Abstract
The interaction of alpha-actinin with lipid films and actin filaments was investigated. First alpha-actinin was incorporated in lipid films at the air/water interface. Injection of alpha-actinin into the subphase of a lipid monolayer led to a significant increase of the surface pressure only for lipid films consisting of a mixture of a negatively charged lipid with a high proportion of diacylglycerol. These alpha-actinin-containing films were transferred onto silanized quartz slides. Photobleaching experiments in the evanescent field allowed quantification of the lateral number density of the lipid-bound alpha-actinin. In combination with the area increase from the monolayer experiments, the photobleaching measurements suggest that alpha-actinin is incorporated into the lipid film in such a way that actin binding sites are accessible from the bulk phase. Binding experiments confirmed that the alpha-actinin selectively binds actin filaments in this configuration. We also showed that, in contrast to actin filaments which are adsorbed directly onto planar surfaces, the alpha-actinin-bound actin filaments are recognized and cleaved by the actin-severing protein gelsolin. Thus we have constructed an in vitro system which opens new ways for investigations of membrane-associated actin-binding proteins and of the physical behavior of actin filaments in the close neighborhood to membranes.
Full text
PDF

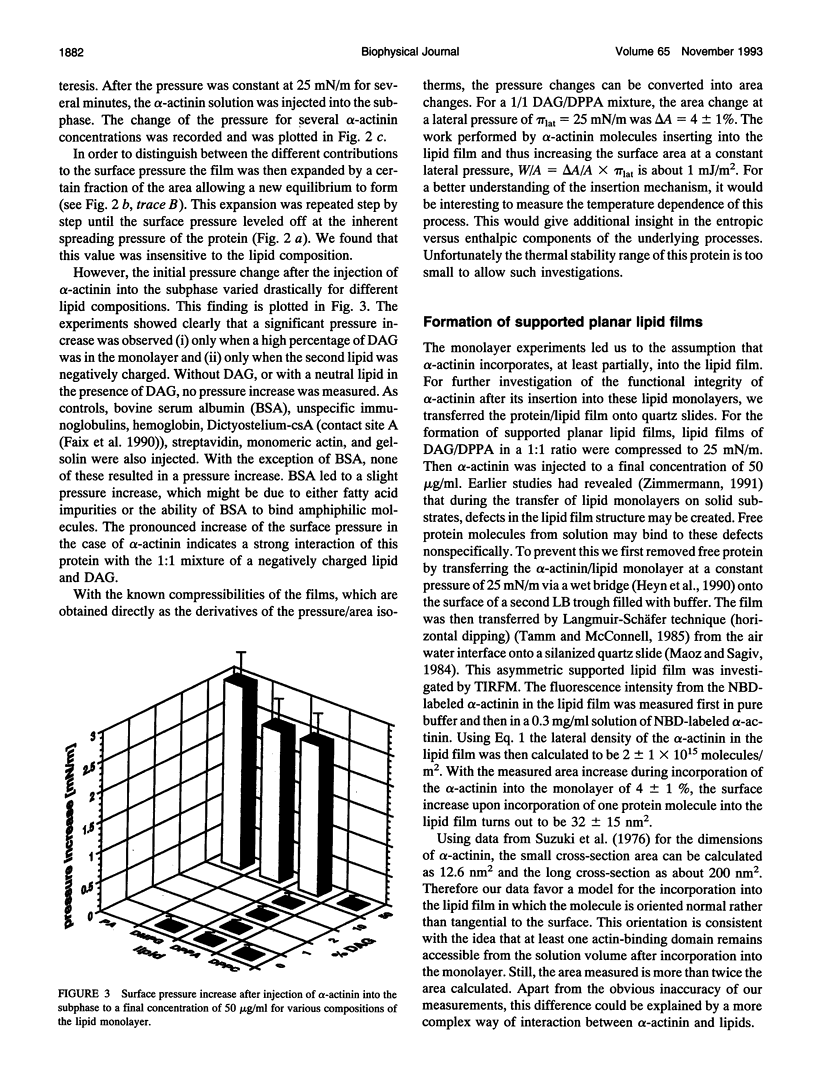
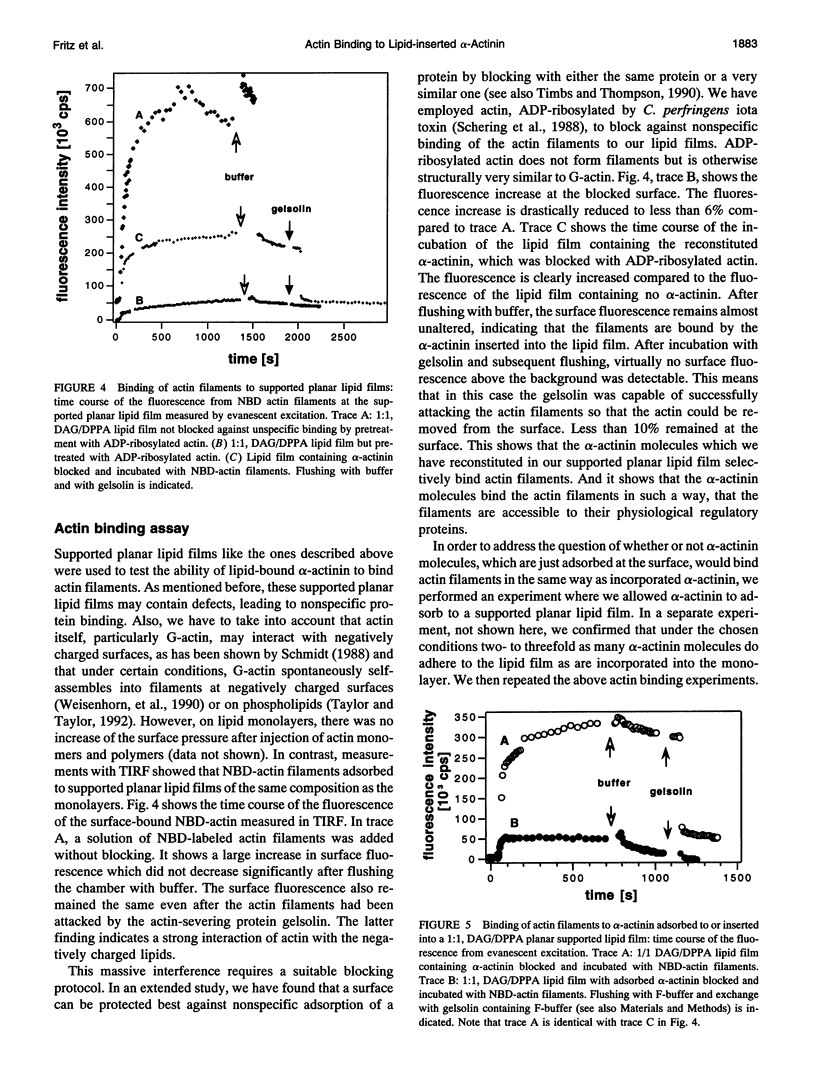


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D., Burghardt T. P., Thompson N. L. Total internal reflection fluorescence. Annu Rev Biophys Bioeng. 1984;13:247–268. doi: 10.1146/annurev.bb.13.060184.001335. [DOI] [PubMed] [Google Scholar]
- Blanchard A., Ohanian V., Critchley D. The structure and function of alpha-actinin. J Muscle Res Cell Motil. 1989 Aug;10(4):280–289. doi: 10.1007/BF01758424. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burn P., Rotman A., Meyer R. K., Burger M. M. Diacylglycerol in large alpha-actinin/actin complexes and in the cytoskeleton of activated platelets. Nature. 1985 Apr 4;314(6010):469–472. doi: 10.1038/314469a0. [DOI] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Burridge K., Feramisco J. R. Non-muscle alpha actinins are calcium-sensitive actin-binding proteins. Nature. 1981 Dec 10;294(5841):565–567. doi: 10.1038/294565a0. [DOI] [PubMed] [Google Scholar]
- Byers T. J., Husain-Chishti A., Dubreuil R. R., Branton D., Goldstein L. S. Sequence similarity of the amino-terminal domain of Drosophila beta spectrin to alpha actinin and dystrophin. J Cell Biol. 1989 Oct;109(4 Pt 1):1633–1641. doi: 10.1083/jcb.109.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraway K. L., Carraway C. A. Membrane-cytoskeleton interactions in animal cells. Biochim Biophys Acta. 1989 May 9;988(2):147–171. doi: 10.1016/0304-4157(89)90017-8. [DOI] [PubMed] [Google Scholar]
