Abstract
During latent infection of humans with Mycobacterium tuberculosis, bacteria persist in the asymptomatic host within granulomas, organized collections of differentiated macrophages, and other immune cells. The mechanisms for persistence remain poorly understood, as is the metabolic and replicative state of the microbes within granulomas. We analyzed the gene expression profile of Mycobacterium marinum, the cause of fish and amphibian tuberculosis, during its persistence in granulomas. We identified genes expressed specifically when M. marinum persists within granulomas. These granuloma-activated genes were not activated in vitro in response to various conditions postulated to be operant in tuberculous granulomas, suggesting that their granuloma-specific activation was caused by complex conditions that could not be mimicked in vitro. In addition to the granuloma-activated genes, the bacteria resident in granulomas expressed a wide range of metabolic and synthetic genes that are expressed during logarithmic growth in laboratory medium. Our results suggest a dynamic host-pathogen interaction in the granuloma, where metabolically active bacteria are kept in check by the host immune system and where the products of granuloma-specific bacterial genes may thwart the host's attempt to completely eradicate the bacteria.
Infection with Mycobacterium tuberculosis, the causative bacterium of human tuberculosis, results predominantly in an asymptomatic persistent infection, often referred to as latency (1–3). Such persistently infected individuals harbor small, relatively stable numbers of the bacteria within lesions called granulomas, which are comprised of organized collections of highly differentiated macrophages as well as other cells of the immune system. Bacteria are seen within macrophages and within a central acellular necrotic region called caseation necrosis in well-organized human tuberculous granulomas (1). Infected individuals are at risk over their lifetime to convert their asymptomatic infection into what is called reactivation tuberculosis, a disease state both potentially fatal and highly contagious.
The long sojourn of the bacteria in the host during asymptomatic infection poses fundamental biological questions. What is the replicative and metabolic state of the bacteria during persistent asymptomatic infection? The bacteria could be in a nonreplicative, metabolically and transcriptionally inactive, and physiological spore-like state. Alternately, the bacteria could be replicatively, transcriptionally, and metabolically active but kept in check by a dynamic equilibrium with the host immune system. Data from various human and animal models and in vitro studies of M. tuberculosis can be interpreted to support the existence of both states (3, 4). A second key question is how a persistent bacterial infection is sustained indefinitely in the granuloma in the face of a robust host immune response. Recent molecular genetic approaches have yielded Mycobacterium proteins and lipids important for virulence and persistence (5). Hence, it can be argued that the bacteria manipulate the host immune response to ensure their persistence in granulomas.
Mycobacterium marinum, a close genomic relative of M. tuberculosis (6), and the cause of tuberculosis in ectothermic hosts such as fish and frogs (7), has been used to study mycobacterial pathogenesis (8–12). M. marinum also causes a well-described infection in humans largely limited to the surfaces of extremities (13). We recently identified M. marinum genes selectively activated in cultured macrophages and/or granulomas by using the technique of differential fluorescence induction (11). One gene, mag 24–1, encoding a protein homologous to the M. tuberculosis repetitive PE-PGRS family, was found to be important for M. marinum's survival in cultured macrophages and frog granulomas (11).
Here, we have used differential fluorescence induction to conduct a broad survey of M. marinum gene expression in granulomas. Two main categories of granuloma-expressed genes were identified. The majority, encoding metabolic and synthetic functions, was also expressed during logarithmic-phase growth in laboratory medium. In addition, genes activated specifically in granulomas were isolated.
Materials and Methods
Bacteria and Macrophage Cultures.
The wild-type M. marinum strain M (14), strain M transformed with plasmids containing various promoter inserts, and the L1D (mag 24–1) mutant strain (11) were grown in OADC-Tw liquid or agar medium, which is Middlebrook 7H9 (Difco) supplemented as described (14, 15). Kanamycin was used at 20 μg/ml. The J774 A.1 or ATCC TIB67 (J774) mouse macrophage cell line was maintained and infected with M. marinum as described (14).
Frog Infections.
Young adult leopard frogs (Rana pipiens) of 5–6 cm length purchased from J. M. Hazen (Alburg, VT) were maintained in accordance with the animal husbandry guidelines of Stanford University. Three frogs each were inoculated i.p. with M. marinum containing one of three gfp promoter-trap libraries (1.5 × 107, 2.3 × 107, and 3.6 × 108 viable M. marinum per frog, respectively) (11) and euthanized at either 8 weeks (four frogs) or 77 weeks (one frog). Livers and spleens were harvested, a small portion was removed for histological analyses (16), and the remainder was homogenized for FACS sorting as described (11). M. marinum colony-forming units were assessed by using a portion of the homogenates (16).
FACS Sorting of Libraries and Analyses.
