. 1) do not always impart such effects because of variability in their density (5, 19), and species considered weak interactors might impart stronger than expected ecological effects in cases of high density (11). Furthermore, theoretical work suggests that the removal of a supposedly weak interactor can sometimes drive other species to extinction (7). Last, the ecological significance of any community change does not always follow patterns of statistical significance. It may therefore be difficult to assign a specific threshold for discretely distinguishing between strong and weak interactors (1, 3, 20), in which case, the actual distribution of per capita interaction strength within communities may be the best way to explore the relative roles of predatory interactions in structuring communities.
Is it possible to estimate the strength of pairwise interactions for describing the distribution of interaction strength in an ecological community? Per capita indices have been developed for estimating the strength of interactions for species pairs (21) by using experimental (1, 9) and observational (2) methods. Estimates of per capita interaction strength, however, are context-specific, and single values may not describe the range of complex interactions between two species (3, 6). Variability in predator and prey size and density may cause subsequent variability in per capita interaction strength. Moreover, the strength of predator–prey interactions can also vary in the presence of competitors and/or higher-order predators. A solution to these problems, as suggested by Ruesink (3), is to focus on the limits of potential trophic effects to define interaction strength. As such, studies of the community-wide distribution of interaction strength may be best approached by examining the maximum per capita interaction strength for all pairwise predator–prey interactions in a trophic guild. Maximum per capita interaction strength can be estimated by using an experimental design in which predators are given only a single prey species population without recruitment (i.e., a nongrowing prey population).
Here we present an experimental estimation of the distribution of predator–prey interaction strengths within a California giant kelp community, and discuss the possible causes and ecological consequences of this distribution. The structure of this community is very dynamic in space and time, driven primarily by fluctuations in the abundance of the dominant canopy-forming giant kelp, Macrocystis pyrifera, and patches of understory kelps and turf algae (14–18). Recent studies have shown that recruitment is a key factor in regulating M. pyrifera population dynamics (18, 22), and thus, in determining the structure and persistence of giant kelp communities (16, 18). Adult M. pyrifera macroscopic sporophytes release microscopic haploid zoospores into the plankton that settle onto the bottom within days and, after germination, develop into microscopic male and female benthic gametophytes. Given adequate light and nutrients, fertilization occurs, and microscopic sporophytes develop and grow to macroscopic size (up to 30 m). Sporophytes are most vulnerable to herbivory at small sizes (15, 16), and grazing on M. pyrifera microscopic sporophytes may play a fundamental role in regulating community dynamics. Our goal was to study the distribution of per capita interaction strength in the species-rich herbivore assemblage characteristic of M. pyrifera forests, its combined importance on the survival of M. pyrifera microscopic sporophytes, and ultimately the recruitment of this dominant habitat-forming species.
Methods
Laboratory experiments were used to estimate the effects of herbivory on survival of M. pyrifera microscopic sporophytes. M. pyrifera sporogenous blades (sporophylls) were collected from a depth of 15 m in the Point Loma kelp forest, San Diego, and transported to the lab in an insulated container within 3 h of collection. Sporophylls were rinsed in seawater that had been passed through a 0.2-μm-pore filter seawater and were desiccated between moist paper towels in the dark for 1 h at 5°C. Zoospore release was stimulated by immersing sporophylls in 15°C filtered seawater for 15 min, after which sporophylls were discarded and the concentration of the resultant zoospore solution was quantified by using a hemacytometer. The zoospore solution was diluted to 103 spores per ml with 0.2-μm-filtered seawater and poured into trays to a depth of 2 cm. Microscope slides lined the bottom of the trays. Zoospores were allowed to settle at 15°C under constant irradiance (25–30 μE⋅m−2⋅s−1) for 24 h, after which the water was replaced with continuous-flow 0.2-μm-filtered seawater at 12°C. The resulting germlings were cultured under the same irradiance, but on a 12-h/12-h photoperiod. Cultures were monitored until the appearance of microscopic sporophytes with ≥8 cells (≈100 μm total length). At this time the mean density (±SE) of sporophytes on the slides was 8.40 ± 0.35 per mm2.
