Abstract
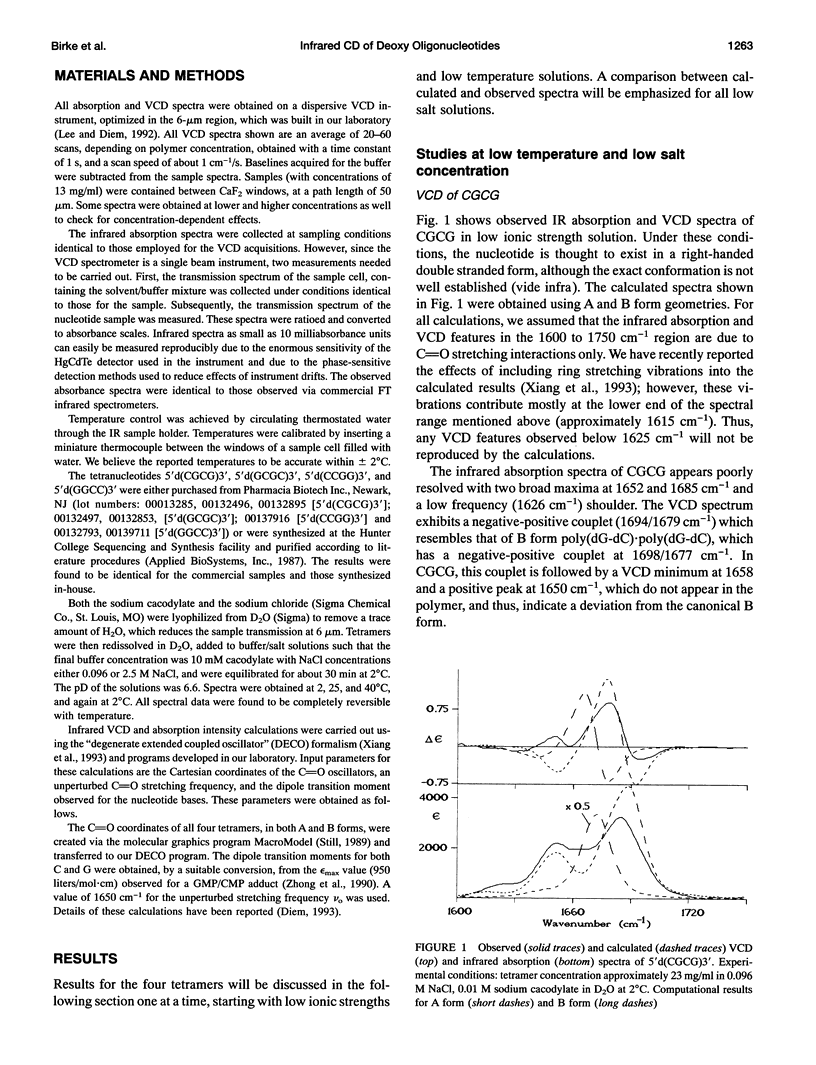
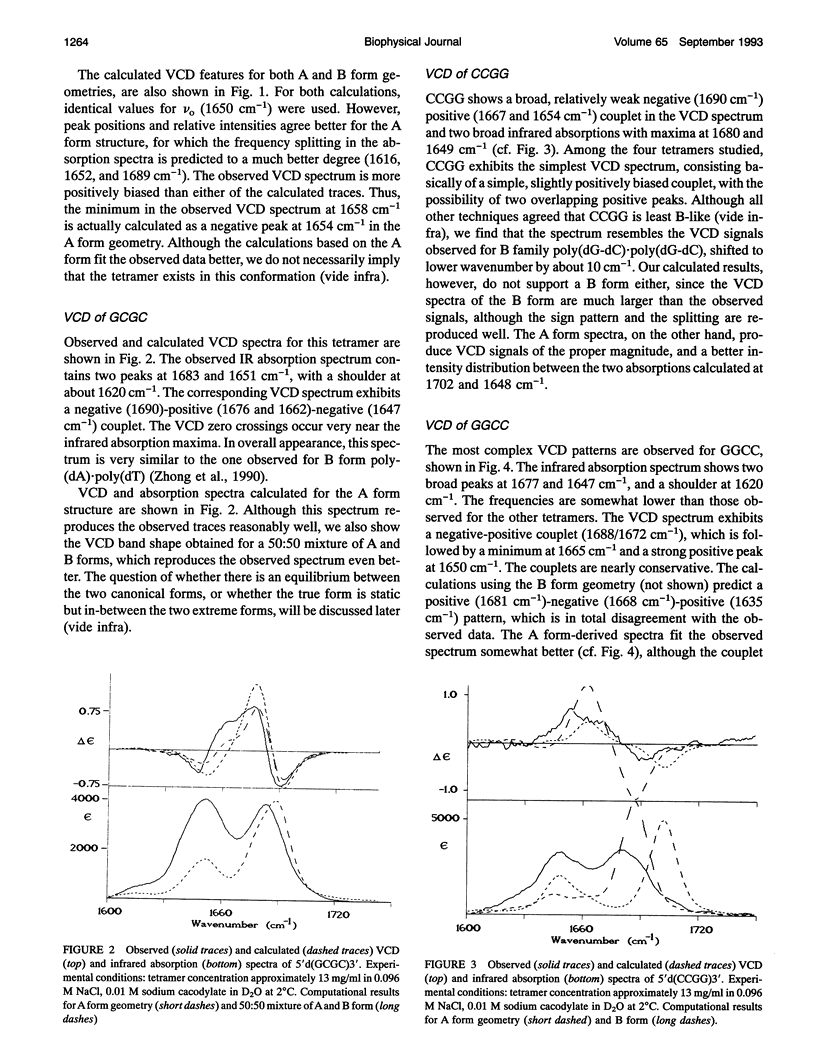
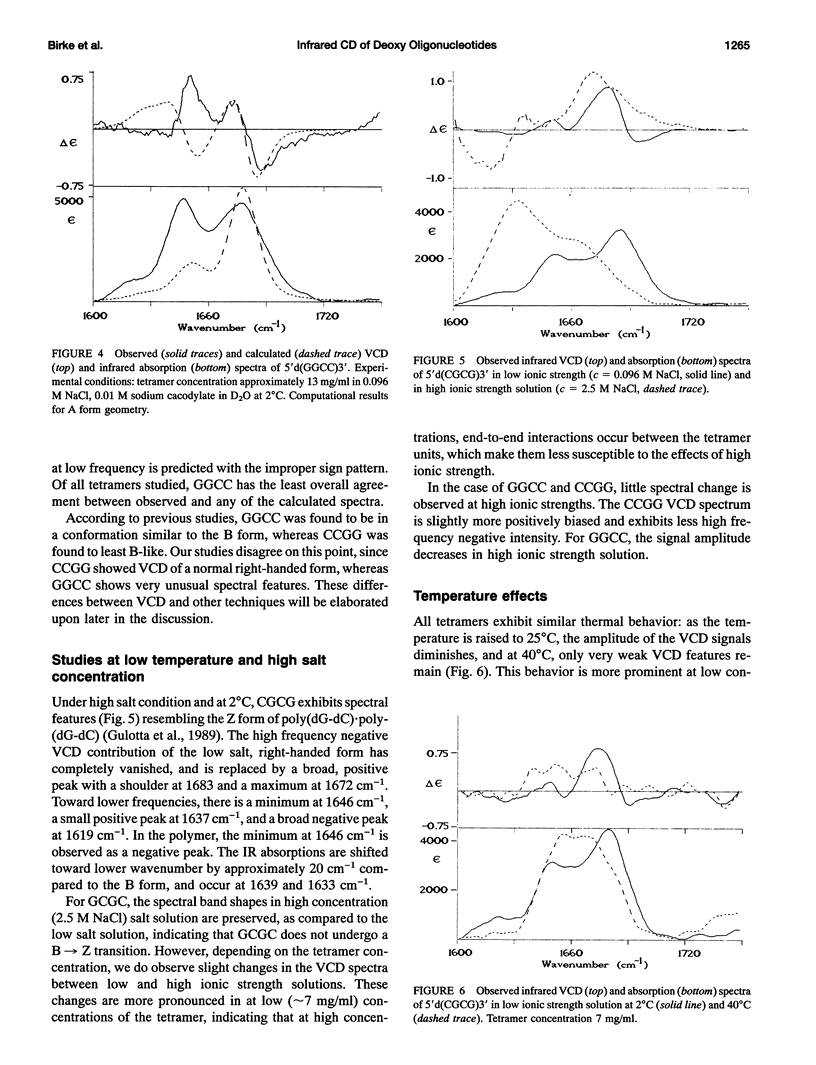
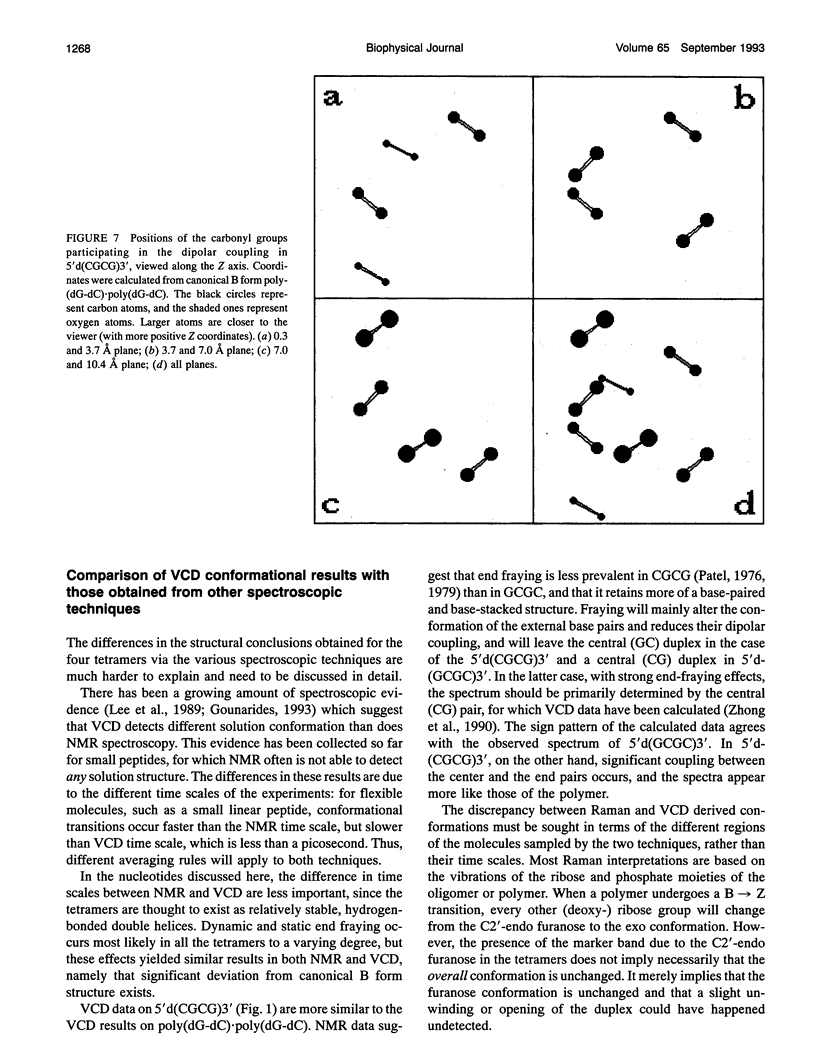
Infrared (vibrational) circular dichroism (VCD) spectra have been obtained for the self-complementary tetranucleotides, 5'd(CGCG)3', 5'd(GCGC)3', 5'd(CCGG)3', and 5'd(GGCC)3'. In buffered aqueous solution at low salt concentration, these tetramers exhibit spectra associated with right-handed polymers, although the spectra differ significantly for the four species. In high salt solution, a B-->Z transition occurs in 5'd(CGCG)3', while the other tetranucleotides appear only slightly altered. Temperature dependent studies of these oligonucleotides reveal a greater amount of thermal stability for the tetramers in low salt than for the high salt solutions. VCD intensities computed via the exciton formalism are compared with observed results.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crawford J. L., Kolpak F. J., Wang A. H., Quigley G. J., van Boom J. H., van der Marel G., Rich A. The tetramer d(CpGpCpG) crystallizes as a left-handed double helix. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4016–4020. doi: 10.1073/pnas.77.7.4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew H. R., Dickerson R. E., Itakura K. A salt-induced conformational change in crystals of the synthetic DNA tetramer d(CpGpCpG). J Mol Biol. 1978 Nov 15;125(4):535–543. doi: 10.1016/0022-2836(78)90315-7. [DOI] [PubMed] [Google Scholar]
- Gulotta M., Goss D. J., Diem M. IR vibrational CD in model deoxyoligonucleotides: observation of the B----Z phase transition and extended coupled oscillator intensity calculations. Biopolymers. 1989 Dec;28(12):2047–2058. doi: 10.1002/bip.360281202. [DOI] [PubMed] [Google Scholar]
- Herbeck R., Zundel G. Influence of temperature and magnesium ions on the secondary and tertiary structures of tRNAPhe and 23 S RNA - infrared investigations. Biochim Biophys Acta. 1976 Jan 5;418(1):52–62. doi: 10.1016/0005-2787(76)90326-9. [DOI] [PubMed] [Google Scholar]
- Howard F. B., Frazier J., Miles H. T. Interbase vibrational coupling in G:C polynucleotide helices. Proc Natl Acad Sci U S A. 1969 Oct;64(2):451–458. doi: 10.1073/pnas.64.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastrup R. V., Young M. A., Krugh T. R. Ethidium bromide complexes with self-complementary deoxytetranucleotides. Demonstration and discussion of sequence preferences in the intercalative binding of ethidium bromide. Biochemistry. 1978 Nov 14;17(23):4855–4865. doi: 10.1021/bi00616a002. [DOI] [PubMed] [Google Scholar]