FACS sorts and analyses were performed as described (11, 17). Details of the complexity of the libraries have been described; they represent ≈5,000 inserts of 0.4–1.2 kb (at most ≈ one-fourth of the M. marinum promoters, assuming it has a similar number of genes as M. tuberculosis) (11). Eight-week infected livers and 77-week infected spleen were analyzed. Approximately 1,500 viable fluorescent bacteria were obtained from sorting the 8-week livers, representing all three libraries. A total of 700 viable fluorescent bacteria were obtained from sorting the 77-week spleen representing one library.
Assay Conditions for the ex Vivo Induction of Individual map and gap Fusions.
The median fluorescence of bacteria grown under different conditions was quantitated with cellquest software (Becton Dickinson) (11, 17). Details of the conditions tested are published as supporting information on the PNAS web site, www.pnas.org.
Fluorescence Microscopy of Infected Macrophages and Granulomas.
J774 cells were seeded onto wells with glass coverslips, infected with the M. marinum promoter strains (11), and processed as described in additional Methods, which are published as supporting information on the PNAS web site.
Results
Analysis of M. marinum Transcriptional Activity in Granulomas.
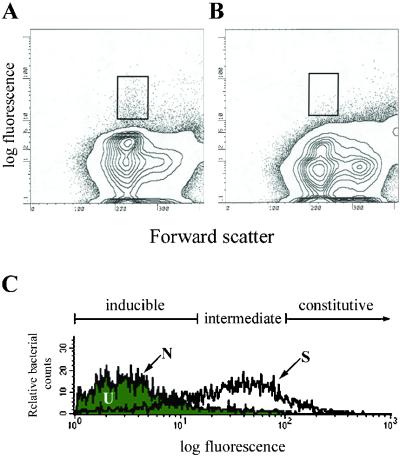
Previously we had focused our differential fluorescence induction analysis of M. marinum from cultured macrophage infections and 8-week granulomas to identify promoters activated selectively in macrophages and/or granulomas (11). Here, we conducted a more comprehensive analysis of M. marinum gene expression in granulomas at 8 and 77 weeks postinfection. Frogs infected with the promoter-trap libraries showed no external signs of disease and had no macroscopic lesions in their organs. The 77-week postinfection spleen was greatly enlarged to approximately 10 times the size of uninfected or 8-week infected spleens. However, it had no other macroscopic changes. Microscopically, the granulomas from both time points consisted predominantly of epithelioid cells as reported (18). The 77-week granulomas had granulocytes interspersed among the epithelioid macrophages. More acid-fast organisms were seen in the 77-week than in the 8-week granulomas although the bacterial colony-forming units were similar at the two time points on a per-weight basis (D. Bouley and L.R., unpublished result). Fluorescent events were sorted from infected livers or spleens, using uninfected organs to determine the sorting gates that would enrich for fluorescent bacteria (Fig. 1 A and B) (11, 17) (population S, see below).
Figure 1.
Fluorescence profiles of the promoter trap library in infected frogs. (A) Fluorescence profile of bacterial promoters isolated from the spleen tissue of frogs infected with the promoter trap library. (B) Fluorescence profile from the spleen tissue of uninfected frogs. (C) Histograms of the promoter trap library before it had been used to infect frogs (N), from infected frog tissue before sorting for fluorescent bacteria (U), and from infected frog tissue immediately after sorting for fluorescent events (S).
To analyze the breadth of bacterial transcriptional activity in granulomas, we examined the fluorescence profile of each promoter-trap library at three points in its experimental history: (i) before it had been used to infect frogs (N, native); (ii) from infected frog tissue before sorting for fluorescent events (U, unsorted); and (iii) the sorted fluorescent events from infected frog tissue (S, sorted) (Fig. 1A). Bacteria/homogenized infected tissue from each of the three conditions were plated on bacteriological agar so as to yield individual colonies.
Several thousand bacterial colonies from each of the three conditions were pooled, grown overnight in bacterial medium, and analyzed by FACS. Population S had a striking shift in the direction of greater fluorescence as compared with population N (Fig. 1C), suggesting that at least some promoters expressed in granulomas were also expressed in logarithmic-phase laboratory culture. The fluorescence histograms of populations U and N were virtually identical (Fig. 1C), suggesting that a wide repertoire of bacterial clones was preserved in the granulomas over extended time periods. Identical FACS profiles were obtained from both the 8-week and the 77-week infected tissues.
Most Genes Expressed in Granulomas Are Also Expressed During Logarithmic-Growth Phase.
FACS analysis of individual colonies isolated from population S confirmed that most colonies were highly fluorescent in logarithmic-phase bacteriological medium (Fig. 2). Such promoters were distinct from the promoter classes we had isolated previously that were selectively expressed in granulomas but not in cultured macrophages (gaps for granuloma-activated promoters), or activated both in granulomas and cultured macrophages (maps for macrophage-activated promoters) (11). We have called the promoters expressed both in the granuloma and during logarithmic-phase laboratory culture caps (constitutively active promoters). The downstream genes of the maps, gaps, and caps are mags, gags, and cags, respectively.
