Abstract
Background
Hepatocellular carcinoma (HCC) causes a large worldwide health burden, needing novel ways to prevention and treatment. Traditional Chinese medicine, which is rich in bioactive substances, has emerged as a viable approach to tackling HCC difficulties. Artemisia rupestris L. (AR), a perennial plant, has received interest for its immunoregulatory qualities and potential protection against viral influenza and hepatocellular cancer.
Methods
In this work, we looked at the pharmacological effects of Artemisia rupestris L. extract (ARE) on HCC mice. We used 16 S rRNA sequencing and computational biology approaches to investigate ARE-induced changes in bacterial composition inside HCC mouse tissues. Furthermore, we used liquid chromatography tandem mass spectrometry (UPLC-MS/MS) to identify metabolic changes caused by ARE.
Results
Our data indicate that ARE affects hepatocellular cancer via several pathways. AR offers a multi-faceted strategy to combating HCC by influencing critical metabolic pathways such α-linolenic acid and glycerophospholipid metabolism.
Conclusions
This research sheds new light on Artemisia rupestris L.‘s anticancer potential, setting the platform for a more in-depth knowledge of its influence on hepatocellular carcinoma using a multi-omics approach.
Keywords: Flavonoids, Hepatocellular carcinoma, Microbiome
Introduction
Hepatocellular carcinoma (HCC) ranks among the most prevalent malignant tumors worldwide and stands as the second leading cause of tumor-related mortality in China [1, 2]. Unfortunately, the present prognosis and treatment options for liver cancer are inadequate. Emerging evidence underscores the critical role of the gut microbiota in liver diseases, including HCC. Dysbiosis of the gut microbiome has been linked to chronic inflammation, metabolic dysfunction, and tumor progression, suggesting that microbiota modulation may offer a promising therapeutic avenue.
Recent experimental study results emphasize the anticancer potential of traditional Chinese medicine (TCM) and its bioactive ingredients [3, 4]. These medicines are thought to inhibit cancer cell growth, slow the cell cycle, induce apoptosis and autophagy, boost immunological responses, modify signaling pathways, and overcome drug resistance in hepatocellular carcinoma cells. Herbal medicine has a broader spectrum of pharmacological effects than single-receptor medicines [5]. TCM herbs like Panax ginseng and Astragali radix have demonstrated efficacy in cancer treatment, partly via microbiota-dependent pathways [6]. These findings highlight the intricate interplay between herbal medicine, the microbiome, and host metabolism—a relationship central to our study.
Artemisia rupestris L. (AR) is a perennial plant of the Artemisia (Compositae) family that is widely spread in Xinjiang (China), Middle Asia, and Europe. AR’s chemical makeup consists mostly of caffeoyl quinic acid derivatives, flavonoids, monomeric and dimeric sesquiterpenoids, fatty acids, penylpropanoids, and others [7, 8]. Notably, recent work suggests that AR and related species (e.g., Artemisia capillaris) may ameliorate HCC by reshaping gut microbial communities and restoring metabolic homeostasis [9, 10]. However, the precise mechanisms linking AR-induced microbiota shifts to antitumor effects remain unexplored.
We wanted to investigate the pharmacological effects and associated alterations in HCC mice after administering Artemisia rupestris L. extract (ARE). We sequenced 16 S rRNA from HCC mouse tissues and used computational biology approaches to quantify the bacteria present. Meanwhile, metabolic disturbances were studied using liquid chromatography tandem mass spectrometry (UPLC-MS/MS) technology.
Artemisia capillaris may indirectly influence hepatocellular carcinoma by modulating the composition and activity of intestinal microbes, thereby affecting critical metabolic pathways such as α-linolenic acid and glycerophospholipid metabolism. This intricate interplay between the herb and the gut microbiota sheds light on novel perspectives for understanding the mechanisms underlying Artemisia capillaris’ effects on hepatocellular cancer. Employing a multi-omics approach allows for a comprehensive investigation, elucidating the intricate molecular networks and interactions involved in this process. These findings not only deepen our understanding of the complex relationship between natural compounds and cancer but also provide a valuable framework for developing targeted therapeutic strategies against hepatocellular carcinoma.