- Chen W. T., Singer S. J. Immunoelectron microscopic studies of the sites of cell-substratum and cell-cell contacts in cultured fibroblasts. J Cell Biol. 1982 Oct;95(1):205–222. doi: 10.1083/jcb.95.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coluccio L. M., Bretscher A. Reassociation of microvillar core proteins: making a microvillar core in vitro. J Cell Biol. 1989 Feb;108(2):495–502. doi: 10.1083/jcb.108.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese J. D., Frieden C. Microheterogeneity of actin gels formed under controlled linear shear. J Cell Biol. 1988 Oct;107(4):1477–1487. doi: 10.1083/jcb.107.4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig S. W., Lancashire C. L., Cooper J. A. Preparation of smooth muscle alpha-actinin. Methods Enzymol. 1982;85(Pt B):316–321. doi: 10.1016/0076-6879(82)85031-3. [DOI] [PubMed] [Google Scholar]
- Critchley D. R., Gilmore A., Hemmings L., Jackson P., McGregor A., Ohanian V., Patel B., Waites G., Wood C. Cytoskeletal proteins in adherens-type cell-matrix junctions. Biochem Soc Trans. 1991 Nov;19(4):1028–1033. doi: 10.1042/bst0191028. [DOI] [PubMed] [Google Scholar]
- Detmers P., Weber A., Elzinga M., Stephens R. E. 7-Chloro-4-nitrobenzeno-2-oxa-1,3-diazole actin as a probe for actin polymerization. J Biol Chem. 1981 Jan 10;256(1):99–105. [PubMed] [Google Scholar]
- Drenckhahn D., Dermietzel R. Organization of the actin filament cytoskeleton in the intestinal brush border: a quantitative and qualitative immunoelectron microscope study. J Cell Biol. 1988 Sep;107(3):1037–1048. doi: 10.1083/jcb.107.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson E. L. Cellular mechanics as an indicator of cytoskeletal structure and function. Annu Rev Biophys Biophys Chem. 1988;17:397–430. doi: 10.1146/annurev.bb.17.060188.002145. [DOI] [PubMed] [Google Scholar]
- Faix J., Gerisch G., Noegel A. A. Constitutive overexpression of the contact site A glycoprotein enables growth-phase cells of Dictyostelium discoideum to aggregate. EMBO J. 1990 Sep;9(9):2709–2716. doi: 10.1002/j.1460-2075.1990.tb07457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami K., Furuhashi K., Inagaki M., Endo T., Hatano S., Takenawa T. Requirement of phosphatidylinositol 4,5-bisphosphate for alpha-actinin function. Nature. 1992 Sep 10;359(6391):150–152. doi: 10.1038/359150a0. [DOI] [PubMed] [Google Scholar]
- Gerisch G., Noegel A. A., Schleicher M. Genetic alteration of proteins in actin-based motility systems. Annu Rev Physiol. 1991;53:607–628. doi: 10.1146/annurev.ph.53.030191.003135. [DOI] [PubMed] [Google Scholar]
- Goll D. E., Dayton W. R., Singh I., Robson R. M. Studies of the alpha-actinin/actin interaction in the Z-disk by using calpain. J Biol Chem. 1991 May 5;266(13):8501–8510. [PubMed] [Google Scholar]