Eight herbivore species were chosen for feeding trials to empirically estimate per capita interaction strength: the amphipod Ampithoe humeralis, the isopod Idotea resecata, the gastropods Lithopoma undosum, Mitrella carinata, and Norrisia norrisi, and the sea urchins Lytechinus anamesus, Strongylocentrotus franciscanus, and Strongylocentrotus purpuratus. All of these species feed on M. pyrifera and were chosen among the most abundant species to represent the major herbivore guilds in Californian M. pyrifera forests. Different biomasses and natural population densities of the herbivores required three different sized aquaria to be used for the feeding trails: (i) 22 × 20 × 6 cm for A. humeralis, I. resecata, M. carinata, and L. anamesus; (ii) 50 × 18 × 15 cm for small S. purpuratus; and (iii) 50 × 25 × 15 cm for L. undosum, N. norrisi, S. franciscanus, and large S. purpuratus. For each species, four aquaria of appropriate size were used as experimental treatments containing only that species, and four size-matched aquaria were used as controls (without herbivores). Densities of herbivores in the experimental treatments were chosen to represent realistic densities as found in the field or higher: 95 A. humeralis per m2; 48 I. resecata per m2; 238 M. carinata per m2; 48 L. anamesus per m2; 8 L. undosum per m2; 16 N. norrisi per m2; 8 S. franciscanus per m2; 24 small (1.9–2.1 cm) S. purpuratus per m2; and 11 large (4.8–5.0 cm) S. purpuratus per m2. Sixteen microscope slides with cultured M. pyrifera microscopic sporophytes were then randomly assigned to each experimental and control aquarium to provide for a consistent initial density of “prey” across treatments and controls (previous studies suggest that varying the initial density does not affect the proportional outcome of grazing). The density of sporophytes was determined on four slides per aquarium at the beginning of the experiment and at 1, 2, and 3 weeks afterward by counting all sporophytes within 10 randomly located fields of view by using a microscope. No herbivore species was able to remove 100% of M. pyrifera microscopic sporophytes by the end of the study. Furthermore, consumption rates did not increase over the 3-week period, suggesting that the herbivores were not starved.
We estimated per capita interaction strength by using the dynamic index of Wootton (2) because M. pyrifera sporophyte mortality was exponential over time. This index gives exact estimates of per capita interaction strength under positive exponential prey growth or negative exponential prey mortality (23):
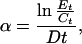 |
1 |
where α is per capita interaction strength, Et and Ct are sporophyte densities in the presence and absence of herbivores at the end of a time interval t, and D is the density of herbivores. For each herbivore species at each time point, we used sporophyte densities averaged across the four control aquaria to estimate Ct, and independently estimated values for treatment aquaria.
We hypothesized that per capita interaction strength will reach an asymptote as individual herbivores increase in size (biomass) because of a decrease in the efficiency of larger or coarser feeding structures for removing M. pyrifera microscopic sporophytes. We therefore used a Michaelis–Menten function to describe the relationship between per capita interaction strength (y; α for each herbivore species averaged across the three time intervals) and herbivore biomass (x; wet mass in g for each of the herbivore species averaged for individuals used in the feeding trials):
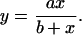 |
2 |
The parameter a is the per capita interaction strength at which grazing saturates (i.e., the maximum realized per capita interaction strength), and b represents the rate at which the function approaches saturation (i.e., half-saturation constant).
We used the parameterized form of Eq. 2 to predict per capita interaction strength from individual biomasses for other herbivores associated with M. pyrifera. Individual biomass estimates were obtained for adults of 37 additional herbivore species that are likely to feed directly on M. pyrifera microscopic sporophytes (18 amphipods, 2 isopods, 16 gastropods, and 1 sea urchin). We did not include herbivorous fishes because the taxa that prey on M. pyrifera (e.g., Hermosilla azurea, Hypsypops rubicundus, Girella nigricans, Medialuna californiensis; refs. 24 and 25) are thought to feed only on macroscopic sporophytes. Abalones (Haliotis spp.) were excluded because they feed on M. pyrifera drift. We also did not include four species of chitons (Cryptochiton stelleri, Ischnochiton intersinctus, Lepidozona cooperi, Tonicella lineata) and one herbivorous asteroid (Asterina miniata) because representatives of polyplacophora and asteroidea were not included in the feeding trials, and therefore their per capita interaction strength may not be functionally predicted by the parameterized version of Eq. 2. The use of adult biomasses, and the fact that herbivores in experimental feeding trials were offered only a single-species prey resource, were consistent with our goal of estimating maximum per capita interaction strength for each species in the analyses.