Materials and methods
Cell culture
The HCC cell line HepG2 was sourced from the Cell Bank of the Chinese Academy of Medical Sciences in Shanghai, China. The cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA, USA) at 37 °C in a humidified atmosphere containing 5% CO2. The culture medium was refreshed every 2–3 days to maintain optimal cell conditions. Passage of cells was performed upon reaching 80–90% confluency using a 0.25% trypsin-EDTA solution to detach cells from the culture vessel. After a 24-hour incubation period, hepatocellular carcinoma (HCC) cells were exposed to lipopolysaccharide (LPS) at a concentration of 100 ng/ml (Sigma-Aldrich) subsequent to 24 h, then given various dosages of ARE (2.5, 5, 10, 20 µg/ml). Serum-free media supplemented with 0.3% dimethyl sulfoxide (DMSO) was used to treat the cells in the control group.
Drug extraction
The AR substance was meticulously measured at a weight of 100 g and subjected to extraction using eight-fold the quantity of water heated to reflux for a duration of 1 h. The filtrates obtained from each of the three extractions were pooled together. Ultimately, a concentration of 0.6 g/mL of the initial medication was achieved through rotary evaporation.
16S rDNA
For each set of rat cecum samples, microbial genomic DNA was extracted using the DNeasy PowerSoil Kit (Qiagen, Germany) following the manufacturer’s protocol, with additional bead-beating steps to ensure optimal lysis of tough bacterial cell walls. DNA concentration and purity were assessed using a NanoDrop spectrophotometer (OD260/280 ratio > 1.8) and 1% agarose gel electrophoresis. The PD entire tree was used to estimate the α-diversity of each group’s gut flora. The rank abundance curves were produced using R software (version 4.1.2). The analysis involved the application of the non-metric multidimensional scaling (NMDS) technique for component analysis, and the linear discriminant analysis effect size (LEfSe) was executed to illustrate significant bacterial alterations. A threshold LDA score of 3 was employed for screening purposes in the linear discriminant analysis (LDA).
Metabolomics analysis
Thaw each set of serum samples thoroughly on ice. Take 50 µL of the sample and combine it with 300 µL of acetonitrile-methanol solution (1:4). Vortex the mixture for 3 min, followed by centrifugation at 4 °C at 12,000 revolutions/minute for 10 min. Place 200 µL of the obtained supernatant into a centrifuge tube labeled with the corresponding number. Ensure accurate matching of the supernatant to its designated tube by using proper labeling, allowing it to sit at -20 °C for 30 min. Subsequently, centrifuge for an additional 3 min at 4 °C and 12,000 revolutions per minute. Post-centrifugation, transfer 180 µL of the supernatant to a tube equipped with the appropriate injection bottle for subsequent online analysis. The chromatographic analysis was performed using a Waters ACQUITY UPLC HSS T3 C18 column (2.1 mm × 100 mm, 1.8 μm). The mobile phase consisted of solvent A (0.1% formic acid in water) and solvent B (0.1% formic acid in acetonitrile), employing a gradient elution method. The gradient program was set as follows: from 0 to 11 min, a linear increase from 5 to 90% B; then from 11 to 12 min, holding at 90% B; followed by a rapid return to 5% B from 12 to 12.1 min; maintaining at 5% B from 12.1 to 14.1 min; and finally, equilibrating at 5% B from 14.1 to 14.5 min. Consistent flow at a rate of 0.4 mL/min was ensured throughout the analysis by maintaining the column temperature at 40 °C. Each analysis involved injecting a sample volume of 2 µL. This standardized approach helped maintain uniformity and accuracy across all experiments.
