Abstract
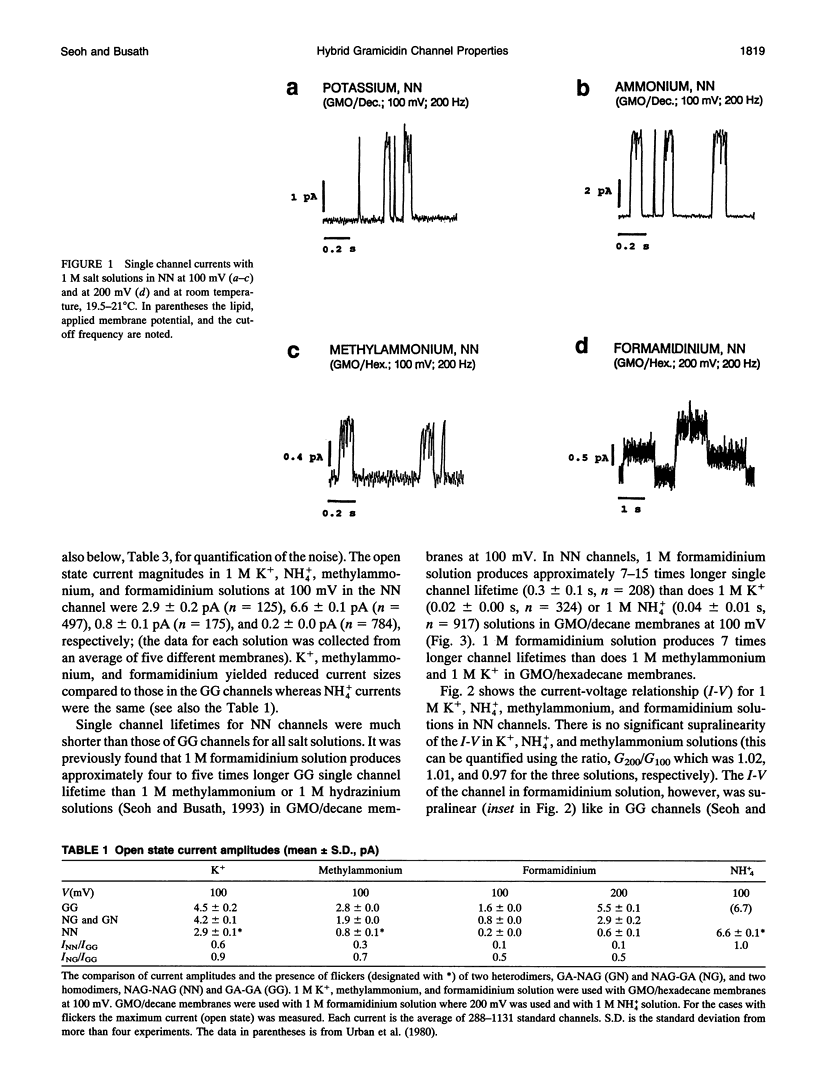
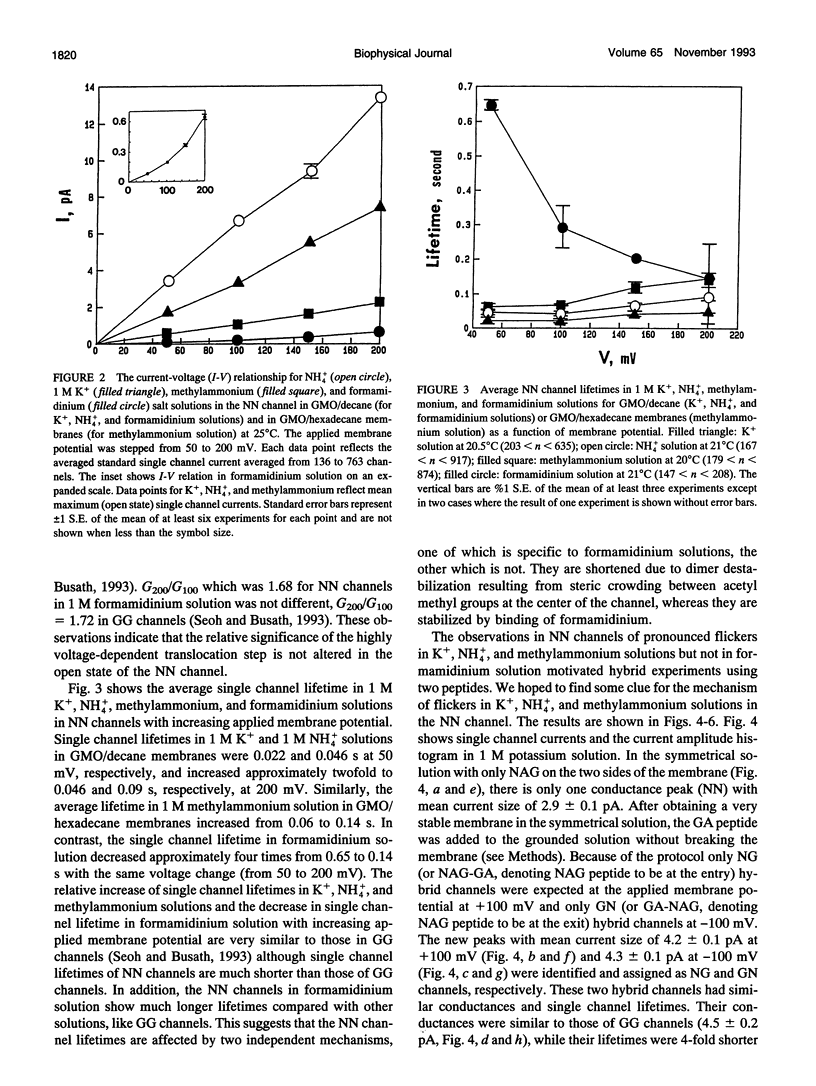
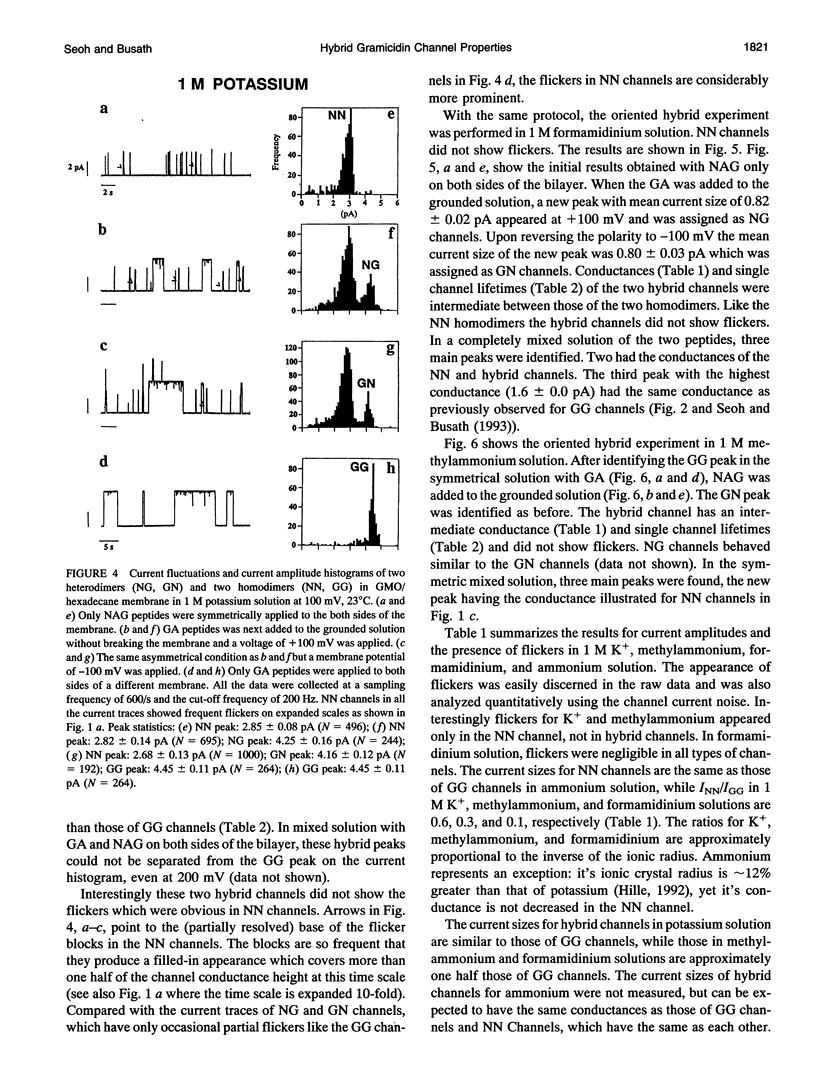
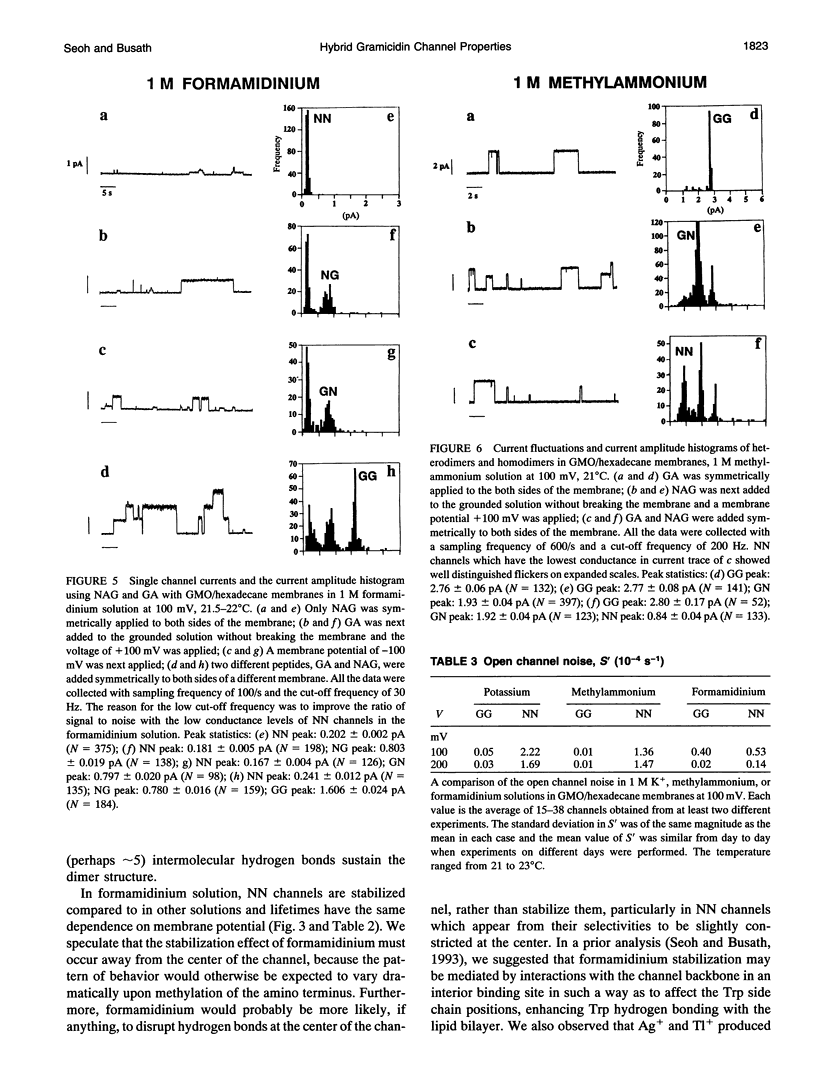
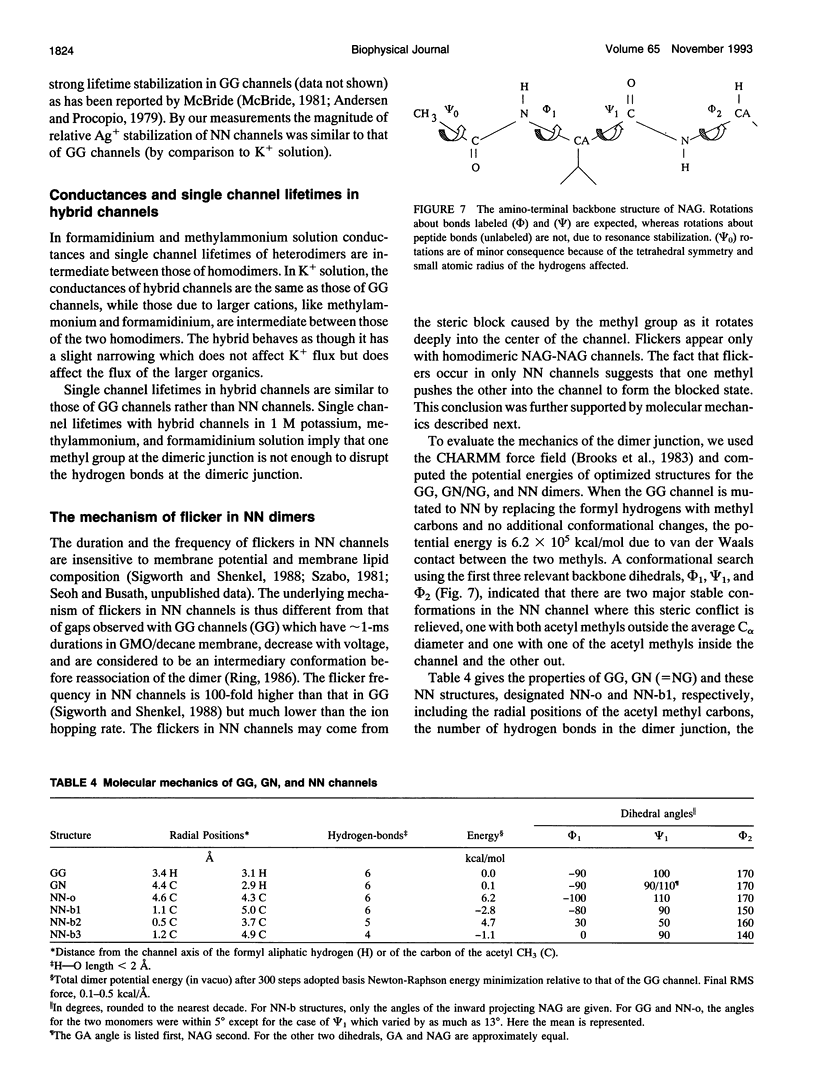
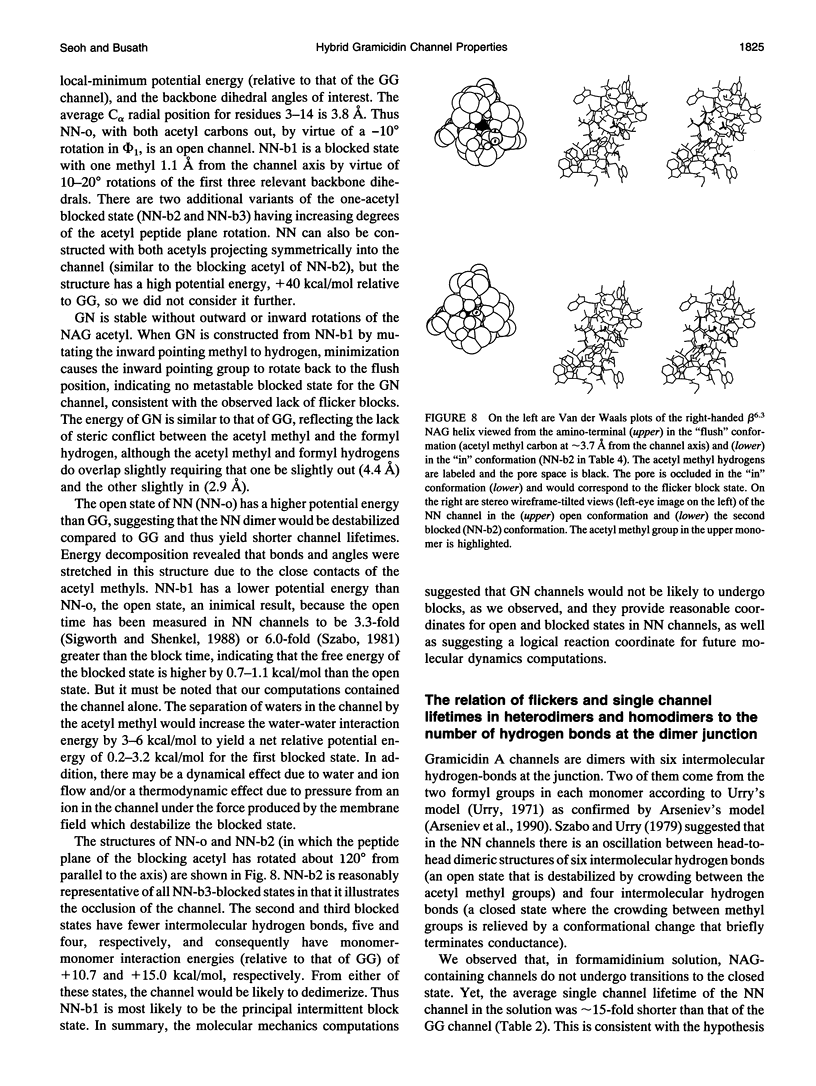
Compared to the N-formyl gramicidin A (GA), the N-acetyl gramicidin A (NAG) channel has unchanged conductance in 1 M NH4+ (gamma NN/gamma GG = 1, conductance ratio) but reduced conductance in 1 M K+ (gamma NN/gamma GG = 0.6) methylammonium (gamma NN/gamma GG = 0.3), and formamidinium (gamma NN/gamma GG = 0.1) solutions. Except with formamidinium, "flicker blocks" are evident even at low cutoff frequencies. For all cations studied, channel lifetimes of N-acetyl homodimers (NN) are approximately 50-fold shorter than those of the GA homodimer (GG). The novel properties of GA channels in formamidinium solution (supralinear current-voltage relations and dimer stabilization (Seoh and Busath, 1993)) also appear in NN channels. The average single channel lifetime in 1 M formamidinium solution at 100 mV is 6-7-fold longer than in K+ and methylammonium solutions and, like in the GA channel, significantly decreases with increasing membrane potential. Experiments with mixtures of the two peptides, GA and NAG, showed