Abstract
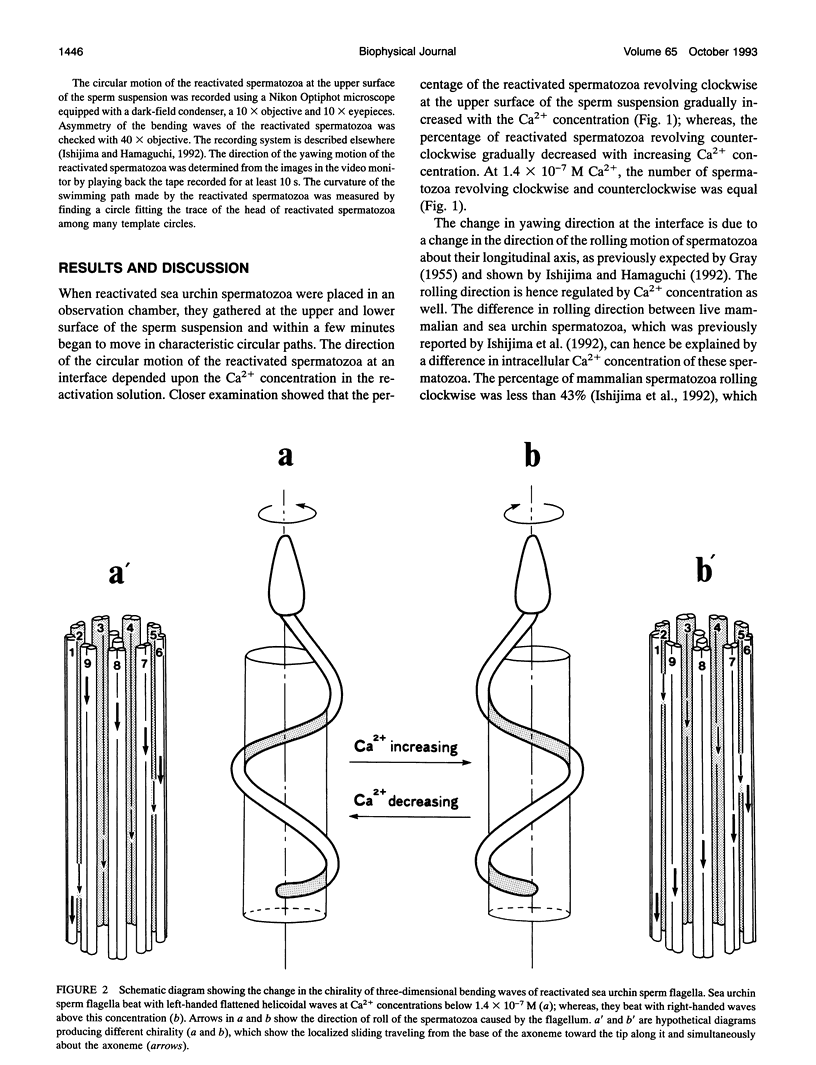
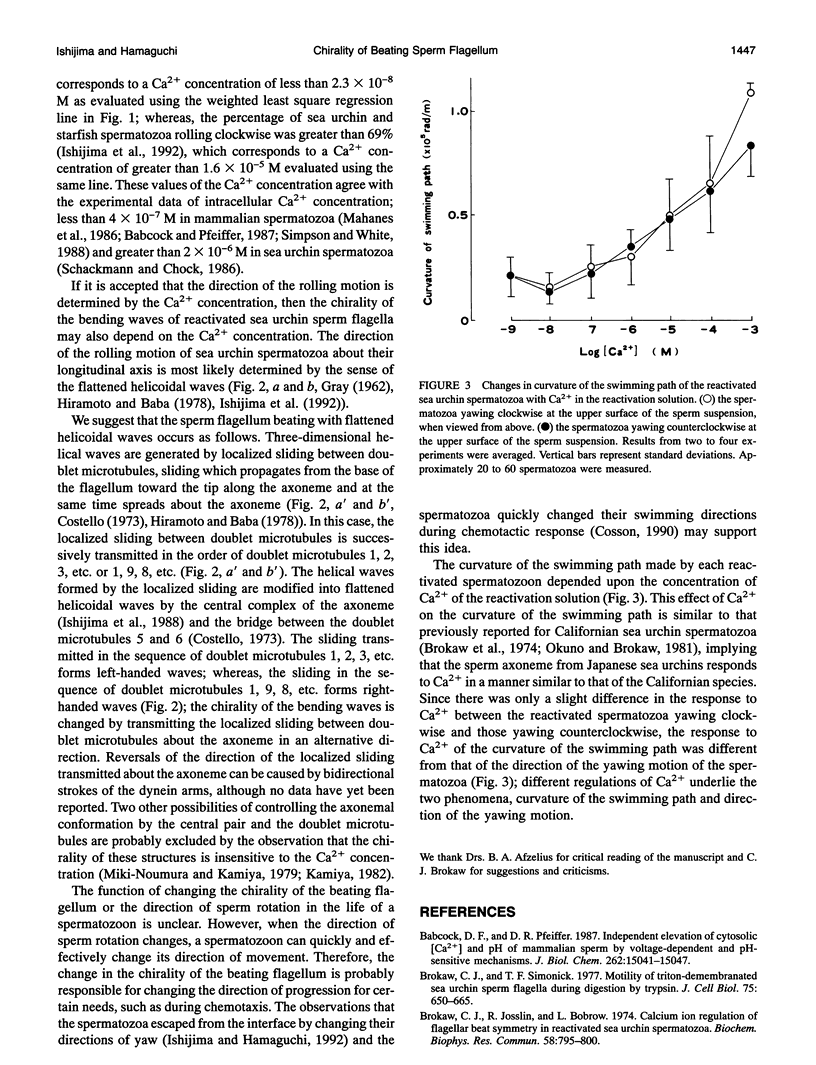
Near an interface, sea urchin spermatozoa swim almost in circles. The direction is usually clockwise at the lower surface of a coverslip and counterclockwise at the upper surface of a glass slide, when viewed from above. Examination of demembranated spermatozoa has shown that Ca2+ regulates the direction of the circular motion of spermatozoa reactivated with adenosine triphosphate (ATP). This finding suggests that Ca2+ changes the chirality of the three-dimensional bending waves of sperm flagella.
Full text
PDF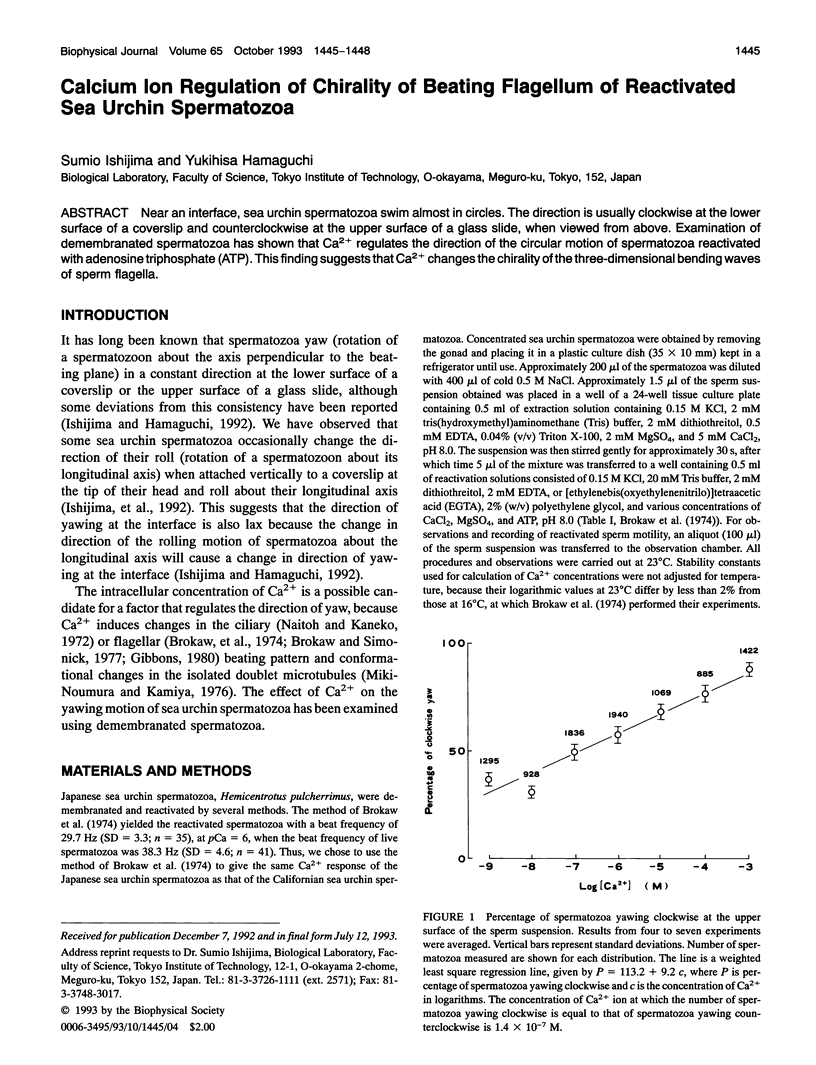

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babcock D. F., Pfeiffer D. R. Independent elevation of cytosolic [Ca2+] and pH of mammalian sperm by voltage-dependent and pH-sensitive mechanisms. J Biol Chem. 1987 Nov 5;262(31):15041–15047. [PubMed] [Google Scholar]
- Brokaw C. J., Simonick T. F. Motility of triton-demembranated sea urchin sperm flagella during digestion by trypsin. J Cell Biol. 1977 Dec;75(3):650–665. doi: 10.1083/jcb.75.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello D. A new theory on the mechanics of ciliary and flagellar motility. II. Theoretical considerations. Biol Bull. 1973 Oct;145(2):292–309. doi: 10.2307/1540041. [DOI] [PubMed] [Google Scholar]