Finally, we estimated potential population interaction strengths for each herbivore species by multiplying maximum per capita interaction strength by field estimates of benthic herbivore density (number of herbivores per m2) from California giant kelp forests. Herbivore densities were taken from our personal data and the literature for 40 of the original 45 species, individually or in species groups. This method resulted in an estimate of the proportion of M. pyrifera microscopic sporophytes removed daily by each herbivore species under realistic population densities; the density data were taken at different times and locations and are not meant to represent species composition and abundance in any given M. pyrifera forest. Although herbivores may exhibit nonlinear predatory functional responses, the assumption of a linear response provides reasonable estimates of species impact when species-specific functional responses are not known (2). Our understanding is that herbivore densities are highly variable in space and time, and our goal was simply to explore the potential community-wide consequences of the pattern of distribution of per capita interaction strength.
Results and Discussion
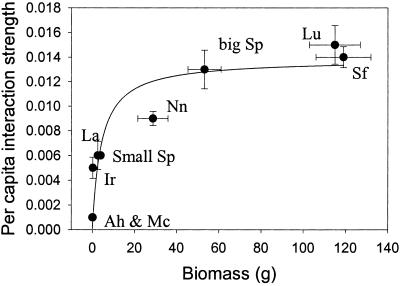
Per capita interaction strengths of the eight experimental herbivore species ranged from 0.06% to 1.5% of M. pyrifera microscopic sporophytes removed per m2 of substrate per herbivore per day (Fig. 1). A. humeralis and M. carinata exhibited the weakest per capita effects (−0.0007 and −0.0006, respectively), whereas L. undosum, S. franciscanus, and S. purpuratus had the strongest per capita effects (−0.0154, −0.0141, and −0.0132, respectively). The nonlinear relationship between per capita interaction strength and individual herbivore biomass had a fit (adjusted r2) of 88% (Fig. 1), which was better than the 77% fit of a linear model. This finding supports the hypothesis that the proportion of M. pyrifera microscopic sporophytes removed per herbivore saturates as herbivore size increases. The Michaelis–Menten saturation curve estimated a maximum (±SE) per capita interaction strength for these invertebrate herbivores of 1.38% ± 0.14% (Fig. 1). Note that the 1.5% estimate for L. undosum, the species with the highest per capita interaction strength, falls within this limit. This observation suggests that the maximum area within a 1-m2 plot that can be efficiently grazed for M. pyrifera microscopic sporophytes by a single herbivore, regardless of taxon, is 1.38% per day. We propose that this asymptotic relationship between per capita interaction strength and herbivore size is caused by increasing predator/prey size ratios that limit the efficiency at which herbivores can remove the microscopic sporophytes. In fact, it is likely that further increases in herbivore size beyond an unknown threshold will yield grazers with feeding structures too coarse to consume microscopic sporophytes. Microscopic sporophytes may therefore occupy a size refuge below which grazing by large herbivores is ineffective. These data suggest that such prey size refuges may yield nonlinear saturating relationships between predator body size and per capita effects on prey.
Figure 1.
Relationship between the biomass of individual herbivores and maximum per capita interaction strength on M. pyrifera microscopic sporophytes. The curve was fit to the data by using a Michaelis–Menten function [Eq. 2; y = 0.0138 x/(4.23 + x)]. Data are means ± 95% confidence intervals. Variability in per capita interaction strength was estimated for each species by bootstrapping the data set 1,000 times (1). Ah, A. humeralis; Ir, I. resecata; Lu, L. undosum; Mc, M. carinata; Nn, N. norrisi; La, L. anamesus; Sf, S. franciscanus; Sp, S. purpuratus.