The ion source was an electrospray ionisation source (ESI) with positive and negative ionisation modes; the ion source voltage was 2 500 V (+) and 1 500 V (-), the flow rate of auxiliary gas was 8 L-min-1, the fragmentation voltage was 135 V, the temperature of the ion source and the temperature of the sheath gas were set at 325 °C, the flow rate of the sheath gas was 11 L-min-1, and the voltage of the nebulising gas was 40 V. The ion source temperature and sheath gas temperature were set at 325 °C, the flow rate of the sheath gas was 11 L-min-1, and the voltage of the nebulising gas was 40 V. The ion source temperature and sheath gas voltage were set at 2 500 V (+) and 1 500 V (-).
RT-qPCR
HCC cells were collected, and total RNA extraction was performed using TRIzol reagent (Invitrogen, Shanghai, China) according to the manufacturer’s instructions. The isolated RNA was subsequently reverse transcribed into cDNA with the PrimeScriptTM RT Reagent Kit (Takara, Shanghai, China). RT-qPCR was carried out using SYBR® Premix-Ex-Taq (Takara, Shanghai, China) and ABI7300 systems. The expression of miR-181c was normalized to the internal reference GAPDH. Relative quantification was conducted by using the 2(−ΔΔCt) method. Primer sequences are as follows: Sirt2 forward 5’-CTGCGGAACTTATTCTCCCAGAC-3’; Sirt2 reverse 5’-CCACCAAACAGATGACTCTGCG-3’; Pge2 forward 5’- GACCACCTCATTCTCCTGGCTA-3’; Pge2 reverse 5’- AACCTAAGAGCTTGGAGGTCCC-3’.
Western blot
Proteins were extracted from HCC cells using RIPA lysis buffer (Beyotime Biotechnology, Shanghai, China). The protein samples were then combined with loading buffer and boiled in water for 10 min to denature them. Following denaturation, the proteins were separated by SDS-PAGE and transferred onto a polyvinylidene fluoride (PVDF) membrane. This process ensures the effective isolation and preparation of proteins for downstream analysis (Millipore, Bedford, MA, USA). Following this, the membranes underwent treatment with 5% defatted milk to obstruct non-specific binding sites. They were then subjected to overnight incubation at 4 °C with primary antibodies to facilitate specific antigen detection. The primary antibody used was anti-Casp3 (diluted at 1:500; ab4051, Abcam, Shanghai, China). Following thorough washing with TBST, the membranes underwent an incubation step with a horseradish peroxidase-linked secondary antibody (Beyotime, Shanghai, China) for 1 h at room temperature. Subsequently, protein bands were visualized utilizing the enhanced chemiluminescence (ECL) kit (Beyotime, Shanghai, China) to complete the detection process.
Animals
All experiments were conducted in accordance with the regulations of the Laboratory Animal Ethics Committee of the Xinjiang Uygur Medical Research Institute of the Xinjiang Uygur Autonomous Region. Six-week-old BALB/c rats were provided by Xinjiang Medical University Laboratory Animal Centre. The rearing conditions were SPF-grade animal house of Xinjiang Uygur Autonomous Region Uygur Medicine Research Institute, with less than 5 animals in each plastic cage box for rats, free to drink and ingest food. The room temperature was kept at 26 °C, relative humidity was 60%, and light and darkness were each 12 h. The rats were kept for 5 d before the experiment, and after general behavioral observation, healthy rats meeting the requirements were selected for the experiment.
Mouse xenografts
All mice were treated using methods authorized by the Shanghai Medical Experimental Animal Care Commission and the Shanghai Cancer Institute. HCC cells (5 × 106 cells per animal) were injected subcutaneously into the right posterior flanks of 6-week-old BALB/c nude mice (six males per group). To compute tumor volume using calliper readings, use the modified ellipsoidal formula: tumor volume = 1/2 length × width. Following tumor development, mice were randomly randomized to a 5-day per week therapy with vehicle or medication combination, with each chemical provided at the same dosage and schedule as the single agent.
