Abstract
Mutations in the crumbs homologue 1 (CRB1) gene cause a specific form of retinitis pigmentosa (RP) that is designated “RP12” and is characterized by a preserved para-arteriolar retinal pigment epithelium (PPRPE) and by severe loss of vision at age <20 years. Because of the early onset of disease in patients who have RP with PPRPE, we considered CRB1 to be a good candidate gene for Leber congenital amaurosis (LCA). Mutations were detected in 7 (13%) of 52 patients with LCA from the Netherlands, Germany, and the United States. In addition, CRB1 mutations were detected in five of nine patients who had RP with Coats-like exudative vasculopathy, a relatively rare complication of RP that may progress to partial or total retinal detachment. Given that four of five patients had developed the complication in one eye and that not all siblings with RP have the complication, CRB1 mutations should be considered an important risk factor for the Coats-like reaction, although its development may require additional genetic or environmental factors. Although no clear-cut genotype-phenotype correlation could be established, patients with LCA, which is the most severe retinal dystrophy, carry null alleles more frequently than do patients with RP. Our findings suggest that CRB1 mutations are a frequent cause of LCA and are strongly associated with the development of Coats-like exudative vasculopathy in patients with RP.
We have described mutations in the CRB1 gene (MIM 604210) in a severe, autosomal recessive form of retinitis pigmentosa (RP) that is designated “RP12” (MIM 600105) (den Hollander et al. 1999). The gene consists of 12 exons and exhibits alternative splicing at its 3′ end (A. I. den Hollander and F. P. M. Cremers, unpublished data). The CRB1 protein contains 19 epidermal growth factor (EGF)-like domains, 3 laminin A globular-like domains, a transmembrane domain, and a 37-amino acid cytoplasmic tail; in addition, it is homologous to the Drosophila crumbs protein. RP12 is a specific form of RP characterized by a preserved para-arteriolar retinal pigment epithelium (PPRPE) in the early-to-middle stages of disease. Patients experience night blindness and develop a progressive loss of their visual field at <10 years of age. Because of early macular involvement, patients have severe visual impairment at <20 years of age. Other features of this type of RP are hyperopia, nystagmus, optic-nerve–head drusen, vascular sheathing, and maculopathy (Heckenlively 1982; van den Born et al. 1994). Mutations have now been identified in 15 patients who have isolated or autosomal recessive of RP with PPRPE (den Hollander et al. 1999; U. Kellner, A. I. den Hollander, Y. J. M. de Kok, L. I. van den Born, F. P. M. Cremers, J. R. Heckenlively, unpublished data).
Leber congenital amaurosis (LCA) is considered the earliest and most severe form of retinal dystrophy, causing blindness or severe visual impairment at birth or during the first months of life. Mutations that lead to LCA have been detected in GUCY2D (MIM 600179) (Perrault et al. 1996), RPE65 (MIM 180069) (Gu et al. 1997; Marlhens et al. 1997; Morimura et al. 1998), CRX (MIM 602225) (Freund et al. 1998; Sohocki et al. 1998; Swaroop et al. 1999), and AIPL1 genes (MIM 604392) (Sohocki et al. 2000a). Mutations in these four genes account for 15%–30% of LCA cases (Perrault et al. 1999; Dharmaraj et al. 2000; Lotery et al. 2000; Sohocki et al. 2001), an indication that more LCA genes await discovery. Because of the early onset of symptoms in patients who have RP with PPRPE—and the observation that mutations in RPE65 and CRX can lead to both LCA and RP (Gu et al. 1997; Morimura et al. 1998; Sohocki et al. 1998; Thompson et al. 2000)—we considered CRB1 to be a good candidate gene for LCA.