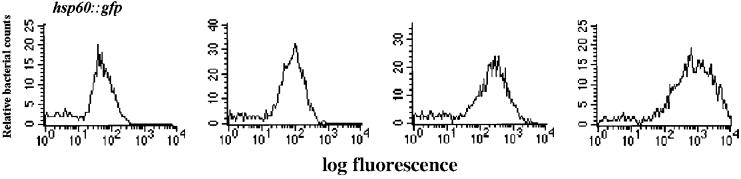
Figure 2.
Fluorescence profiles of caps expressed in the granulomas of infected frogs. Histograms of bacterial green fluorescent protein (GFP)-promoter fusions isolated from the tissue of infected frogs that retain fluorescence equal to or greater than an hsp60∷GFP fusion when plated on bacterial media. Represented are fluorescence profiles of three caps with different fluorescence intensities.
To be conservative in our classification of individual promoter fusions as caps and gaps, stringent cut-offs for peak fluorescence were used (Fig. 1C). The level of the M. tuberculosis hsp60∷gfp fusion expressed in M. marinum served as the cut-off for the initial granuloma sorts and for classification of the individual caps (Fig. 2). Clones with intermediate levels of fluorescence (Fig. 1C) were not analyzed further. Most individual colonies arising from population S were caps (Fig. 2, Table 1).
Table 1.
Classification of promoters active in granulomas
| Frog | caps | Intermediate | gaps | Total | % caps |
|---|---|---|---|---|---|
| 1 | 14 | 3 | 5 | 22 | 63 |
| 2 | 13 | 4 | 0 | 17 | 76 |
| 3 | 31 | 12 | 4 | 47 | 66 |
Frogs 1, 2, and 3 were each infected with one of the three libraries and analyzed at 8 weeks postinfection.
The cap inserts had sequence homologies to M. tuberculosis genes encoding a variety of functions (Table 3, which is published as supporting information on the PNAS web site). In addition, several of the genes had identity to M. tuberculosis genes of unknown function (Table 3). A limited comparison of caps isolated from granulomas (caps 8 and 77) to highly fluorescent promoters isolated by FACS sorting directly from the promoter-trap library before infection (library N in Fig. 1C, caps 0 in Table 3) revealed that similar functional classes of caps were represented in the library before and after infection. Three fluorescent promoter fusions isolated from the unsorted library were also isolated from the granulomas (Table 3), suggesting an overrepresentation of some inserts in the native libraries. Despite this likelihood, only three of 40 caps were isolated more than once from the granuloma sorts (caps 8–20, 8–24/77–4, and 8–25/77–5 in Table 3). Taken together, our results suggest that the caps are comprised of a wide repertoire of M. marinum genes.
Isolation of Additional gaps.
Candidate gaps were identified either directly from the individual colonies derived from population S or by pooling these colonies into DMEM and resorting by FACS for nonfluorescent colonies (Fig. 1C). An additional 14 and six candidate gaps were isolated at 8 and 77 weeks, respectively (Table 2). Because potential gap fusions isolated from granulomas could be gaps or maps (11), each was screened individually for activity in bacterial medium, DMEM, J774 macrophages, and granulomas (Fig. 3). gap and map fusions were distinguished on the basis of intramacrophage fluorescence. None of the newly isolated gaps were expressed in J774 cells. Two gaps isolated at 8 weeks were reisolated at 77 weeks (Table 2). However, only gap 77–4 was the identical insert to gap 3.13. gap 7–33 and gap 77.3 represented different inserts containing the ald promoter and gene.
Table 2.