Statistical analysis
Statistical analyses were conducted using GraphPad Prism 8 software (GraphPad Software, San Diego, CA, USA). In this study, comparisons between two groups were conducted using the two-tailed Student’s t-test for normally distributed data and the Mann-Whitney U test for non-normally distributed data. For comparisons involving more than two groups, the Kruskal-Wallis test was applied for non-parametric data, while one-way ANOVA with Tukey’s multiple comparison test was used for parametric data. Statistical significance was defined as p-values below 0.05.
Results
ARE inhibited tumors growth in nude mice
To investigate the in vivo impact of ARE, we established a subcutaneous xenograft tumor model by subcutaneously injecting human HCC cells, which were subsequently treated with or without ARE. When comparing the tumor volume of the ARE group to that of control, the difference was significant (p <.01) (Fig. 1A), as well as significantly lower tumor weight at the conclusion of the trial (p <.05) (Fig. 1B). These findings provide more evidence that ARE has a protective impact in HCC.
Fig. 1.
Effect of ARE on tumor volume and tumor weight. The data are expressed as the mean ± standard deviation (SD) (n = 10). A Tumor growth rate. B Tumor weight. *P <.5; **P <.01; ***P <.05
Distinct tumor-associated microbiome signatures in hepatocellular carcinoma mice
We used alpha and beta diversity analysis to compare the taxonomic composition and microbial diversity of gut microbiota of the control and treatment groups. The 16s rDNA gene sequencing yielded a median of 167,073 clean reads from six fecal samples. The bulk of length distributions varied between 401 and 440 (Fig. 2A). Figure 2B shows the alpha diversity findings (Chao, Shannon, and Simpson index). The Chao index measures the abundance of operational taxonomic units (OTUs), which represents species richness. Figure 2C depicts the relative abundance of dominating species among samples. At the species level, Alistipes and Odoribacter were considerably underrepresented in the treated mice (Fig. 2D).
Fig. 2.
Analyzing the microbial community composition present within HCC tumors. A Length distribution of clean reads obtained from 16s rDNA gene sequencing of fecal samples, represented as a boxplot. B Alpha diversity metrics including Chao, Shannon, and Simpson indices, indicating species richness and diversity among samples. C Relative abundance of dominating species in control and treatment groups. D Comparison of species-level abundance, highlighting underrepresentation of Alistipes and Odoribacter in treated mice. E Weighted UniFrac PCoA revealing clustering patterns between control and treatment groups. F LEfSe analysis identifying discriminative microbial taxa at various taxonomic levels (phylum, class, order, family, and genus), with emphasis on five species distinguishing between experimental groups (LDA > 2, p <.05). PCoA: principal coordinates analysis; OTU: Operational Taxonomic Unit
Next, we employed beta diversity analysis to construct the weighted UniFrac principal coordinates analysis (PCoA), which revealed clustering between two groups (Fig. 2E). The LEfSe analysis unveiled noteworthy variations in the prevalence of microbial taxa at multiple taxonomic tiers, encompassing phylum, class, order, family, and genus levels. This comprehensive examination provided insights into the differential microbial composition across these taxonomic categories, shedding light on potential associations with the studied condition. Five species in particular were shown to be discriminative, suggesting that they may be used to differentiate between experimental groups (LDA > 2, p <.05) (Fig. 2F.).
Metabolomics analysis
Multivariate statistical analysis
PCA was conducted on the UHPLC-MS/MS data of serum from the control and ARE groups, and the findings revealed that the control and ARE groups’ metabolites were well separated (Fig. 3A). Second, Partial Least Squares Discrimination Analysis (PLS-DA) was used, with the control and ARE groups separated into two areas. Figure 3C shows that the PLS-DA model performed well in terms of goodness-of-fit and predictive ability (R2X = 0.737, R2Y = 0.995, Q2 = 0.912, P <.005). Figure 3 shows the proportion of detected metabolites. Lipids and lipid-like compounds were the most often recognized metabolites, followed by organic acids and derivatives.