Fifty-two unrelated patients with LCA were ascertained by ophthalmologists from six centers in the Netherlands, Germany, and the United States. The diagnosis of LCA is made when patients are nonseeing or visually inattentive in infancy and have a nonrecordable electroretinogram when investigated at <1 year of age (Foxman et al. 1985). In early stages, the fundus is, typically, blond; however, in three patients (13067, 16507, and 16690), there was a preservation of the retinal pigment epithelium (RPE) that is characteristic for RP with PPRPE. We used 25 primer sets to screen exons 1–11 of the CRB1 gene by single-strand conformation analysis, as described elsewhere (den Hollander et al. 1999), but we replaced the primer set for exon 5 by primers 5′-TAATTCAACACCTTTGACTTAGC-3′ and 5′-TGCCATAAAATACCAGAAAGTC-3′. Primers used to amplify exon 12 were 5′-CCTGAGTAGTTCCATTGTCC-3′ and 5′-ATTCACAGTGTGTGGATCCC-3′. Products that migrated differently through the gel were analyzed by sequencing. When only one allele was identified in a patient, the patient's sample was also subjected to sequence analysis of all 26 amplicons and of the promoter region of CRB1, which contains several putative photoreceptor-gene regulatory sites (A. I. den Hollander and F. P. M. Cremers, unpublished data), with primers 5′-GTAAAAATCAGCTATAGAAATTGC-3′ and 5′-TTTTCTGTTCATAAATTATATTCCC-3′ (−800 to −345), and primers 5′-TAAGTTTTCTTCTGTCTTGGCC-3′ and 5′-CTGAGGTAGAAGATGAGAAGG-3′ (−421 to +179).
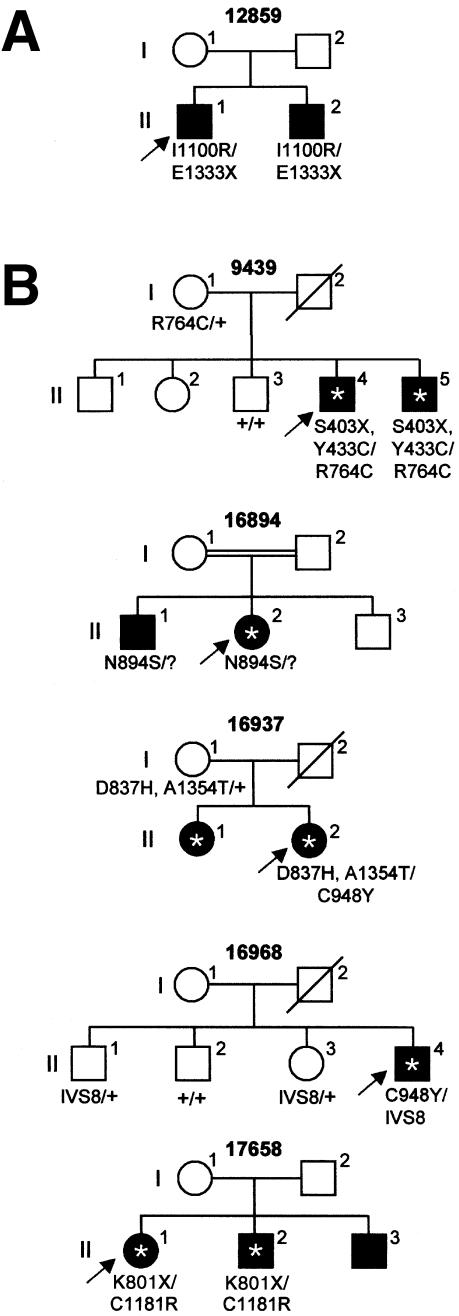
In eight patients with LCA, nine distinct sequence changes in the coding region or splice sites of CRB1 were found (table 1; homozygous Thr821Met polymorphism in proband 12864 not depicted—see below). Two of the changes (Lys801stop and Cys948Tyr) had been identified by us previously (den Hollander et al. 1999; U. Kellner, A. I. den Hollander, Y. J. M. de Kok, L. I. van den Born, F. P. M. Cremers, J. R. Heckenlively, unpublished data) in patients who had RP with PPRPE, but seven of them (748-754del, Thr821Met, Ile1100Arg, Glu1111stop, 4013+1G→T, Arg1331His, and Glu1333stop) had not been identified previously and were not found in 180 control chromosomes. For two probands (12859 and 12864), DNA samples of family members were available for cosegregation analysis (for patient 12859, see fig. 1A). Proband 12864 and one of her unaffected sisters were homozygous for Thr821Met, which suggested that this sequence change is not pathogenic. In patient 12872, only one allele was identified. No sequence changes were detected in the 800 bp that precede the transcription-start site, which suggested that the second allele may be present in other, unidentified regulatory elements or splice variants of the gene or may involve a large deletion that was not detected by PCR analysis.