Characteristics of granuloma-activated promoters/genes
| Promoter construct | M. tuberculosis homolog* | Protein features | Putative granuloma-specific function |
|---|---|---|---|
| gap 3.13† | Rv0133 | Puromycin N-acetyltransferase | Unknown |
| gap 3.19 | PPE family | Glycine-rich repetitive protein | Antigenic variation, virulence (25, 30) |
| gap 7‡ | Rv0631c (recC) | RecC (exoribonuclease V) | RecA-mediated DNA repair (26, 27) |
| gap 7.32 | Rv1874 | Unknown | Unknown |
| gap 7.33† | Rv2780 (ald) | Alanine/glycine dehydrogenase | Synthesis of alanine and glycine |
| gap 15 | Rv0811c | Conserved hypothetical protein | Unknown |
| gap 25‡ | Rv0321 (dcd) | Deoxycytidine deaminase | Conversion of dCTP to dUTP (27–29) |
| gap 26‡ | Rv1205 | Conserved hypothetical protein | Unknown |
| gap 40ठ| Rv1106c | Cholesterol dehydrogenase | Cholesterol metabolism |
| gap 45 | Rv3106 (fprA) | Adrenodoxin and NADPH ferredoxin reductase | Restoration of cellular redox balance during oxidative stress (27) |
| gap 2-8¶ | Rv3709c (ask) | Aspartokinase | Formation of homoserine for lysine, threonine, and methionine synthesis (27) |
| gap 2-11¶ | Rv2179c | Unknown | Unknown |
| gap 2-12¶ | Rv0014c (pknB) | Serine/threonine protein kinase | Yersinia and Pseudomonas virulence (34–36) |
| gap 2-33¶ | Rv0265c (fecB2) | Iron transporter protein | Iron acquisition (27) |
| gap 2A¶ | Rv3451 | Probable cutinase | Possible cell surface modification |
| gap 77-1¶ | Rv0012 | Possible cell division protein | Cell division |
| gap 77-2¶ | Rv0826/Rv1645 | Conserved hypothetical proteins | Unknown |
| gap 77-3† | Rv2780 (ald) | Alanine/glycine dehydrogenase | Synthesis of alanine and glycine (30–33) |
| gap 77-4† | Rv0133 | Puromycin N-acetyltransferase | Unknown |
| map 24‡ | Rv3812/1651c | Glycine-rich repetitive proteins | Antigenic variation, virulence |
| map 25‡ | Rv3416 (whiB3) | Transcriptional activator | Transcriptional activator of early sporulation |
| map 49‡ | Rv1200 | Transmembrane (metabolite) transport protein | Transport in or out of phagocytic vacuole |
| map 83ঠ| Rv0575c | Oxidoreductase | Counter oxidative stress in macrophage |
| map 85‡ | PE | Subfamily of PE-PGRS family | Antigenic variation, virulence |
gaps 77-1 to 77-4 isolated at 77 weeks postinfection. All other gaps isolated at 8 weeks postinfection.
Genes described by Cole et al. (30). Homologies were considered relevant only if they were in the right location and orientation for the putative promoter in the insert to be driving expression of that gene. maps 15, 62 and 86, gaps 7.21 and 4, and one candidate gap isolated at 8 weeks and four candidate gaps isolated at 77 weeks postinfection did not have relevant homologies although the inserts had homology to M. tuberculosis genes in other regions or directions. However, homologies to downstream genes contained within the map 24 and gap 15 promoter insert fragments became apparent only after sequencing beyond the insert into a cloned genomic fragment.
Isolated at both 8 weeks and 77 weeks postinfection. gaps 3.13 and 77-4 are identical inserts whereas gaps 7.33 and 77-3 are different inserts that contain the ald promoter fusion.
Originally described in Ramakrishnan et al. (11).
Homology does not start at the beginning of the M. tuberculosis protein.
Not confirmed individually for activity in granulomas.
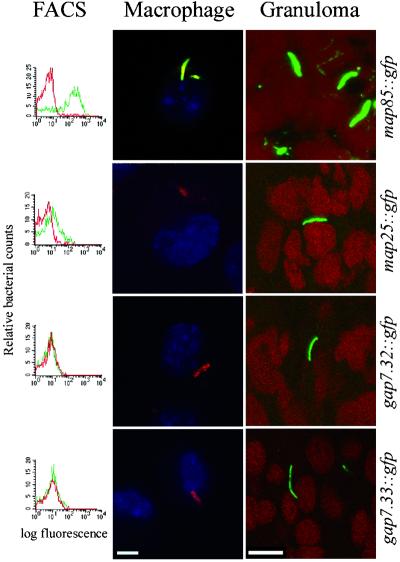
Figure 3.
Analysis of individual promoter fusions. Individual promoter fusions isolated from frog granulomas in the library screen were analyzed for activity in DMEM, J774 macrophages, and granulomas. Red lines in the FACS histograms represent fluorescence in DMEM; green lines indicate fluorescence in macrophages. Macrophage confocal images are overlays of red (bacterial membrane protein), green [for expression of the green fluorescent protein (GFP) fusion in the promoter constructs], and blue (macrophage nuclear staining). Therefore GFP expression in macrophages renders the bacteria yellow. In the absence of GFP expression, they are red fluorescent. Granuloma confocal images are overlays of red (macrophage nuclear stain) and green (for expression of the GFP promoter fusion). The maps and gaps have minimal fluorescence in laboratory medium. map 85 is fluorescent in both macrophages (yellow) and granulomas (green), whereas the two gaps are fluorescent only in granulomas (green) and not in macrophages (red). map 25 straddles the classification between a map and a gap, being only slightly activated in macrophages (red with faint yellow) and green in the granuloma. (Bars represent 10 microns.)
Individual Physiological Signals Activating the maps and gaps.
Individual map and gap fusions were tested for promoter activity under various in vitro growth conditions to try to elucidate signals that activate them in cultured macrophages or the granuloma. Individual test stimuli were chosen based on existing data or hypotheses about the environment of the macrophages or the granuloma.