- Gibbons B. H. Intermittent swimming in live sea urchin sperm. J Cell Biol. 1980 Jan;84(1):1–12. doi: 10.1083/jcb.84.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishijima S., Hamaguchi M. S., Naruse M., Ishijima S. A., Hamaguchi Y. Rotational movement of a spermatozoon around its long axis. J Exp Biol. 1992 Feb;163:15–31. doi: 10.1242/jeb.163.1.15. [DOI] [PubMed] [Google Scholar]
- Ishijima S., Hamaguchi Y. Relationship between direction of rolling and yawing of golden hamster and sea urchin spermatozoa. Cell Struct Funct. 1992 Oct;17(5):319–323. doi: 10.1247/csf.17.319. [DOI] [PubMed] [Google Scholar]
- Kamiya R. Extrusion and Rotation of the central-pair microtubules in detergent-treated Chlamydomonas flagella. Prog Clin Biol Res. 1982;80:169–173. doi: 10.1002/cm.970020732. [DOI] [PubMed] [Google Scholar]
- Mahanes M. S., Ochs D. L., Eng L. A. Cell calcium of ejaculated rabbit spermatozoa before and following in vitro capacitation. Biochem Biophys Res Commun. 1986 Jan 29;134(2):664–670. doi: 10.1016/s0006-291x(86)80471-5. [DOI] [PubMed] [Google Scholar]