Table 1.
CRB1 Mutations in Patients Who Have LCA or RP with Coats-like Exudates
|
Allele 1 |
Allele 2 |
||||
| Disease andPatient Number | Inheritance | Mutation | Effect | Mutation | Effect |
| LCA: | |||||
| 12831 | Isolated | 2978G→A | Cys948Tyr | 2978G→A | Cys948Tyr |
| 12859 | Recessive | 3434T→G | Ile1100Arg | 4132G→T | Glu1333stop |
| 12862 | Isolated | 2536A→T | Lys801stop | 2536A→T | Lys801stop |
| 12872 | Recessive | 4127G→T | Arg1331His | ? | |
| 13067 | Isolated | 3466G→T | Glu1111stop | 4013+1G→T | Splice defect |
| 16507 | Isolated | 2978G→A | Cys948Tyr | 2978G→A | Cys948Tyr |
| 16690 | Isolated | 748-754del | Frameshift | 2536A→T | Lys801stop |
| RP with Coats- like exudates: | |||||
| 9439 | Recessive | 1343C→G, 1433A→G | Ser403stop, Tyr433Cys | 2425C→T | Arg764Cys |
| 16894 | Recessive | 2816A→G | Asn894Ser | ? | |
| 16937 | Recessive | 2644G→C, 4195G→A | Asp837His, Ala1354Thr | 2978G→A | Cys948Tyr |
| 16968 | Isolated | 2978G→A | Cys948Tyr | 2978+5G→A | Splice defect |
| 17658 | Recessive | 2536A→T | Lys801stop | 3676T→C | Cys1181Arg |
Figure 1.
Cosegregation analysis of CRB1 mutations in (A) one family with LCA and (B) five families with RP and Coats-like exudates. A question mark (?) denotes the unidentified second allele; an asterisk (*) denotes patients with RP who have developed the Coats-like complication. Arrows indicate probands. IVS8 denotes the splice-site mutation of exon 8, 2978+5G→A.
Of 13 alleles identified in patients with LCA, 7 were nonsense, frameshift, or splice-site mutations, which is a larger proportion than is found on CRB1 alleles of patients who have RP with PPRPE (4 of 30 alleles; den Hollander et al. 1999; U. Kellner, A. I. den Hollander, Y. J. M. de Kok, L. I. van den Born, F. P. M. Cremers, J. R. Heckenlively, unpublished data). In three patients we identified null mutations on both CRB1 alleles, which suggests that LCA is the most severe phenotype that can be associated with mutations in CRB1. Two patients with LCA were homozygous for the mutation (Cys948Tyr) that is most frequently found in patients who have RP with PPRPE. In four patients who had RP with PPRPE, this mutation was found in combination with another missense mutation; and in one patient who had RP with PPRPE, it was found together with a splice-site mutation (2978+5G→A) that does not necessarily render the mutant splice site completely inactive (den Hollander et al. 1999). These findings suggest that Cys948Tyr is a severe mutation that leads to a severe phenotype when it is present homozygously. Cys948Tyr changes the 4th conserved cysteine residue of the 14th EGF-like domain of CRB1, which is involved in the formation of disulfide bridges and thus in the correct folding of the EGF-like domain.
Pathogenic CRB1 mutations were identified in 7 (13%) of 52 unrelated patients with LCA from the Netherlands, Germany, and the United States. Mutations in GUCY2D, RPE65, AIPL1, and CRX account for 6%–20%, 3%–16%, 7%, and 2%–3% of LCA cases, respectively (Morimura et al. 1998; Dharmaraj et al. 2000; Lotery et al. 2000; Perrault et al. 2000; Sohocki et al. 2000b; Thompson et al. 2000), which suggests that CRB1 mutations contribute significantly to the etiology of LCA.