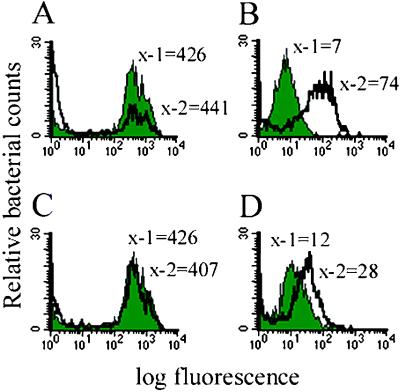
The phagosome of cultured macrophages within which the pathogenic Mycobacteria, including M. marinum, reside has a pH of 6.1–6.5 (8, 19). Therefore the maps were tested for activation in bacteriological media at pH 6.25. FACS analysis revealed that two fusions, map 24 and map 85, both activating genes of the PE/PE-PGRS family (11), were induced (Fig. 4 A and B, and data not shown).
Figure 4.
Fluorescence profiles of promoters under in vitro inducing conditions. Shaded peaks correspond to control conditions (pH 7.0 and 200 μM Mg2+) whereas unshaded peaks correspond to inducing conditions (either pH 6.25 or 20 μM Mg2+). x-1 and x-2 represent the median fluorescence of bacteria in each of the two conditions. (A and B) The msp12 constitutive promoter and the map 24 promoter at pH 7.0 and pH 6.25, respectively. (C and D) The msp12 constitutive promoter and map 25 at 200 μM and 20 μM Mg2+, respectively.
M. marinum phagosomes are frequently fused to lysosomes in granulomas, suggesting that the bacteria may be subjected to an even more acidic environment in vivo than in cultured macrophages (18). Therefore, the M. marinum maps and gaps were tested for activation over a pH range of 5.5 to 7.0 to include a more acidic environment. Interestingly, map 24 showed a pH-dependent temporal profile for activation in that increased fluorescence was seen by 3 h at pH 5.5, by 11 h at pH 6.0, and by 24 h at pH 6.25. However, the same maximal magnitude of activation was seen at each pH. map 85 activation was seen only by 24 h in pH 5.5–7.0. No other maps or gaps were activated at either pH 6.25 or pH 5.5.
Based on evidence that the Mycobacterium phagosomal environment may be low in magnesium (Mg2+), we analyzed the maps in bacteriological media containing either high (200 μM) or low (20 μM) Mg2+ (41). Only map 25 demonstrated increased fluorescence in 20 μM Mg2+ (Fig. 4 C and D) with an activation level equal to that in macrophages (Fig. 3). None of the gaps were activated in low Mg2+. No maps or gaps were activated by the combination of low Mg2+ and low pH.
Because the granuloma environment has been proposed to be of low oxygen tension (4, 20) the M. marinum gap fusions were subjected to hypoxic growth conditions. No activation was observed in log-phase bacteria incubated at 0.1%, 1%, or 4% oxygen for 24 and 48 h. Similarly, none of the gap fusions were induced in stationary phase.
Ultrastructural studies have revealed that M. marinum resides within activated macrophages in granulomas (18). Furthermore, the expression of the M. tuberculosis isocitrate lyase gene (icl) is increased in activated macrophages (21). We therefore tested the gap fusions for activity in J774 macrophages treated with various concentrations of IFN-γ. None of the gap fusions were activated under these conditions. Similarly, the gap fusions were not activated in mouse peritoneal macrophages with or without IFN-γ stimulation. We tested next whether the gaps could be activated by the addition of factors released by the host during its response to infection by pathogenic Mycobacteria. No activation was seen when splenocyte supernatants from M. tuberculosis-infected mice were added to the J774 cell cultures before infection of the cells by the M. marinum gap strains. The activation level of the maps within these different cellular conditions was the same as that in untreated J774 cells.
Growth of the mag 24–1 Mutant Is Not Compromised in Acidic Medium.
Because map 24 was activated in laboratory growth media at pH 5.5–6.25, we determined whether expression of its downstream gene mag 24–1 was important for survival or growth of M. marinum under acidic conditions. We found no difference in the growth of the mag 24–1 mutant, L1D (11), and wild-type M. marinum in OADC-Tw medium at pH 7.0 and 6.25 (Fig. 5A, which is published as supporting information on the PNAS web site) and pH 6.0 up to 7 days (data not shown). Both the wild-type and L1D mutant strains grew more slowly at pH 6.25 and pH 6.0 than at pH 7.0, attaining stationary phase approximately 48 h later (Fig. 5A). The L1D mutant and wild-type bacteria exhibited a significant growth difference in J774 cells by 7 days (Fig. 5B), as shown (11).
Discussion
M. marinum causes a silent long-term infection in frogs with relatively stable numbers of bacteria isolated from the granulomas (≈107 bacteria/g liver or spleen tissue) over many months (16). We have used differential fluorescence induction to examine global bacterial gene expression as well as to identify genes expressed specifically in granulomas. Bacteria from whole organs (rather than isolated granulomas) were analyzed. However, microscopic studies have shown that virtually all bacteria reside in granulomas (18). Our analysis is not saturating as we have screened less than a fourth of the M. marinum genes. This is corroborated by the fact that most of the promoters isolated in the cap screen were unique. Furthermore, we failed to reisolate all of the maps in the gap screen even though all of the maps are active in granulomas and present in the libraries (11). This finding suggests that M. marinum has many more genes expressed in the granuloma.
