Abstract
Here we provide the first evidence, to our knowledge, that estradiol (E2) affects learning and memory via the newly discovered estrogen receptor β (ERβ). In this study, ERβ knockout (ERβKO) and wild-type littermates were tested for spatial learning in the Morris water maze after ovariectomy, appropriate control treatment, or one of two physiological doses of E2. Regardless of treatment, all wild-type females displayed significant learning. However, ERβKOs given the low dose of E2 were delayed in learning acquisition, and ERβKOs administered the higher dose of E2 failed to learn the task. These data show that ERβ is required for optimal spatial learning and may have implications for hormone replacement therapy in women.
The steroid hormone estradiol (E2) either is required for or significantly modulates many behaviors, including cognitive behaviors (1–9). Learning tasks that involve reference memory tend to be impaired by E2 (1,7, 8). In contrast, low levels of E2 may facilitate working memory (5, 9). In women, changes in ability on visuospatial tasks and memory recall over the menstrual cycle have been documented (10, 11). Also, beneficial effects of estrogen replacement on cognition have been noted in normally aging women (12, 13) and in women suffering from dementia associated with Alzheimer's disease (14, 15).
How estrogen acts on learning and memory is not clear. Estrogen binding has been reported throughout the rodent brain, including the hippocampus and cortex (16). Both characterized nuclear estrogen receptors (ERα and -β) are present in extrahypothalamic regions (17, 18). In addition, in vitro work suggests that estrogen acts in a nongenomic fashion in hippocampal tissues (19, 20). Mice lacking functional ERα have severe deficits in several aspects of reproduction, including behavior (21–24). On the other hand, ERβ knockout mice (ERβKO) are subfertile, and sexual behavior is normal (25, 26). Thus, there has been speculation that ERβ regulates estrogen's nonreproductive functions in the central nervous system (21, 27, 28).
Here we describe our work on spatial learning in female ERβKO mice. When treated with doses of E2 that do not affect learning in wild-type (WT) littermates, ERβKO mice had impaired ability to escape from the Morris water maze. Interactions between ERα and -β may contribute to the learning response; to test the hypothesis that lack of ERβ influences the level of ERα protein, mouse brains were examined for ERα immunoreactivity (ERα-ir), with emphasis on the hippocampus (HIPP).
Methods
Animals.
Female mice (ages 5–7 mos) were of mixed 129/J and C57BL/6J background. Subjects were generated by crossing heterozygotic mating pairs carrying a single copy of the disrupted ERβ gene (25). The resulting offspring were genotyped by PCR amplification of tail DNA. WT and ERβ disrupted (ERβKO) littermates were used in these studies. The ERβ gene disruption was created by Neo insertion into exon 3 (25), thus the following three primers were used: one from intron 2 (5′-GGAGTAGAAACAAGCAATCCAGACATC-3′), another from the 3′ end of the Neo insert (5′-GCAGCCTCTGTTCCACATACACTTC-3′), and a third from exon 3 (5′-AGAATGTTGCACTGCCCCTGCTGCT-3′). A 665-bp band (intron 2 and exon 3 primers) was amplified for homozygous WT mice, a 500-bp band (intron 2 and Neo primers) for homozygous mutant mice, and both bands for heterozygous mice.
After surgery, mice were individually housed in polycarbonate cages, maintained on a 12:12-h light/dark cycle (lights off at 1800 h Eastern Daylight Time), and received food (Purina mouse chow no. 5001) and water ad libitum.
Surgery and Hormone Administration.
All mice (WT, n = 25, and ERβKO, n = 30) were ovariectomized (OVX) under ketamine/xylazine anesthesia (20/2 mg per 25 g body weight). At time of surgery, each female was randomly assigned to a treatment group and received either an E2 17β-filled or an empty Silastic implant (controls). E2 implants were made in Silastic tubing via two methods. One type of implant was made by packing 5 mm of crystalline 17β-E2 into Silastic tubing (i.d. 1.02 × o.d. 2.16 mm). We anticipated that this would yield a relatively high dose of E2 in plasma. The other implants were produced by first dissolving 17β-E2 in sesame oil vehicle (2.5 μg/0.025 ml) and then infusing 0.025 ml into a Silastic implant (i.d. 1.02 × o.d. 2.16 mm). Both ends of the Silastic tubing were glued with adhesive. Dilution of E2 in sesame oil was done to create a relatively low concentration of E2 in plasma. Thus a total of six groups were formed with 8–13 individuals per group. Silastic implants were administered s.c. and were placed in the midscapula region.
Ten days after surgery, each animal was tested for behavior by observers that were uninformed as to the genotype of the animals. Three to four days after behavior testing was concluded mice were deeply anesthetized with sodium pentobarbital (10 mg/kg) and quickly decapitated. Blood was collected and spun, and plasma frozen for E2 assay. Brains were rapidly removed and immersion fixed in 5% acrolein. Uteri were removed, cleaned of fat and connective tissue, and weighed. Animal care was conducted in accordance with the University of Virginia Animal Care and Use Committee guidelines.
Water Maze Training.