To determine whether mutations in the CRB1 gene are a common cause of RP in the Dutch population, we screened 97 unrelated patients who had isolated or autosomal recessive RP for the presence of Cys948Tyr and Arg764Cys. These mutations had been previously identified in 5 and 3 CRB1 alleles, respectively, of a total of 30 CRB1 alleles of unrelated patients who had RP with PPRPE (den Hollander et al. 1999; U. Kellner, A. I. den Hollander, Y. J. M. de Kok, L. I. van den Born, F. P. M. Cremers, J. R. Heckenlively, unpublished data). The presence of the nucleotide alteration 2425C→T (Arg764Cys) was analyzed by allele-specific oligonucleotide (ASO) hybridization (Shuber et al. 1993), using wild-type primer 5′-AATATATCCGTGTCTGG-3′ and mutant primer 5′-AATATATCTGTGTCTGG-3′. The presence of 2978G→A (Cys948Tyr), was analyzed with the amplification-refractory mutation system (Newton et al. 1989) with sense primers 5′-ATTATCACCTTCTCTCATTAGG-3′ (wild-type allele) or 5′-ATTATCACCTTCTCTCATTAGA-3′ (mutant allele) and antisense primer 5′-GTGCCATCATTCACTGACTG-3′. One of the 97 patients (patient 9439) carried the Arg764Cys mutation on one allele. Sequencing of the 12 exons of CRB1 revealed two more sequence changes (Ser403stop and Tyr433Cys), both of which are located on the second allele, as determined by allele-specific PCR (data not shown) and segregation analysis (fig. 1).
As a complication of RP, proband 9439 (individual II-4) (fig. 2) and his affected brother (II-5) had developed a Coats-like exudative vasculopathy, which caused additional loss of vision. Coats-like exudative vasculopathy, a relatively rare complication of RP, can develop in later stages of the disease and is characterized by vascular abnormalities (aneurysmal dilations and telangiectatic retinal veins), yellow extravascular lipid depositions, and retinal detachment. Patients with RP who develop Coats-like changes show a wide spectrum of disorders, ranging from mild visual difficulties or nyctalopia, as observed in classical RP, to the other extreme, in which a proliferative vasculopathy develops. If untreated, this proliferative vasculopathy may result in a painful blind eye caused by rubeosis, retinal neovascularization, or serous retinal detachment. It has been suggested that genetic factors may be involved in RP with Coats-like exudative vasculopathy (Khan et al. 1988). We therefore hypothesized that CRB1 mutations may be associated with the development of this complication of RP, and we ascertained eight additional isolated or autosomal recessive patients who had RP with Coats-like exudative vasculopathy. These patients were tested for mutations in CRB1 by sequence analysis of all 26 amplicons. Five of the eight patients had sequence changes, and clinical descriptions of these patients are summarized in table 2.
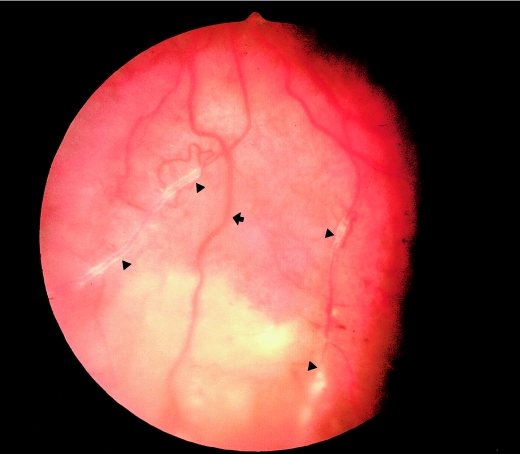
Figure 2.
Fundus photograph of the inferior part of the left eye of a patient who had RP with unilateral Coats-like exudative vasculopathy (proband 9439; individual II-4). Note the widespread subretinal yellowish-white deposits between the neural retina and RPE in the lower part. This region of the fundus is out of focus as a result of the elevation of tissue caused by subretinal accumulation of fluid. Triangles (▴) indicate retinal vessels with white sheathing that is indicative of vasculitis. The RPE shows some preservation near retinal arterioles as seen in patients who had RP with PPRPE (arrow).
Table 2.