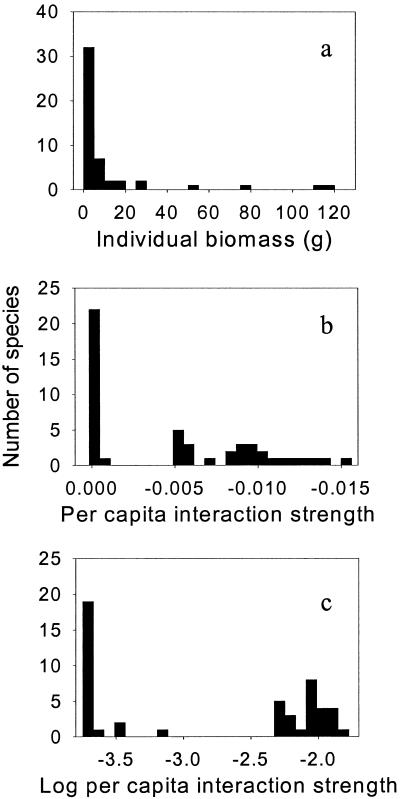
The distribution of average adult individual biomass for the overall assemblage of 45 herbivore species was highly skewed because of the presence of many small amphipod and isopod taxa in the community, and the relative rarity of large gastropod and sea urchin taxa (Fig. 2a). The predicted distribution of herbivore per capita interaction strengths was more bimodal (Fig. 2b). Greater than half of the herbivore species had predicted per capita interaction strengths <0.1%, whereas predicted per capita interaction strengths for the stronger group ranged from 0.5% to 1.5%. As such, there was an apparent break between the weakest herbivore–kelp interactions in this community, the amphipods and isopods, and the strongest ones, the gastropods and sea urchins. This distinction was even clearer given the logarithms of the absolute values of per capita interaction strength (Fig. 2c). The benefit of using logarithms is that distinctions between stronger and weaker interactions can be made on the scale of orders of magnitude. In this herbivore assemblage, the median strength of the stronger (0.90%) and weaker (0.02%) groups of interactions differed by >1 order of magnitude, providing a potential empirical threshold for separating the strongest and weakest interactions in this community. The existence of this threshold may actually be unique given that the bimodal distribution of per capita interaction strengths is in striking contrast to the log-normal distributions previously reported (1, 2, 12). This finding suggests the existence of subsystems within the community (26) composed of species with similar per capita interaction strength. Furthermore, unlike these other studies, the number of strong herbivore–kelp interactors is roughly equivalent to that of the weak interactors, suggesting that the previous observation that strong species interactions are rare in natural communities is not general.
Figure 2.
Frequency distributions of average adult biomass (g) of taxa considered grazers on M. pyrifera microscopic sporophytes (a); daily per capita interaction strength (b); and logarithm of daily per capita interaction strength (c). Data are for the 8 experimental species and 37 species whose individual biomasses could be determined from the literature.
Given single-point estimates of present-day adult herbivore biomasses and abundance, the maximum combined impact of herbivores on kelp recruitment was estimated to be 28.7% of M. pyrifera microscopic sporophytes removed per m2 per day (Table 1). Most M. pyrifera forests, however, have only subsets of these species in the community, herbivores that feed preferentially on other algal species, or herbivores that feed at lower rates because of the presence of predators, and therefore M. pyrifera microscopic sporophytes are likely to experience even less of a grazing impact. This observation suggests that although herbivory may not be trivial, an herbivore assemblage with similar biomass distributions and abundances is likely not capable of inhibiting giant kelp recruitment. In many situations, however, in situ herbivore densities can be observed that are much higher than those presented in Table 1, with drastic implications for the implied impact of grazing on kelp recruitment. For example, although the density of L. anamesus is generally low in California M. pyrifera forests (Table 1), very high L. anamesus densities (60 per m2) have been observed in localized regions of the San Onofre kelp forest in California (31). Our experimental estimates of daily per capita interaction strength predict that such L. anamesus aggregations alone can potentially remove 34.2% of M. pyrifera microscopic sporophytes per m2 day. Population explosions of amphipods, isopods, and sea urchins have also been observed (32, 33), with catastrophic impacts on kelp recruitment. This finding supports the idea that the realized impact of an herbivore depends not only on its per capita interaction strength, but also on its density.
Table 1.