- Heyn S. P., Tillmann R. W., Egger M., Gaub H. E. A miniaturized micro-fluorescence film balance for protein-containing lipid monolayers spread from a vesicle suspension. J Biochem Biophys Methods. 1991 Feb-Mar;22(2):145–158. doi: 10.1016/0165-022x(91)90027-t. [DOI] [PubMed] [Google Scholar]
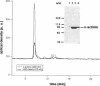
- Houk T. W., Jr, Ue K. The measurement of actin concentration in solution: a comparison of methods. Anal Biochem. 1974 Nov;62(1):66–74. doi: 10.1016/0003-2697(74)90367-4. [DOI] [PubMed] [Google Scholar]
- Janmey P. A., Hvidt S., Lamb J., Stossel T. P. Resemblance of actin-binding protein/actin gels to covalently crosslinked networks. Nature. 1990 May 3;345(6270):89–92. doi: 10.1038/345089a0. [DOI] [PubMed] [Google Scholar]
- Jockusch B. M., Burger M. M., DaPrada M., Richards J. G., Chaponnier C., Gabbiani G. alpha-Actinin attached to membranes of secretory vesicles. Nature. 1977 Dec 15;270(5638):628–629. doi: 10.1038/270628a0. [DOI] [PubMed] [Google Scholar]
- Jockusch B. M., Isenberg G. Interaction of alpha-actinin and vinculin with actin: opposite effects on filament network formation. Proc Natl Acad Sci U S A. 1981 May;78(5):3005–3009. doi: 10.1073/pnas.78.5.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Actin, alpha-actinin, and tropomyosin interaction in the structural organization of actin filaments in nonmuscle cells. J Cell Biol. 1976 Feb;68(2):202–219. doi: 10.1083/jcb.68.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E., Burridge K. Alpha-actinin: immunofluorescent localization of a muscle structural protein in nonmuscle cells. Cell. 1975 Nov;6(3):289–298. doi: 10.1016/0092-8674(75)90180-4. [DOI] [PubMed] [Google Scholar]
- Lu M. H., DiLullo C., Schultheiss T., Holtzer S., Murray J. M., Choi J., Fischman D. A., Holtzer H. The vinculin/sarcomeric-alpha-actinin/alpha-actin nexus in cultured cardiac myocytes. J Cell Biol. 1992 Jun;117(5):1007–1022. doi: 10.1083/jcb.117.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean-Fletcher S., Pollard T. D. Identification of a factor in conventional muscle actin preparations which inhibits actin filament self-association. Biochem Biophys Res Commun. 1980 Sep 16;96(1):18–27. doi: 10.1016/0006-291x(80)91175-4. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Modular organization of actin crosslinking proteins. Trends Biochem Sci. 1991 Mar;16(3):87–92. doi: 10.1016/0968-0004(91)90039-x. [DOI] [PubMed] [Google Scholar]
- McConnell H. M., Watts T. H., Weis R. M., Brian A. A. Supported planar membranes in studies of cell-cell recognition in the immune system. Biochim Biophys Acta. 1986 Jun 12;864(1):95–106. doi: 10.1016/0304-4157(86)90016-x. [DOI] [PubMed] [Google Scholar]