three main conductance peaks. Oriented hybrids were formed utilizing the principle that monomers remain in one leaflet of the bilayer (O'Connell et al., 1990). With GA at the polarized side and NAG at the grounded side, at positive potentials (in which case hybrids were designated GN) and at negative potentials (in which case hybrids were designated NG), channels had the same conductances and channel properties at all potentials studied. Flicker blocks were not evident in the hybrid channels, which suggests that both N-acetyl methyl groups at the junction of the dimer are required to cause flickers. Channel lifetimes in hybrids are only approximately threefold shorter than those of the GG channels, and channel conductances are similar to those of GG rather than NN channels. We suggest that acetyl-acetyl crowding at the dimeric junction in NN channels cause dimer destabilization, flickers, and increased selectivity in N-acetyl gramicidin channels.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apell H. J., Bamberg E., Alpes H. Dicarboxylic acid analogs of gramicidin A: dimerization kinetics and single channel properties. J Membr Biol. 1979 Nov 30;50(3-4):271–285. doi: 10.1007/BF01868893. [DOI] [PubMed] [Google Scholar]
- Bamberg E., Apell H. J., Alpes H. Structure of the gramicidin A channel: discrimination between the piL,D and the beta helix by electrical measurements with lipid bilayer membranes. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2402–2406. doi: 10.1073/pnas.74.6.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberg E., Benz R. Voltage-induced thickness changes of lipid bilayer membranes and the effect of an electrin field on gramicidin A channel formation. Biochim Biophys Acta. 1976 Mar 19;426(3):570–580. doi: 10.1016/0005-2736(76)90400-4. [DOI] [PubMed] [Google Scholar]
- Bamberg E., Janko K. The action of a carbonsuboxide dimerized gramicidin A on lipid bilayer membranes. Biochim Biophys Acta. 1977 Mar 17;465(3):486–499. doi: 10.1016/0005-2736(77)90267-x. [DOI] [PubMed] [Google Scholar]
- Busath D., Szabo G. Low conductance gramicidin A channels are head-to-head dimers of beta 6.3-helices. Biophys J. 1988 May;53(5):689–695. doi: 10.1016/S0006-3495(88)83150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifu A. S., Koeppe R. E., 2nd, Andersen O. S. On the supramolecular organization of gramicidin channels. The elementary conducting unit is a dimer. Biophys J. 1992 Jan;61(1):189–203. doi: 10.1016/S0006-3495(92)81826-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman G., Enos B., Hägglund J., Sandblom J. Gramicidin as an example of a single-filing ionic channel. Ann N Y Acad Sci. 1980;339:8–20. doi: 10.1111/j.1749-6632.1980.tb15964.x. [DOI] [PubMed] [Google Scholar]
- Finkelstein A., Andersen O. S. The gramicidin A channel: a review of its permeability characteristics with special reference to the single-file aspect of transport. J Membr Biol. 1981 Apr 30;59(3):155–171. doi: 10.1007/BF01875422. [DOI] [PubMed] [Google Scholar]
- Goodall M. C. Thickness dependence in the action of gramicidin A on lipid bilayers. Arch Biochem Biophys. 1971 Nov;147(1):129–135. doi: 10.1016/0003-9861(71)90318-3. [DOI] [PubMed] [Google Scholar]
- Hemsley G., Busath D. Small iminium ions block gramicidin channels in lipid bilayers. Biophys J. 1991 Apr;59(4):901–907. doi: 10.1016/S0006-3495(91)82303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladky S. B., Haydon D. A. Ion transfer across lipid membranes in the presence of gramicidin A. I. Studies of the unit conductance channel. Biochim Biophys Acta. 1972 Aug 9;274(2):294–312. doi: 10.1016/0005-2736(72)90178-2. [DOI] [PubMed] [Google Scholar]
- Läuger P. Ion transport through pores: a rate-theory analysis. Biochim Biophys Acta. 1973 Jul 6;311(3):423–441. doi: 10.1016/0005-2736(73)90323-4. [DOI] [PubMed] [Google Scholar]