We tested whether the bacteria were in a predominantly quiescent state in the granuloma as judged by their transcriptional activity therein and observed a wide spectrum of bacterial genes expressed. Indeed, the majority of gene fusions expressed in granulomas were also expressed in logarithmic-phase bacteria grown in laboratory medium (Table 1). Many of these caps represented genes encoding proteins active in key metabolic and synthetic pathways, including cell wall synthesis, as well as transcriptional regulators (Table 3). From the wide repertoire of gene fusions expressed and their putative products, we conclude that at least a predominant population of bacteria are metabolically active in M. marinum granulomas. Evidence of transcriptional activity in a mouse model of tuberculosis latency (where culturable M. tuberculosis were not present) was also found when transcriptional analysis of four selected M. tuberculosis genes was performed (22, 23). Ultrastructural studies also suggest that M. marinum turnover occurs in the granuloma over extended time periods (18).
The bacteria also express a number of genes (gaps) selectively during granuloma persistence (Table 2) (11). Of these, only a few are also activated in cultured macrophages (maps). The diverse gene classes comprising the gaps and maps appear similar to those comprising the caps expressed in granulomas (Tables 1 and 2). Metabolic and synthetic functions, stress response genes, including oxidoreductases, and homologues of M. tuberculosis genes of unknown function were found both among the caps, and the maps and gaps (Tables 2 and 3). Interestingly, distinct members of the large PE/PE-PGRS and PPE gene families were isolated both as caps, and maps or gaps (Tables 1 and 2). Some M. tuberculosis PE-PGRS genes are also constitutively expressed (24). Individual members of these two families play a role in Mycobacterium persistence in vivo (11, 24, 25). Thus, it will be especially interesting to determine whether the selective expression of certain family members in granulomas determines their specific role in bacterial persistence in vivo.
Some of the gaps provide clues to the nature of the granuloma environment. gaps 7 (recC), 45 (fprA), 25 (dcd), and 7.33 (ald) reveal homologies to bacterial genes encoding proteins likely involved in stress or starvation response pathways (26–29). The up-regulation of dcd, a pyrimidine salvage pathway gene, may reflect a granuloma environment lacking in pyrimidines.
Granuloma-specific activation of the M. marinum ald gene encoding alanine/glycine dehydrogenase (30, 31) is of particular interest. In M. tuberculosis this enzyme catalyzes the formation of alanine and/or glycine (31, 32) and is a major secreted antigen that is not secreted by the nonpathogenic vaccine strain M. bovis, bacillus Calmette–Guérin (33). Also of particular interest is the granuloma induction of the M. tuberculosis pknB homologue encoding a protein serine/threonine kinase (PSTK) (30, 34). PSTKs are involved in the host survival of the pathogens Yersinia pseudotuberculosis (YopO or YpkA) and Pseudomonas aeruginosa (PpkA) (35, 36).
Our efforts to identify in vitro signals up-regulating map expression have met with limited success, suggesting that many maps respond to complex environmental cues within macrophages. The obvious candidate signals of low pH and magnesium, thought to be operant in the vacuolar environment, revealed activation of three maps. Furthermore, the finding that mag 24–1 mutant was compromised for intramacrophage growth but not for growth at low pH, suggests a scenario where mag 24–1 expression is induced by the low pH it first encounters in the phagosome in preparation to counter a different adversity therein.
The signals activating the gaps have proved even more recalcitrant to dissection, consistent with the more complex milieu of the granuloma as compared with cultured macrophages. Despite the finding that M. marinum resides within macrophages of varying degrees of activation in granulomas (18), activation of infected macrophages with IFN-γ or splenocyte supernatants failed to activate the gaps. These results suggest that the bacterial milieu during intramacrophage persistence in the granuloma is different from that of activated macrophages in vitro culture. Electron microscopic findings of a complex organization and cellular diversity in the granuloma support this idea (18).
Stationary phase and particularly hypoxia, postulated to be operant in tuberculous granulomas (3, 4), failed to induce the gaps. Even gap 7.33 (ald) was not induced despite reports of increased glycine dehydrogenase activity in hypoxic M. tuberculosis cultures (20). However, our results are not surprising in light of a recent microarray study of the M. tuberculosis transcriptional response to hypoxia where neither icl nor ald were induced (37). Furthermore, none of the M. marinum gap homologues were induced in M. tuberculosis by hypoxia (37). Thus, it is possible that the induction of the ald- and icl-encoded activities in the long-term M. tuberculosis hypoxic cultures, was in response to stimuli other than pure hypoxia (20). It is also important to note that there is no experimental evidence that the granuloma environment is hypoxic (38, 39).