- Miki-Noumura T., Kamiya R. Conformational change in the outer doublet microtubules from sea urchin sperm flagella. J Cell Biol. 1979 May;81(2):355–360. doi: 10.1083/jcb.81.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki-Noumura T., Kamiya R. Shape of microtubules in solutions. Exp Cell Res. 1976 Feb;97(2):451–453. doi: 10.1016/0014-4827(76)90642-x. [DOI] [PubMed] [Google Scholar]
- Naito Y., Kaneko H. Reactivated triton-extracted models o paramecium: modification of ciliary movement by calcium ions. Science. 1972 May 5;176(4034):523–524. doi: 10.1126/science.176.4034.523. [DOI] [PubMed] [Google Scholar]
- Schackmann R. W., Chock P. B. Alteration of intracellular [Ca2+] in sea urchin sperm by the egg peptide speract. Evidence that increased intracellular Ca2+ is coupled to Na+ entry and increased intracellular pH. J Biol Chem. 1986 Jul 5;261(19):8719–8728. [PubMed] [Google Scholar]
- Simpson A. M., White I. G. Measurement and manipulation of cytoplasmic free calcium of ram and boar spermatozoa using quin 2. Cell Calcium. 1988 Feb;9(1):45–56. doi: 10.1016/0143-4160(88)90037-1. [DOI] [PubMed] [Google Scholar]