Fig. 3.
Multivariate Analysis of Serum Metabolomic Profiles. A PCA plot displaying the separation of metabolites in serum samples between groups. B PLS-DA plot demonstrating distinct clustering of the control group and the ARE group. C Pie chart of identified metabolites. PCA: Principal Component Analysis; PLS-DA: Partial Least Squares Discriminant Analysis
Differential metabolite screening
Metabolites with fold changes of ≥ 2 or ≤ 0.5 were tested. A comprehensive analysis revealed that there were anticipated to be 285 metabolites showing significant differences between the blank and model groups.
A total of 285 metabolites exhibited significant differences between the control group and the group treated with ARE. Among these, 145 metabolites were found to be up-regulated, while 140 were down-regulated in the ARE group compared to the control group (Fig. 4A). This color-coded classification shows how distinct classes of metabolites react to ARE therapy in HCC samples (Fig. 4B). Figure 4C shows the top 21 metabolites that changed significantly in response to ARE therapy in HCC mouse tissues. It showed xanthosine, 5-hpete, dihydrothymine, phosphorylcholine and glycerophosphate were most up-regulated, while Pe(16:1e/14-hdohe), Pe(18:2e/7-hdohe), pristanic acid, 1,2-distearoyl-sn-glycero-3-phospho-l-serine and isoanhydroicaritin were most down-regulated.
Fig. 4.
Differential metabolite analysis between the control group and the ARE group. Metabolites were considered significantly different if they exhibited a fold change ≥ 2 or ≤ 0.5, coupled with P <.05.. A The volcano plot visually represents the differential metabolites, with each point denoting a specific metabolite. This plot serves as a graphical depiction of the relationship between fold change and statistical significance for each metabolite, with those exhibiting up-regulation depicted in vibrant red and down-regulation in striking blue. B Differential metabolites were distinctly color-coded based on their assigned categories. C This bar plot illustrates the top 21 metabolites exhibiting significant alterations
Association and pathway analysis of differential metabolites
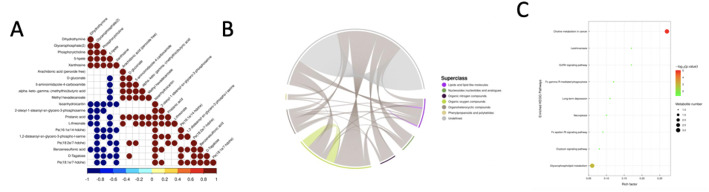
We expanded our analysis by looking at the interrelationships between substantially changed metabolites. The results of this correlation analysis are displayed as a correlation heatmap, which provides a visual representation of the relationships between metabolites (Fig. 5A). The strong positive correlations (red) between upregulated metabolites (e.g., xanthosine, 5-HPETE) and downregulated lipids (e.g., PE derivatives) suggest ARE induces coordinated metabolic reprogramming. This aligns with ARE’s known effects on glycerophospholipid metabolism. Chord diagrams, in addition, give an accessible portrayal of the co-regulatory links between distinct metabolites(Fig. 5B). The thickest chords represent correlations surviving FDR correction (q < 0.05). Furthermore, KEGG enrichment analysis identified key metabolic pathways that were strongly impacted by ARE. Notable pathways, including choline metabolism, glycerophospholipid metabolism, the GnRH signaling system, necroptosis, the oxytocin signaling route, and starch metabolism, showed substantial changes (Fig. 5C).
Fig. 5.