In conclusion, our results provide a picture of the granuloma where the bacteria are metabolically active, expressing a multitude of genes also expressed during logarithmic-growth phase in laboratory medium. They also express granuloma-specific genes among whose products may combat oxidative stress, nutrient stress, and low pH. The repertoire of the gaps indicates the bacteria have adapted a highly complex state. For instance, on the one hand, the activation of whiB3 encoding putative sporulation-related functions would suggest acquisition of a quiescent state (40). On the other hand, activation of genes encoding enzymes involved in protein and peptidoglycan synthesis, metabolite and iron transporters, kinases and a putative cell division protein suggest enhanced activity of certain metabolic, synthetic, and cell cycle pathways. It is also important to bear in mind that the function of these gene products in M. marinum may be different from the proposed functions based on homology to other bacterial proteins.
The bacteria are in heterogeneous niches in the granuloma as judged by ultrastructural studies (18). Therefore, it is possible that there is heterogeneous expression of each of the gaps within the granuloma, such that any given gap is expressed only by a subset of bacteria dictated by its particular microenvironment. In any case, the state of the mycobacteria in the granuloma would appear to defy classification as spore-like or vegetative, quiescent or active. Rather the bacteria may have orchestrated a pattern of gene expression leading to a unique physiological state to allow their long-term survival in the granuloma. Mutational analysis of the gaps coupled with further dissection of their activating signals should provide insight into the persistence mechanisms of the pathogenic Mycobacteria, including M. tuberculosis.
Supplementary Material
Acknowledgments
We thank N. Salama, C. Cosma, D. Sherman, J. Ernst, S. Miller, and M. Bergman for valuable discussions; E. Johnson and D. Sherman for help with the hypoxia experiments; J. Ernst and M. Bergman for providing splenocyte supernatants and mouse peritoneal macrophages, respectively; N. Ghori, M. Amieva, and S. L. Palmieri for advice on confocal microscopy; K. Klein and D. Cunanan for excellent technical assistance; and N. Salama and C. Cosma for a critical reading of the manuscript. This work was supported by National Institutes of Health Grant R01 AI 36396 (to L.R.). K.C. is supported by a National Science Foundation Graduate Research Fellowship.
Abbreviations
- gap
granuloma-activated promoter
- map
macrophage-activated promoter
- cap
constitutively active promoter
References
- 1.Dannenberg A M., Jr Hospital Practice. 1993;28:51–58. doi: 10.1080/21548331.1993.11442738. [DOI] [PubMed] [Google Scholar]
- 2.Flynn J L, Chan J. Infect Immun. 2001;69:4195–4201. doi: 10.1128/IAI.69.7.4195-4201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manabe Y C, Bishai W R. Nat Med. 2000;6:1327–1329. doi: 10.1038/82139. [DOI] [PubMed] [Google Scholar]
- 4.Wayne L G, Sohaskey C D. Annu Rev Microbiol. 2001;55:139–163. doi: 10.1146/annurev.micro.55.1.139. [DOI] [PubMed] [Google Scholar]
- 5.Glickman M S, Jacobs W R., Jr Cell. 2001;104:477–485. doi: 10.1016/s0092-8674(01)00236-7. [DOI] [PubMed] [Google Scholar]
- 6.Tonjum T, Welty D B, Jantzen E, Small P L. J Clin Microbiol. 1998;36:918–925. doi: 10.1128/jcm.36.4.918-925.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark H F, Shepard C C. J Bacteriol. 1963;86:1057–1069. doi: 10.1128/jb.86.5.1057-1069.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker L P, George K M, Falkow S, Small P L. Infect Immun. 1997;65:1497–1504. doi: 10.1128/iai.65.4.1497-1504.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Etr S H, Yan L, Cirillo J D. Infect Immun. 2001;69:7310–7317. doi: 10.1128/IAI.69.12.7310-7317.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pagan-Ramos E, Song J, McFalone M, Mudd M H, Deretic V. J Bacteriol. 1998;180:4856–4864. doi: 10.1128/jb.180.18.4856-4864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramakrishnan L, Federspiel N A, Falkow S. Science. 2000;288:1436–1439. doi: 10.1126/science.288.5470.1436. [DOI] [PubMed] [Google Scholar]
- 12.Talaat A M, Reimschuessel R, Wasserman S S, Trucksis M. Infect Immun. 1998;66:2938–2942. doi: 10.1128/iai.66.6.2938-2942.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Travis W D, Travis L B, Roberts G D, Su D W, Weiland L W. Arch Pathol Lab Med. 1985;109:1109–1113. [PubMed] [Google Scholar]