Clinical Features of Patients with RP and Coats-like Exudates[Note]
| Patient and Age at Onset of RP | Coats Reaction and Age at Onseta | PPRPE | Refractionb |
| 9439, <10 years | U, 20 years | Yes | +5.25 |
| 16894, 28 years | U, 30 years | No | −6.00 |
| 16937, >10 years | U, 27 years | No | +4.50 |
| 16968, <10 years | U, 20 years | ? | ? |
| 17658, <10 years | B, 20 years | No | +1.00 |
Note.— ? = unknown.
B = bilateral; U = unilateral.
Spherical equivalent.
In one patient, we identified three sequence changes; in two patients, we found compound heterozygous mutations; and in one patient, we identified one allele (table 1). Screening of the promoter region of CRB1 in the patient with one allele revealed no sequence changes. Cosegregation analysis in family members of all five probands confirmed autosomal recessive inheritance of CRB1 mutations (fig. 1). Of the 10 different sequence changes identified in the five patients who had RP with Coats-like exudates, five mutations (Ser403stop, Arg764Cys, Lys801stop, Cys948Tyr, and 2978+5G→A) have been identified previously in patients who had RP with PPRPE. The other five changes (Tyr433Cys, Asp837His, Asn894Ser, Cys1181Arg, and Ala1354Thr) had not previously been identified in patients who had RP with PPRPE or in patients with LCA, and the changes were not found in 180 control chromosomes.
Coats-like exudative vasculopathy occurs in only 1.2%–3.6% of patients with RP (Khan et al. 1988). Among patients who had both RP and PPRPE and were described by Van den Born et al. (1994), 2 (8.3%) of 24 had Coats-like changes, and CRB1 mutations were found in these patients (den Hollander et al. 1999). At least one patient described in the present study (patient II-4 in family 9439) had RP with Coats-like changes and PPRPE. However, the disorders of two patients (patient II-2 in family 16894 and patient II-2 in family 16937) were clearly distinct from RP with PPRPE. In both patients, the onset of RP occurred when patients were >10 years old. Neither patient showed a preservation of the RPE surrounding the arterioles, and one patient was highly myopic.
Our findings show that CRB1 mutations are associated with Coats-like exudative vasculopathy in patients who have RP with or without the PPRPE phenotype. These findings demonstrate that patients with PPRPE should be checked regularly for the Coats-like complication. Furthermore, the routine screening of patients with autosomal recessive or isolated RP may be important because of its ability to reveal those patients who are at increased risk of developing exudative retinal detachment. If the process is detected before it becomes proliferative, cryotherapy can be used to prevent further progression.
Not all affected siblings of patients who have RP with Coats-like exudative vasculopathy develop the Coats-like complication (e.g., families 16894 and 17658 [fig. 1]). This finding, together with the observation that most patients with CRB1 mutations had developed unilateral Coats-like exudates, strengthens the idea that CRB1 mutations are an important risk factor for the development of the Coats-like reaction and that other genetic or environmental factors may be involved. Interestingly, no CRB1 mutations were identified in 13 (45%) of 29 patients who had RP with PPRPE (U. Kellner, A. I. den Hollander, Y. J. M. de Kok, L. I. van den Born, F. P. M. Cremers, J. R. Heckenlively, unpublished data), and in the present study we did not detect CRB1 mutations in four of nine patients who had RP with Coats-like exudates, which suggests that another gene may be involved in these two specific forms of RP.
We found no obvious genotype-phenotype correlation when we compared mutations in patients who had both RP and PPRPE with those in patients who had LCA or RP with Coats-like exudates. However, the absence of clear-cut null mutations on both CRB1 alleles of 15 patients who had RP with PPRPE and of 5 patients who had RP with Coats-like exudates, together with the presence of null mutations on both CRB1 alleles in 3 patients with LCA, suggests that LCA may be associated with complete loss of function of CRB1. In contrast, patients who have RP with PPRPE and patients who have RP with Coats-like exudates may have residual CRB1 function.