Average adult biomass (g wet mass), per capita interaction strength (PCIS, % removed per m2 per herbivore per day), average density (individuals per m2), and absolute impact of each taxon (% removed per m2 per day)
| Taxa | Biomass | PCIS | Density | Impact |
|---|---|---|---|---|
| Amphipoda | ||||
| A. humeralis | 0.22 | −0.0007 | 1.250 | −0.0009 |
| Other Gammaridean amphipods∗ | 0.08 | −0.0003 | 22.12 | −0.0066 |
| Isopoda | ||||
| I. resecata | 0.24 | −0.0051 | 0.600 | −0.0031 |
| Limnoria algarum | 0.11 | −0.0004 | 7.110 | −0.0025 |
| Paracerceis cordata | 0.10 | −0.0003 | 1.400 | −0.0005 |
| Gastropoda | ||||
| Calliostoma annulatum | 9.56 | −0.0097 | 0.25029 | −0.0024 |
| Calliostoma canaliculatum | 12.00 | −0.0104 | 0.16029 | −0.0016 |
| Calliostoma ligulatum | 7.60 | −0.0090 | 0.46029 | −0.0041 |
| Diodora arnoldi | 6.03 | −0.0082 | 0.17529 | −0.0014 |
| Lithopoma gibberosum | 29.56 | −0.0122 | 0.20029 | −0.0024 |
| L. undosum | 110.00 | −0.0154 | 0.180 | −0.0028 |
| M. carinata | 4.16 | −0.0006 | 0.630 | −0.0004 |
| N. norrisi | 29.53 | −0.0090 | 0.023 | −0.0002 |
| Tegula aureotincta | 15.09 | −0.0109 | 1.70030 | −0.0185 |
| Tegula brunnea | 9.60 | −0.0097 | 1.50029 | −0.0146 |
| Tegula eiseni | 7.597 | −0.0090 | 21.9030 | −0.1971 |
| Tegula montereyi | 18.92 | −0.0114 | 0.13029 | −0.0015 |
| Tegula pulligo | 12.02 | −0.0104 | 0.40029 | −0.0041 |
| Tricolia spp. (2) | 2.74 | −0.0055 | 0.23029 | −0.0013 |
| Echinoidea | ||||
| L. anamesus | 2.55 | −0.0057 | 0.003 | −1.42 10−5 |
| S. franciscanus | 119.50 | −0.0141 | 0.014 | −0.0002 |
| S. purpuratus | 53.17 | −0.0132 | 1.463 | −0.0190 |
| Total | −0.2870 |
Biomass was estimated in this study or taken from literature where indicated by numerical superscripts. Bold PCIS values are from feeding trials; nonbold values are predicted from average adult biomass. Average adult biomass was estimated in this study for the experimental species, and from the literature (27–28) for the other species. Densities are for California M. pyrifera kelp forests estimated in this study or literature where indicated by numerical superscripts. Other species included in Fig. 2 for which density data were not available were Amphipods: Amphilochus spp., Ampithoe plea, Ampithoe rubricata, Aoroides columbae, Batea transversa, Ceradocus spinicauda, Corophium spp., Cymadusa uncinata, Elasmopus antennatus, Ericthonius brasiliensis, Hyale frequens, Jassa falcata, Maera ascensionis, Maera inaequipes, Maera simile, Microjassa litotes, Pleusirus secorrus, and Podocerus cristatus; Gastropods: Amphithalmus inclusus, Amphithalmus tenuis, Barleeia californica, and Lottia ocracea; and the Echinoid Centrostephanus coronatus.
Species-specific biomass estimates were available allowing all amphipod species to be included individually in Fig. 2.
Once the average size of an individual herbivore approaches the size at which per capita interaction strength saturates, the population impact of the species as a whole may decrease with further increases in individual size because of the negative relationship between individual size and population density (34). The asymptotic nature of the per capita interaction strength curve in Fig. 1 suggests that differently sized species above the asymptote will have the same per capita interaction strength. However, 1 kg of S. purpuratus per m2 will have more than double the impact on M. pyrifera microscopic sporophyte survival (24.8% removed per m2 per day) than 1 kg of S. franciscanus over the same area (11.8%), simply because there are more S. purpuratus. Although the herbivore densities required to consume 100% of M. pyrifera recruitment are probably rare in nature, the steep size threshold in the relationship between maximum per capita interaction strength and herbivore size clearly identifies intermediate-sized herbivores as important (e.g., small gastropods, Tegula spp.), and generally unappreciated, grazers in M. pyrifera forests. The potential role of these grazers is enhanced by the fact that larger herbivores, such as sea urchins and large gastropods, are heavily fished in southern and central California (35), and can be under strong predation pressure by higher-order predators (e.g., sea otters in central California; refs. 36 and 37). Similar community roles have been shown for amphipods in Atlantic algal assemblages (38). That smaller species can have similar per capita effects as large species, but ultimately have greater population impacts, emphasizes the importance of having multiple levels of information for estimating the strength of trophic interactions.