Metabolic interaction and pathway enrichment analysis. A Correlation heat map showing the relationships between substantially different metabolites. Each cell’s color represents the strength and direction of the association between metabolite pairs. B Chord diagrams depicting the co-regulatory interactions among different metabolites. The breadth and color of the chords indicate the intensity and type of the correlations, creating an understandable visual representation of the linkages. C A bar plot displaying major metabolic pathways discovered using KEGG enrichment analysis
Effect of ARE on Sirt2 and PGE2 in serum of rats
After administration of ARE, the mRNA levels of Sirt2 and PGE2 in the blood of rats in the model group exhibited a notable decrease compared to those in the control group (P <.01) (Fig. 6A and B). Moreover, the protein expression level showed similar reduction in ARE groups compared to control group (Fig. 6C).
Fig. 6.
A mRNA expression of Sirt2 B mRNA expression of PGE2 C Expression of SIRT2 protein in HCC cells. The expression levels were measured by qPCR. The expression levels were normalized to GAPDH level. Data are shown as mean ± SD and were analyzed by ordinary one-way ANOVA. *P <.5; **P <.01; ***P <.05
Discussion
Using a multi-omics approach, we investigated the diverse pharmacological effects of Artemisia rupestris L. extract (ARE) on hepatocellular carcinoma (HCC). The study of traditional Chinese medicine, particularly AR, has gained traction owing to its possible therapeutic effects in cancer therapy. Our results demonstrated that AR has a significant influence on HCC, affecting both the makeup of gut bacteria and important metabolic pathways. The normal human intestine contains about 100 trillion microbiotas with a total weight of 1 ~ 2 kg [11]. These microbiotas have vital functions in the human body, such as digestion and absorption, immunological modulation, maintaining the intestinal mucosal barrier, and intestinal homeostasis [12–14].
Identifying AR’s impact on various metabolic pathways, including α-linolenic acid, glycerophospholipid, starch, and sucrose metabolism, sheds light on its probable methods of action. These pathways are known to play important roles in cancer development, and AR regulation indicates a complicated interaction between the herbal extract and HCC metabolism. The complex interplay between herbal components and metabolic pathways emphasizes the significance of a thorough knowledge of traditional Chinese medicine’s holistic effects in cancer treatment. Our data demonstrate that ARE significantly suppresses Sirt2 expression at both transcriptional and translational levels (Fig. 6). This aligns with recent evidence that Sirt2, a NAD+-dependent deacetylase, acts as a dual regulator in HCC. Sirt2 modulates lipid metabolism (e.g., α-linolenic acid pathway) and mitochondrial function, which are perturbed in HCC. The downregulation of PGE2 by ARE (Fig. 6) may indicate that PGE2 as a key node connecting ARE’s anti-inflammatory effects to its microbiome-modulating properties.
We also used modern sequencing technology and computational biology tools to investigate the complex link between AR, gut microbiota, and HCC. The observed alterations in intestinal bacteria point to an indirect impact of AR on HCC via the gut microbiome. Understanding the dynamics of this relationship may lead to the development of tailored treatment techniques that take advantage of the complex interplay between herbal medicine, host metabolism, and the microbiome.
Furthermore, our comprehensive analysis sheds light on the intricate mechanisms underlying Artemisia rupestris L.’s anticancer effects specifically in HCC. Through the integration of multi-omics data, including genomics, transcriptomics, proteomics, and metabolomics, we have unraveled the dynamic interplay between AR, the gut microbiota, and key metabolic pathways implicated in HCC progression.
By elucidating the specific pathways modulated by AR, our study lays a solid foundation for future investigations aimed at harnessing the therapeutic potential of traditional Chinese medicine in cancer treatment. These findings not only deepen our understanding of herbal medicine but also offer valuable insights into the molecular intricacies governing AR’s anticancer activities in the context of hepatocellular carcinoma.
Moreover, our research highlights the importance of a holistic approach in cancer therapy, emphasizing the significance of considering the intricate network of interactions between the host, the tumor microenvironment, and therapeutic agents derived from natural sources like AR. As we delve further into the complexities of AR-mediated anticancer effects, novel therapeutic strategies targeting HCC may emerge, offering hope for improved outcomes and enhanced quality of life for patients battling this devastating disease.