- Meyer R. K., Aebi U. Bundling of actin filaments by alpha-actinin depends on its molecular length. J Cell Biol. 1990 Jun;110(6):2013–2024. doi: 10.1083/jcb.110.6.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. K., Schindler H., Burger M. M. alpha-Actinin interacts specifically with model membranes containing glycerides and fatty acids. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4280–4284. doi: 10.1073/pnas.79.14.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura N., Asano A. Isolation and characterization of a conserved actin-binding domain from rat hepatic actinogelin, rat skeletal muscle, and chicken gizzard alpha-actinins. J Biol Chem. 1986 Aug 15;261(23):10680–10687. [PubMed] [Google Scholar]
- Mooseker M. S., Coleman T. R. The 110-kD protein-calmodulin complex of the intestinal microvillus (brush border myosin I) is a mechanoenzyme. J Cell Biol. 1989 Jun;108(6):2395–2400. doi: 10.1083/jcb.108.6.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooseker M. S. Organization, chemistry, and assembly of the cytoskeletal apparatus of the intestinal brush border. Annu Rev Cell Biol. 1985;1:209–241. doi: 10.1146/annurev.cb.01.110185.001233. [DOI] [PubMed] [Google Scholar]
- Otey C. A., Pavalko F. M., Burridge K. An interaction between alpha-actinin and the beta 1 integrin subunit in vitro. J Cell Biol. 1990 Aug;111(2):721–729. doi: 10.1083/jcb.111.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee J. D., Spudich J. A. Purification of muscle actin. Methods Enzymol. 1982;85(Pt B):164–181. doi: 10.1016/0076-6879(82)85020-9. [DOI] [PubMed] [Google Scholar]
- Parry D. A., Dixon T. W., Cohen C. Analysis of the three-alpha-helix motif in the spectrin superfamily of proteins. Biophys J. 1992 Apr;61(4):858–867. doi: 10.1016/S0006-3495(92)81893-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavalko F. M., Burridge K. Disruption of the actin cytoskeleton after microinjection of proteolytic fragments of alpha-actinin. J Cell Biol. 1991 Aug;114(3):481–491. doi: 10.1083/jcb.114.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavalko F. M., Otey C. A., Simon K. O., Burridge K. Alpha-actinin: a direct link between actin and integrins. Biochem Soc Trans. 1991 Nov;19(4):1065–1069. doi: 10.1042/bst0191065. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Cooper J. A. Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu Rev Biochem. 1986;55:987–1035. doi: 10.1146/annurev.bi.55.070186.005011. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Cooper J. A. Methods to characterize actin filament networks. Methods Enzymol. 1982;85(Pt B):211–233. doi: 10.1016/0076-6879(82)85022-2. [DOI] [PubMed] [Google Scholar]
- Radmacher M., Tillamnn R. W., Fritz M., Gaub H. E. From molecules to cells: imaging soft samples with the atomic force microscope. Science. 1992 Sep 25;257(5078):1900–1905. doi: 10.1126/science.1411505. [DOI] [PubMed] [Google Scholar]