- 14.Ramakrishnan L, Falkow S. Infect Immun. 1994;62:3222–3229. doi: 10.1128/iai.62.8.3222-3229.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs W R, Jr, Kalpana G V, Cirillo J D, Pascopella L, Udani R A, Jones W D, Jr, Barletta R G, Bloom B R. Methods Enzymol. 1991;204:537–555. doi: 10.1016/0076-6879(91)04027-l. [DOI] [PubMed] [Google Scholar]
- 16.Ramakrishnan L, Valdivia R H, McKerrow J H, Falkow S. Infect Immun. 1997;65:767–773. doi: 10.1128/iai.65.2.767-773.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valdivia R H, Ramakrishnan L. Methods Enzymol. 2000;326:47–73. doi: 10.1016/s0076-6879(00)26046-1. [DOI] [PubMed] [Google Scholar]
- 18.Bouley D M, Ghori N, Mercer K L, Falkow S, Ramakrishnan L. Infect Immun. 2001;69:7820–7831. doi: 10.1128/IAI.69.12.7820-7831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sturgill-Koszycki S, Schlessinger P H, Chakraborty P, Haddix P L, Collins H L, Fok A K, Allen R D, Gluck S L, Heuser J, Russell D G. Science. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 20.Wayne L G, Lin K Y. Infect Immun. 1982;37:1042–1049. doi: 10.1128/iai.37.3.1042-1049.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKinney J D, Honer zu Bentrup K, Munoz-Elias E J, Miczak A, Chen B, Chan W T, Swenson D, Sacchettini J C, Jacobs W R, Jr, Russell D G. Nature (London) 2000;406:735–738. doi: 10.1038/35021074. [DOI] [PubMed] [Google Scholar]
- 22.Hu Y, Mangan J A, Dhillon J, Sole K M, Mitchison D A, Butcher P D, Coates A R. J Bacteriol. 2000;182:6358–6365. doi: 10.1128/jb.182.22.6358-6365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pai S R, Actor J K, Sepulveda E, Hunter R L, Jaganath C. Microb Pathog. 2000;28:335–342. doi: 10.1006/mpat.2000.0353. [DOI] [PubMed] [Google Scholar]
- 24.Brennan M J, Delogu G, Chen Y, Bardarov S, Kriakov J, Alavi M, Jacobs W., Jr Infect Immun. 2001;69:7326–7333. doi: 10.1128/IAI.69.12.7326-7333.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camacho L R, Ensergueix D, Perez E, Gicquel B, Guilhot C. Mol Microbiol. 1999;1999:257–267. doi: 10.1046/j.1365-2958.1999.01593.x. [DOI] [PubMed] [Google Scholar]
- 26.Eggleston A K, West S C. Trends Genet. 1996;12:20–26. doi: 10.1016/0168-9525(96)81384-9. [DOI] [PubMed] [Google Scholar]
- 27.Lynch A S, Lin E C C. In: Escherichia coli and Salmonella typhimurium. Neidhardt F C, editor. Washington, DC: Am. Soc. Microbiol.; 1996. pp. 1526–1538. [Google Scholar]
- 28.Neuhard J, Thomassen E. J Bacteriol. 1971;105:657–665. doi: 10.1128/jb.105.2.657-665.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Donovan G A, Edlin G, Fuchs J A, Neuhard J, Thomassen E. J Bacteriol. 1971;105:666–672. doi: 10.1128/jb.105.2.666-672.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Grodon S V, Eiglmeier K, Gas S, Barry C E., III Nature (London) 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 31.Usha V, Jayaraman R J, Toro J C, Hoffner S E, Das K S. Can J Microbiol. 2002;48:7–13. doi: 10.1139/w01-126. [DOI] [PubMed] [Google Scholar]
- 32.Hutter B, Singh M. Biochem J. 1999;343:669–672. [PMC free article] [PubMed] [Google Scholar]
- 33.Raynaud C, Etienne G, Peyron P, Laneelle M A, Daffe M. Microbiology. 1998;144:577–587. doi: 10.1099/00221287-144-2-577. [DOI] [PubMed] [Google Scholar]
- 34.Av-Gay Y, Jamil S, Drews S J. Infect Immun. 1999;67:5676–5682. doi: 10.1128/iai.67.11.5676-5682.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Li C, Yang H, Mushegian A, Jin S. J Bacteriol. 1998;180:6764–6768. doi: 10.1128/jb.180.24.6764-6768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hakansson S, Galyov E E, Rosqvist R, Wolf-Watz H. Mol Microbiol. 1996;20:593–603. doi: 10.1046/j.1365-2958.1996.5251051.x. [DOI] [PubMed] [Google Scholar]
- 37.Sherman D R, Voskuil M, Schnappinger D, Liao R, Harrell M I, Schoolnik G K. Proc Natl Acad Sci USA. 2001;98:7534–7539. doi: 10.1073/pnas.121172498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan J, Flynn J. In: Nitric Oxide and Infection. Fang F C, editor. New York: Plenum; 1999. pp. 281–310. [Google Scholar]
- 39.Loebel R, Shorr E, Richardson H. J Bacteriol. 1933;26:167–173. doi: 10.1128/jb.26.2.167-200.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis N K, Chater K F. Mol Gen Genet. 1992;232:351–358. doi: 10.1007/BF00266237. [DOI] [PubMed] [Google Scholar]
- 41.McLean I W, Nakane P K. J Histochem Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.