Our procedures have been described in detail (8). Briefly, each mouse was tested over 4 consecutive days before lights off during the lighted portion of the light/dark cycle. Animals were trained in a black circular pool (120 cm in diameter) located in a lit room containing a number of two- and three-dimensional visual cues. Pool water was maintained at 23 ± 2°C. A black escape platform (10.5 cm in diameter) was submerged 1.5 cm below the surface of the water. The location of the platform remained the same throughout the 4-day training period.
On the first day, each mouse was given a 30-sec free swim and then assisted to the platform where she remained for a 30-sec rest. This pattern was repeated four times, once from each equidistant release site. Training consisted of three blocks of four trials each for 4 consecutive days. Each trial lasted for 60 sec or ended sooner if the mouse reached the submerged platform, thus escaping from the swim maze. If a mouse failed to find the platform in 60 sec, she was assisted to it. Between trials, each mouse rested on the platform for 20 sec. Mice were released in random order from one of the four release sites. Data collected for each trial were: latency to escape from the water maze (find the submerged escape platform), and whether the mouse succeeded in finding the platform at all during the 60-sec trial.
After data were collected and analyzed for the spatial version of the task, a separate group of five ERβKO mice were run on the cued version. In this test, the escape platform is 5 cm above the surface of the water and is clearly marked with a large white flag. All competing visual cues in the room are removed. The rationale for this test is that mice that failed to find the escape platform in the spatial test should be able to locate the platform when it is visible. Because this task is relatively simple to learn, it is conducted only over a 3-day interval.
Immunocytochemisty.
Because the largest behavioral effects were noted between the control females and those in the high E2 dose group, we limited the histological analysis to brains collected from these animals (both genotypes, n = 6–11 per group). Brains fixed in 5% acrolein were cryoprotected overnight in 30% sucrose at 4°C and quickly frozen by using 2-methyl butane cooled in dry ice. Brains were stored at −70°C until they were sectioned.
Frozen brains were sectioned coronally at 30 μm and divided into a series of three wells. One well of tissue (one-third of the sections collected) was processed by using a rabbit antiserum specific to ERα (C1355) made against the last 14 amino acids of the C-terminal region of the ERα protein (29). There is no homology between this region of ERα and -β, respectively, thus there is no crossreactivity with ERβ. We have validated the use of this antiserum in mouse brain previously (30).
Immunoreactivity was visualized by using the Vector Elite ABC method (Vector Laboratories). Our methods have been described in detail (30). Nickel intensified diaminobenzidine (DAB) solution (0.25% nickel ammonium sulfate/0.05% DAB), activated by 0.001% hydrogen peroxide was used as the chromogen. Sections were rinsed, mounted onto gelatin coated slides, dehydrated, and coverslipped.
Immunocytochemical Data Analysis.
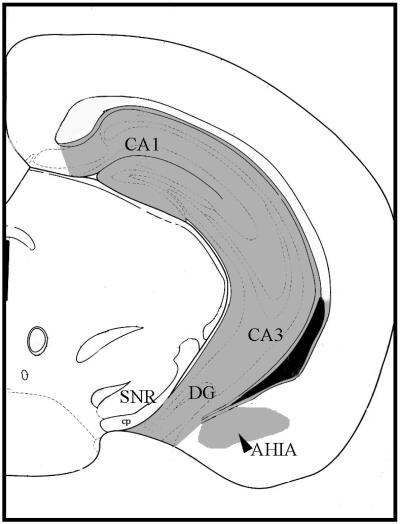
An observer uninformed as to the genotype and treatment of the animals scored the tissues. Immunoreactive cells were visualized by using an Olympus (New Hyde Park, NY) BX60 microscope attached to an Optronics charge-coupled device camera. The image analyses were conducted with metamorph software (Universal Imaging, Media, PA). The number of ERα-ir neurons was counted. The images were captured and saved for each animal from unilateral matched sections of each brain by using well-defined landmarks and a mouse brain atlas (figure 54 of ref. 31; interaural coordinate = 1.00 mm, Bregma = −2.80 mm). Absolute cell counts and counts per microns squared were made. The landmarks include the shape and size of the lateral ventricles and the cerebral peduncle and the periaqueductal gray. Two regions were quantified in the same section. The regions included the total dorsal-to-ventral extent of the dentate gyrus, CA1–3 in the hippocampus (referred to as HIPP) and the amygdalohippocampal area (AHIA; Fig. 1).
Figure 1.
Cameralucida representation of the section we selected for the ERα-ir cell quantification, adapted from ref. 31 (figure 54; interaural coordinate = 1.00 mm, Bregma = −2.80 mm). The extensive shading dorsal to ventral that includes the CA1–3 and dentate gyrus represents the region of the hippocampus in which ER-ir cells were counted. Lateral to this area the small shaded region represents the AHIA. CA1, CA1 field of hippocampus; CA3, CA3 field of hippocampus; SNR, reticular substantia nigra; cp, cerebral peduncle.
E2 Assay.