- Kölkenbeck K., Zundel G. The significance of the 2' OH group and the influence of cations on the secondary structure of the RNA backbone. Biophys Struct Mech. 1975 May 30;1(3):203–219. doi: 10.1007/BF00535757. [DOI] [PubMed] [Google Scholar]
- Lee O., Roberts G. M., Diem M. IR vibrational CD in alanyl tripeptide: indication of a stable solution conformer. Biopolymers. 1989 Oct;28(10):1759–1770. doi: 10.1002/bip.360281009. [DOI] [PubMed] [Google Scholar]
- Patel D. J. Helix-coil transition of the dG-dC-dG-dC self-complementary duplex and complex formation with daunomycin in solution. Biopolymers. 1979 Mar;18(3):553–569. doi: 10.1002/bip.1979.360180307. [DOI] [PubMed] [Google Scholar]
- Patel D. J. Proton and phosphorus NMR studies of d-CpG(pCpG)n duplexes in solution. Helix-coil transition and complex formation with actinomycin-D. Biopolymers. 1976 Mar;15(3):533–558. doi: 10.1002/bip.1976.360150310. [DOI] [PubMed] [Google Scholar]
- Patel D. J. d-CpCpGpG and d-GpGpCpC self-complementary duplexes: Nmr studies of the helix-coil transition. Biopolymers. 1977 Aug;16(8):1635–1656. doi: 10.1002/bip.1977.360160804. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Quadrifoglio F., Manzini G., Vasser M., Dinkelspiel K., Crea R. Conformational stability of alternating d (CG) oligomers in high salt solution. Nucleic Acids Res. 1981 May 11;9(9):2195–2206. doi: 10.1093/nar/9.9.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. A., Peticolas W. L. A temperature-dependent Z to B to single-strand transition in d(CGCG). Biopolymers. 1989 Sep;28(9):1625–1636. doi: 10.1002/bip.360280911. [DOI] [PubMed] [Google Scholar]
- Thomas G. A., Peticolas W. L. Sequence dependence of conformations of self-complementary duplex tetradeoxynucleotides containing cytosine and guanine. Biochemistry. 1984 Jul 3;23(14):3202–3207. doi: 10.1021/bi00309a014. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Wang L., Keiderling T. A. Vibrational circular dichroism studies of the A-to-B conformational transition in DNA. Biochemistry. 1992 Oct 27;31(42):10265–10271. doi: 10.1021/bi00157a013. [DOI] [PubMed] [Google Scholar]
- Zhong W. X., Gulotta M., Goss D. J., Diem M. DNA solution conformation via infrared circular dichroism: experimental and theoretical results for B-family polymers. Biochemistry. 1990 Aug 14;29(32):7485–7491. doi: 10.1021/bi00484a018. [DOI] [PubMed] [Google Scholar]