RP with PPRPE, RP with Coats-like exudative vasculopathy, and LCA represent different (but partly overlapping) clinical entities, as evidenced by the presence of the PPRPE characteristics in some patients with LCA and the higher-than-average incidence of Coats-like changes in patients who had RP with PPRPE. Because our genotype-phenotype comparison did not reveal conclusive evidence of qualitative or quantitative differences in CRB1 function in these patient groups, functional studies of CRB1 are necessary to shed light on this intriguing issue. We suggest that other genetic—and possibly environmental—factors influence the expression of CRB1 mutations, thereby contributing to the wide spectrum of features that have been described in the present study.
Acknowledgments
We thank Carolien Vink and Bellinda van den Helm for their technical assistance. A.I.d.H. and Y.J.M.d.K. were supported by the Foundation Fighting Blindness. J.R.H. was supported by a Center grant from the Foundation Fighting Blindness and a Clinician-Scientist Award from Research to Prevent Blindness.
Electronic Database Information
References
- den Hollander AI, ten Brink JB, de Kok YJM, van Soest S, van den Born LI, van Driel MA, van de Pol TJR, Payne AM, Bhattacharya SS, Kellner U, Hoyng CB, Westerveld A, Brunner HG, Bleeker-Wagemakers EM, Deutman AF, Heckenlively JR, Cremers FPM, Bergen AAB (1999) Mutations in a human homologue of Drosophila crumbs cause retinitis pigmentosa (RP12). Nat Genet 23:217–221 [DOI] [PubMed] [Google Scholar]
- Dharmaraj S, Silva E, Pina AL, Li YY, Yang J-M, Carter RC, Loyer M, El-Hilali H, Traboulsi E, Sundin O, Zhu D, Koenekoop RK, Maumenee IH (2000) Mutational analysis and clinical correlation in Leber congenital amaurosis. Ophthalmic Genet 21:135–150 [PubMed] [Google Scholar]
- Foxman SG, Heckenlively JR, Bateman JB, Wirtschafter JD (1985) Classification of congenital and early onset retinitis pigmentosa. Arch Ophthalmol 103:1502–1506 [DOI] [PubMed] [Google Scholar]
- Freund CL, Wang Q-L, Chen S, Muskat BL, Wiles CD, Sheffield VC, Jacobson SG, McInnes RR, Zack DJ, Stone EM (1998) De novo mutations in the CRX homeobox gene associated with Leber congenital amaurosis. Nat Genet 18:311–312 [DOI] [PubMed] [Google Scholar]
- Gu S, Thompson DA, Srikumari CRS, Lorenz B, Finckh U, Nicoletti A, Murthy KR, Rathmann M, Kumaramanickavel G, Denton MJ, Gal A (1997) Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat Genet 17:194–197 [DOI] [PubMed] [Google Scholar]
- Heckenlively JR (1982) Preserved para-arteriole retinal pigment epithelium (PPRPE) in retinitis pigmentosa. Br J Ophthalmol 66:26–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan JA, Ide CH, Strickland MP (1988) Coats'-type retinitis pigmentosa. Surv Ophthalmol 32:317–332 [DOI] [PubMed] [Google Scholar]
- Lotery AJ, Namperumalsamy P, Jacobson SG, Weleber RG, Fishman GA, Musarella MA, Hoyt CS, Héon E, Levin A, Jan J, Lam B, Carr RE, Franklin A, Radha S, Andorf JL, Sheffield VC, Stone EM (2000) Mutation analysis of 3 genes in patients with Leber congenital amaurosis. Arch Ophthalmol 118:538–543 [DOI] [PubMed] [Google Scholar]
- Marlhens F, Bareil C, Griffoin J-M, Zrenner E, Amalric P, Eliaou C, Liu S-Y, Harris E, Redmond TM, Arnaud B, Claustres M, Hamel CP (1997) Mutations in RPE65 cause Leber's congenital amaurosis. Nat Genet 17:139–141 [DOI] [PubMed] [Google Scholar]