Author contributions
Lina You designed the study and analyzed the experimental data. WuKui Huang conducted the clinical assessments and interpreted the patient outcomes. ShuYing Chen performed statistical analysis and drafted the manuscript. All authors reviewed, edited, and approved the final version of the manuscript.
Funding
This study was financially supported by Natural Science Foundation of Xinjiang Uygur Autonomous Region, 2022D 01C521; 2022D01C510.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of The Affiliated Cancer Hospital of Xinjiang Medical University (grant number: K-2023059). The protocol was approved by the Laboratory Animal Ethics Committee of the Xinjiang Uygur Medical Research Institute, Xinjiang Uygur Autonomous Region, in accordance with the relevant national and institutional guidelines and regulations on laboratory animal welfare. Written informed consent was obtained from individual or guardian participants.
Consent for publication
This manuscript has not been published or presented elsewhere in part or in entirety, and is not under consideration by another journal. All the authors have approved the manuscript and agree with submission to your esteemed journal.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lina You and WuKui Huang are co-first author.
References
- 1.Nagaraju GP, Dariya B, Kasa P, Peela S. El-Rayes, epigenetics in hepatocellular carcinoma. Semin Cancer Biol. 2022;86:622–32. [DOI] [PubMed] [Google Scholar]
- 2.Zhou J, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 Edition). Liver Cancer. 2020;9:682–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li JJ, Liang Q, Sun GC. Traditional Chinese medicine for prevention and treatment of hepatocellular carcinoma: A focus on epithelial-mesenchymal transition. J Integr Med. 2021;19:469–77. [DOI] [PubMed] [Google Scholar]
- 4.Hao X, et al. Inhibition of APOC1 promotes the transformation of M2 into M1 macrophages via the ferroptosis pathway and enhances anti-PD1 immunotherapy in hepatocellular carcinoma based on single-cell RNA sequencing. Redox Biol. 2022;56:102463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei L, et al. Frontier progress of the combination of modern medicine and traditional Chinese medicine in the treatment of hepatocellular carcinoma. Chin Med. 2022;17:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Qiu H, Li C, Cai P, Qi F. The positive role of traditional Chinese medicine as an adjunctive therapy for cancer. Biosci Trends. 2021;15:283–98. [DOI] [PubMed] [Google Scholar]
- 7.Cao X et al. Quality assessment of Artemisia rupestris L. Using quantitative analysis of Multi-Components by single marker and fingerprint analysis. Molecule. 2022;27. [DOI] [PMC free article] [PubMed]
- 8.Song WX, Ji TF, Si YK, Su YL. Studies on chemical constituents in herb from Artemisia rupestris. Zhongguo Zhong Yao Za Zhi. 2006;31:1790–2. [PubMed] [Google Scholar]
- 9.Zhang S, et al. Exploring the molecular mechanism of Artemisia rupestris L. for the treatment of hepatocellular carcinoma via PI3K/AKT pathway. J Ethnopharmacol. 2024;322:117572. [DOI] [PubMed] [Google Scholar]
- 10.Li Q, Weng X, Zhao B, Yang Y, Zhang A. Immunoregulatory properties of the cultivated Artemisia rupestris L. polysaccharide as a potential adjuvant. Carbohydr Polym. 2022;291:119525. [DOI] [PubMed] [Google Scholar]
- 11.Ruppe E, Andremont A. Causes, consequences, and perspectives in the variations of intestinal density of colonization of multidrug-resistant Enterobacteria. Front Microbiol. 2013;4. [DOI] [PMC free article] [PubMed]
- 12.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and Bacteria cells in the body. PLoS Biol. 2016;14:e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sacks D, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. 2018;13:612–32. [DOI] [PubMed] [Google Scholar]
- 14.Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474:1823–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.