- O'Connell A. M., Koeppe R. E., 2nd, Andersen O. S. Kinetics of gramicidin channel formation in lipid bilayers: transmembrane monomer association. Science. 1990 Nov 30;250(4985):1256–1259. doi: 10.1126/science.1700867. [DOI] [PubMed] [Google Scholar]
- Ring A. Brief closures of gramicidin A channels in lipid bilayer membranes. Biochim Biophys Acta. 1986 Apr 25;856(3):646–653. doi: 10.1016/0005-2736(86)90160-4. [DOI] [PubMed] [Google Scholar]
- Russell E. W., Weiss L. B., Navetta F. I., Koeppe R. E., 2nd, Andersen O. S. Single-channel studies on linear gramicidins with altered amino acid side chains. Effects of altering the polarity of the side chain at position 1 in gramicidin A. Biophys J. 1986 Mar;49(3):673–686. doi: 10.1016/S0006-3495(86)83694-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seoh S. A., Busath D. The permeation properties of small organic cations in gramicidin A channels. Biophys J. 1993 Apr;64(4):1017–1028. doi: 10.1016/S0006-3495(93)81467-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark G., Strässle M., Takácz Z. Temperature-jump and voltage-jump experiments at planar lipid membranes support an aggregational (micellar) model of the gramicidin A ion channel. J Membr Biol. 1986;89(1):23–37. doi: 10.1007/BF01870893. [DOI] [PubMed] [Google Scholar]
- Szabo G., Urry D. W. N-acetyl gramicidin: single-channel properties and implications for channel structure. Science. 1979 Jan 5;203(4375):55–57. doi: 10.1126/science.83000. [DOI] [PubMed] [Google Scholar]
- Urry D. W. Molecular perspectives of monovalent cation selective transmembrane channels. Int Rev Neurobiol. 1979;21:311–334. doi: 10.1016/s0074-7742(08)60642-x. [DOI] [PubMed] [Google Scholar]
- Urry D. W. The gramicidin A transmembrane channel: a proposed pi(L,D) helix. Proc Natl Acad Sci U S A. 1971 Mar;68(3):672–676. doi: 10.1073/pnas.68.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B. A., Veatch W. R., Blout E. R. The effects of lipid environment, ion-binding and chemical modifications on the structure of the gramicidin transmembrane channel. Biophys J. 1982 Jan;37(1):197–199. doi: 10.1016/S0006-3495(82)84667-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein S., Wallace B. A., Blout E. R., Morrow J. S., Veatch W. Conformation of gramicidin A channel in phospholipid vesicles: a 13C and 19F nuclear magnetic resonance study. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4230–4234. doi: 10.1073/pnas.76.9.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]