- Morimura H, Fishman GA, Grover SA, Fulton AB, Berson EL, Dryja TP (1998) Mutations in the RPE65 gene in patients with autosomal recessive retinitis pigmentosa or Leber congenital amaurosis. Proc Natl Acad Sci USA 95:3088–3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton CR, Graham A, Heptinstall LE, Powell SJ, Summers C, Kalsheker N, Smith JC, Markham AF (1989) Analysis of any point mutation in DNA: the amplification refractory mutation system (ARMS). Nucleic Acids Res 17:2503–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault I, Rozet J-M, Calvas P, Gerber S, Camuzat A, Dollfus H, Châtelin S, Souied E, Ghazi I, Leowski C, Bonnemaison M, Le Paslier D, Frézal J, Dufier J-L, Pittler S, Munnich A, Kaplan J (1996) Retinal-specific guanylate cyclase gene mutations in Leber's congenital amaurosis. Nat Genet 14:461–464 [DOI] [PubMed] [Google Scholar]
- Perrault I, Rozet J-M, Gerber S, Ghazi I, Ducroq D, Souied E, Leowski C, Bonnemaison M, Dufier J-L, Munnich A, Kaplan J (2000) Spectrum of retGC1 mutations in Leber’s congenital amaurosis. Eur J Hum Genet 8:578–582 [DOI] [PubMed] [Google Scholar]
- Perrault I, Rozet J-M, Gerber S, Ghazi I, Leowski C, Ducroq D, Souied E, Dufier J-L, Munnich A, Kaplan J (1999) Leber congenital amaurosis. Mol Genet Metab 68:200–208 [DOI] [PubMed] [Google Scholar]
- Shuber AP, Skoletsky J, Stern R, Handelin BL (1993) Efficient 12-mutation testing in the CFTR gene: a general model for complex mutation analysis. Hum Mol Genet 2:153–158 [DOI] [PubMed] [Google Scholar]
- Sohocki MM, Bowne SJ, Sullivan LS, Blackshaw S, Cepko CL, Payne AM, Bhattacharya SS, Khaliq S, Mehdi SQ, Birch DG, Harrison WR, Elder FFB, Heckenlively JR, Daiger SP (2000a) Mutations in a new photoreceptor-pineal gene on 17p cause Leber congenital amaurosis. Nat Genet 24:79–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohocki MM, Daiger SP, Bowne SJ, Rodriquez JA, Northrup H, Heckenlively JR, Birch DG, Mintz-Hittner H, Ruiz RS, Lewis RA, Saperstein DA, Sullivan LS (2001) Prevalence of mutations causing retinitis pigmentosa and other inherited retinopathies. Hum Mutat 17:42–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohocki MM, Perrault I, Leroy BP, Payne AM, Dharmaraj S, Bhattacharya SS, Kaplan J, Maumenee IH, Koenekoop R, Meire FM, Birch DG, Heckenlively JR, Daiger SP (2000b) Prevalence of AIPL1 mutations in inherited retinal degenerative disease. Mol Genet Metab 70:142–150 [DOI] [PubMed] [Google Scholar]
- Sohocki MM, Sullivan LS, Mintz-Hittner HA, Birch D, Heckenlively JR, Freund CL, McInnes RR, Daiger SP (1998) A range of clinical phenotypes associated with mutations in CRX, a photoreceptor transcription-factor gene. Am J Hum Genet 63:1307–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaroop A, Wang Q-L, Wu W, Cook J, Coats C, Xu S, Chen S, Zack DJ, Sieving PA (1999) Leber congenital amaurosis caused by a homozygous mutation (R90W) in the homeodomain of the retinal transcription factor CRX: direct evidence for the involvement of CRX in the development of photoreceptor function. Hum Mol Genet 8:299–305 [DOI] [PubMed] [Google Scholar]
- Thompson DA, Gyürüs P, Fleischer LL, Bingham EL, McHenry CL, Apfelstedt-Sylla E, Zrenner E, Lorenz B, Richards JE, Jacobson SG, Sieving PA, Gal A (2000) Genetics and phenotypes of RPE65 mutations in inherited retinal degeneration. Invest Ophthalmol Vis Sci 41:4293–4299 [PubMed] [Google Scholar]
- van den Born LI, van Soest S, van Schooneveld MJ, Riemslag FCC, de Jong PTVM, Bleeker-Wagemakers EM (1994) Autosomal recessive retinitis pigmentosa with preserved para-arteriolar retinal pigment epithelium. Am J Ophthalmol 118:430–439 [DOI] [PubMed] [Google Scholar]