Our conclusion that the distribution of herbivore–kelp interaction strengths is bimodal, with a relatively equal ratio of weaker and stronger interactions, is based on the analysis of only those species that are considered potential grazers of M. pyrifera microscopic sporophytes. The importance of herbivores that graze on M. pyrifera microscopic gametophytes or macroscopic sporophytes was not studied, but such herbivores are likely also important for regulating kelp recruitment. Our estimation of the distribution of interaction strengths is also biased toward negative impacts because we ignored the presence of either null or positive interactors, the latter of which may actually enhance M. pyrifera microscopic sporophyte survival by preying on herbivores or competitors. As with most systems, our view of these properties of kelp forest communities and food webs is scale-dependent and partial to logistical limitations. Despite recent laudable efforts to provide a theoretical framework to estimate per capita interaction strength in multitrophic-level communities (6), determination of the total distribution of interaction strength within complex natural communities may be practical only for simple communities composed of only a few species. Moreover, the theoretical speculation that communities dominated by weak interactions are more stable and resistant to invasion (6, 7) needs to be tested on species-rich communities. For instance, this hypothesis alone does not explain why Mediterranean benthic communities composed of hundreds of species, in which apparently only a few species are strong interactors (39), have been invaded repeatedly by benthic algae (40, 41); or why the loss of a few species can result in ecosystem shifts, as in kelp forests concerning sea otters (5) and coral reefs concerning a few species of herbivores (42, 44).
These limitations, however, should not deter ecologists from seeking such a holistic view of trophic interactions in nature. We predict that future advances and insight will come by focusing attention on identifying subsystems of species that have important roles in community organization (26), keeping in mind that although large and charismatic species can be strong interactors, populations of smaller and relatively inconspicuous species may have effects of similar or greater magnitude.
Acknowledgments
We thank P. Edwards, L. Foote, A. Hobday, R. McConnaughey, K. Riser, S. Rumsey, and E. Yee for help in the field and in the laboratory. We benefited from discussions with and comments by P. Dayton, L. Ferry-Graham, J. Jackson, R. Paine, J. Stachowicz, G. Sugihara, N. Tsutsui, and D. Wesson. To all we are very grateful. Funding for this research was provided through a postdoctoral grant from the Catalan Government (Commission for Universities and Research) and a Professional Association of Diving Instructors Foundation Grant (to E.S.), and a University of California Faculty Fellowship (to M.G.).
References
- 1.Paine R T. Nature (London) 1992;355:73–75. [Google Scholar]
- 2.Wootton J T. Ecol Monogr. 1997;67:45–64. [Google Scholar]
- 3.Ruesink J L. Proc Natl Acad Sci USA. 1998;95:6843–6847. doi: 10.1073/pnas.95.12.6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paine R T. Am Nat. 1969;103:91–93. [Google Scholar]
- 5.Estes J A, Duggins D O. Ecol Monogr. 1995;65:75–100. [Google Scholar]
- 6.McCann K, Hastings A, Huxel G R. Nature (London) 1998;395:794–798. [Google Scholar]
- 7.Kokkoris G D, Troumbis A Y, Lawton J H. Ecol Lett. 1999;2:70–74. [Google Scholar]
- 8.May R T. Stability and Complexity in Model Ecosystems. Princeton, NJ: Princeton Univ. Press; 1973. [PubMed] [Google Scholar]
- 9.Navarrete S A, Menge B A. Mar Ecol Progr Ser. 1996;66:409–429. [Google Scholar]
- 10.Navarrete S A, Menge B A, Daley B A. Ecology. 2000;81:2264–2277. [Google Scholar]
- 11.Berlow E L. Nature (London) 1999;398:330–334. [Google Scholar]
- 12.Fagan W F, Hurd L E. Ecology. 1994;75:2022–2032. [Google Scholar]
- 13.Raffaelli D G, Hall S J. In: Food Webs, Integration of Patterns and Dynamics. Polis G, Winemiller K, editors. New York: Chapman & Hall; 1995. pp. 185–191. [Google Scholar]
- 14. North, W. J. (1971) Beihefte Nova Hedwigia32.