- Sato M., Schwarz W. H., Pollard T. D. Dependence of the mechanical properties of actin/alpha-actinin gels on deformation rate. 1987 Feb 26-Mar 4Nature. 325(6107):828–830. doi: 10.1038/325828a0. [DOI] [PubMed] [Google Scholar]
- Schering B., Bärmann M., Chhatwal G. S., Geipel U., Aktories K. ADP-ribosylation of skeletal muscle and non-muscle actin by Clostridium perfringens iota toxin. Eur J Biochem. 1988 Jan 15;171(1-2):225–229. doi: 10.1111/j.1432-1033.1988.tb13780.x. [DOI] [PubMed] [Google Scholar]
- Schmidt C. F., Zimmermann R. M., Gaub H. E. Multilayer adsorption of lysozyme on a hydrophobic substrate. Biophys J. 1990 Mar;57(3):577–588. doi: 10.1016/S0006-3495(90)82573-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small J. V. Geometry of actin-membrane attachments in the smooth muscle cell: the localisations of vinculin and alpha-actinin. EMBO J. 1985 Jan;4(1):45–49. doi: 10.1002/j.1460-2075.1985.tb02315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Goll D. E., Singh I., Allen R. E., Robson R. M., Stromer M. H. Some properties of purified skeletal muscle alpha-actinin. J Biol Chem. 1976 Nov 10;251(21):6860–6870. [PubMed] [Google Scholar]
- Takahashi K., Hattori A. Alpha-actinin is a component of the Z-filament, a structural backbone of skeletal muscle Z-disks. J Biochem. 1989 Apr;105(4):529–536. doi: 10.1093/oxfordjournals.jbchem.a122701. [DOI] [PubMed] [Google Scholar]
- Tamm L. K., McConnell H. M. Supported phospholipid bilayers. Biophys J. 1985 Jan;47(1):105–113. doi: 10.1016/S0006-3495(85)83882-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K. A., Taylor D. W. Formation of 2-D paracrystals of F-actin on phospholipid layers mixed with quaternary ammonium surfactants. J Struct Biol. 1992 Mar-Apr;108(2):140–147. doi: 10.1016/1047-8477(92)90013-z. [DOI] [PubMed] [Google Scholar]
- Timbs M. M., Thompson N. L. Slow rotational mobilities of antibodies and lipids associated with substrate-supported phospholipid monolayers as measured by polarized fluorescence photobleaching recovery. Biophys J. 1990 Aug;58(2):413–428. doi: 10.1016/S0006-3495(90)82387-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts T. H., Gaub H. E., McConnell H. M. T-cell-mediated association of peptide antigen and major histocompatibility complex protein detected by energy transfer in an evanescent wave-field. Nature. 1986 Mar 13;320(6058):179–181. doi: 10.1038/320179a0. [DOI] [PubMed] [Google Scholar]
- Weisenhorn A. L., Drake B., Prater C. B., Gould S. A., Hansma P. K., Ohnesorge F., Egger M., Heyn S. P., Gaub H. E. Immobilized proteins in buffer imaged at molecular resolution by atomic force microscopy. Biophys J. 1990 Nov;58(5):1251–1258. doi: 10.1016/S0006-3495(90)82465-6. [DOI] [PMC free article] [PubMed] [Google Scholar]