- 15.Dayton P K. Annu Rev Ecol Syst. 1985;16:215–245. [Google Scholar]
- 16.Dayton P K, Currie V, Gerrodette T, Keller B D, Rosenthal R, Ven Tresca D. Ecol Monogr. 1984;54:253–289. [Google Scholar]
- 17.Dayton P K, Tegner M J, Parnell P E, Edwards P B. Ecol Monogr. 1992;62:421–445. [Google Scholar]
- 18.Dayton P K, Tegner M J, Edwards P B, Riser K L. Mar Ecol Prog Ser. 1999;69:219–250. [Google Scholar]
- 19.Konar B. Mar Ecol Prog Ser. 2000;199:271–280. [Google Scholar]
- 20.Power M E, Tilman D, Estes J A, Menge B A, Bond W J, Mills L S, Daily G, Castilla J C, Lubchenco J, Paine R T. Bioscience. 1996;46:609–620. [Google Scholar]
- 21.Laska M S, Wootton J T. Ecology. 1998;79:461–476. [Google Scholar]
- 22.Graham M H, Harrold C, Lisin S, Light K, Watanabe J M, Foster M S. Mar Ecol Prog Ser. 1997;148:269–279. [Google Scholar]
- 23.Berlow E L, Navarrete S A, Briggs C J, Power M E, Menge B A. Ecology. 1999;80:2206–2224. [Google Scholar]
- 24.Sturm E A, Horn M H. Mar Biol. 1998;132:515–522. [Google Scholar]
- 25.Hobson E S, Chess J R. Env Biol Fish. 2001;60:411–457. [Google Scholar]
- 26.Paine R T. J Anim Ecol. 1980;49:669–685. [Google Scholar]
- 27.Coyer J A. Ph.D. Thesis. Los Angeles: University of California; 1979. [Google Scholar]
- 28.Morris R H, Abbott D P, Haderlie E C. Intertidal Invertebrates of California. Stanford, CA: Stanford Univ. Press; 1980. [Google Scholar]
- 29.Anonymous. Diablo Canyon Power Plant Thermal Effects Monitoring Program Analysis Report. San Francisco: Tenera; 1997. [Google Scholar]
- 30.Schmitt R J. Ecology. 1987;68:1887–1897. doi: 10.2307/1939880. [DOI] [PubMed] [Google Scholar]
- 31.Dean T A, Jacobsen F R, Thies K, Lagos S L. Mar Ecol Prog Ser. 1988;48:99–102. [Google Scholar]
- 32.Tegner M J, Dayton P K. Adv Ecol Res. 1987;17:243–279. [Google Scholar]
- 33.Watanabe J M, Harrold C. Mar Ecol Prog Ser. 1991;71:125–141. [Google Scholar]
- 34.Blackburn T M, Brown V K, Doube B M, Greenwood J D, Lawton J H, Stork N E. J Anim Ecol. 1993;62:519–528. [Google Scholar]
- 35.Dayton P K, Tegner M J, Edwards P B, Riser K L. Ecol Appl. 1998;8:309–322. [Google Scholar]
- 36.Lowry L F, Pearse J S. Mar Biol. 1973;23:213–219. [Google Scholar]
- 37.Laur D R, Ebeling A W, Coon D A. In: The Community Ecology of Sea Otters. VanBlaricom G R, Estes J A, editors. Berlin: Springer; 1988. pp. 151–168. [Google Scholar]
- 38.Duffy J E, Hay M E. Ecol Monogr. 2000;70:237–263. [Google Scholar]
- 39.Sala E, Boudouresque C F, Harmelin-Vivien M. Oikos. 1998;82:425–439. [Google Scholar]
- 40.Verlaque M. Oceanologica Acta. 1994;17:1–23. [Google Scholar]
- 41.Meinesz A, Hesse B. Oceanologica Acta. 1991;14:415–426. [Google Scholar]
- 42.Hughes T P. Science. 1994;265:1547–1551. doi: 10.1126/science.265.5178.1547. [DOI] [PubMed] [Google Scholar]
- 43.Jackson J B C. Coral Reefs. 1997;16:S23–S32. [Google Scholar]
- 44.Jackson J B C, Kirby M X, Berger W H, Bjorndal K A, Botsford L W, Bourque B J, Bradbury R H, Cooke R, Erlandson J, Estes J A, et al. Science. 2001;293:629–638. doi: 10.1126/science.1059199. [DOI] [PubMed] [Google Scholar]