All samples were assayed in singlicate in a single assay. A commercial assay kit (Ultra-Estradiol, Diagnostic Systems Laboratories, Webster, TX) was used. The theoretical sensitivity of the assay is 0.6 pg/ml, and the standard curve ranges from 1.5 to 150 pg/ml. Maximum binding was 28%, and intraassay variability was 10.4%.
Statistics.
All behavior data were analyzed by repeated-measures ANOVA by using genotype, E2 dose, and test day as the treatment factors. Specific genotype differences were analyzed over time and on specific test days with two-way ANOVA. We conducted the behavioral analyses on the average escape latency per day for each subject. In addition, we calculated a success score based on the number of times each day the mouse was able to locate the platform during each 60-sec trial. The maximum score was 12, and the minimum was 0. The immunocytochemical data were subjected to three-way ANOVA. The three factors were: immunocytochemical run (sections were developed in two runs separated by several months), genotype, and hormone treatment. E2 concentrations in plasma and uterine weights were analyzed via two-way ANOVA. Bonferroni's planned comparisons corrected for multiple comparisons were conducted to assess group differences.
Results
E2 Treatment Impedes Escape Behavior in ERβKO Mice.
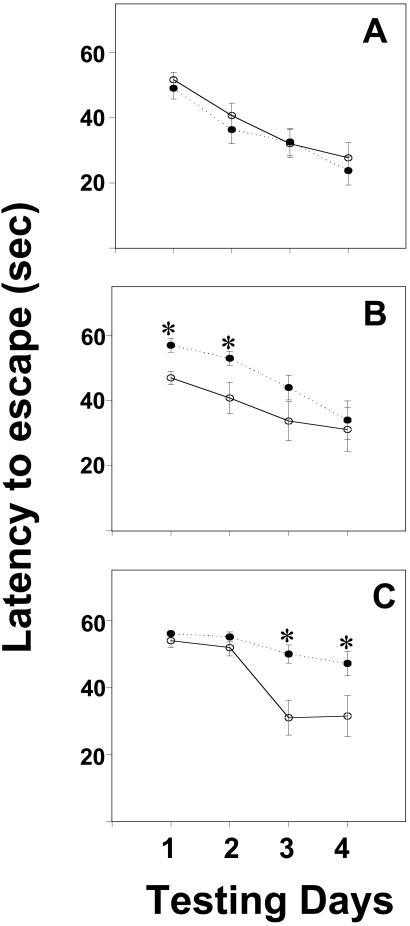
OVX ERβKO mice treated with E2 escaped from the Morris water maze more slowly than WT littermates (Fig. 2). A two-way repeated-measures ANOVA revealed significant effects of hormone dose [F(2,220) = 5.60, P < 0.0065] and genotype [F(1,220) = 4.47, P < 0.04] on latency to find the submerged escape platform. When control OVX WT and ERβKO mice were compared, no differences were found in escape latencies for any of the testing days [F(1,17) = 0.54, 0.54, 0.01, and 0.36 on days 1, 2, 3, and 4, respectively]. However, when females that received E2 were examined, an effect of genotype was found [F(1,152) = 9.23, P < 0.005], as well as a three-way interaction between genotype, hormone dose, and test day [F(3,152) = 4.06, P < 0.01, Fig. 2].
Figure 2.
Changes in latencies (group means ± SEM) to reach the escape platform in the Morris water maze over the 4-day training period. Data from WT mice are represented by open circles and solid lines. ERβKO data are represented by closed circles and dashed lines. In A, data from OVX mice (n = 8 WT, n = 9 ERβKO) are compared. B features data from OVX control mice receiving a low dose of E2 (n = 9 WT, n = 8 ERβKO). Finally, in C, data from females receiving the higher dose of E2 are shown (n = 8 WT, n = 13 ERβKO). *, significantly different from WT females (comparisons on the same day).
WT mice given the low dose of E2 were faster to escape than ERβKO animals on test days 1 [F(1,17) = 11.42, P < 0.005] and 2 [F(1,17) = 4.92, P < 0.045]. By the last 2 test days, no differences between the genotypes were noted [F(1,17) = 1.97, 0.10 on days 3 and 4, respectively]. In contrast, in the high E2 dose group significant differences were noted on the final 2 testing days [F(1,21) = 12.74, P < 0.002 on day 3 and F(1,21) = 5.35, P < 0.035 on day 4]. Again, ERβKO mice took longer to find the platform and thus escaped from the maze more slowly compared with WT mice. No such differences existed during the first 2 test days [F(1,21) = 1.38, 1.33 for days 1 and 2; Fig. 2].
Among WT females, no differences in escape latencies were attributed to hormone treatment [F(2,100) = 0.36]. However, latencies to escape depended on E2 treatment in ERβKO females [F(2,120) = 12.37, P < 0.0002], and an interaction between dose and test day was noted [F(6,120) = 2.62, P < 0.025]. ERβKOs receiving the higher dose of E2 were slower to escape on days 2, 3, and 4 compared with untreated OVX ERβKOs (P < 0.05). In addition, ERβKOs that received the lower E2 dose escaped more slowly than untreated females on day 2 and faster than females in the high E2 group on day 4 (P < 0.05).
E2 Inhibits Successful Location of the Escape Platform in ERβKO Mice.
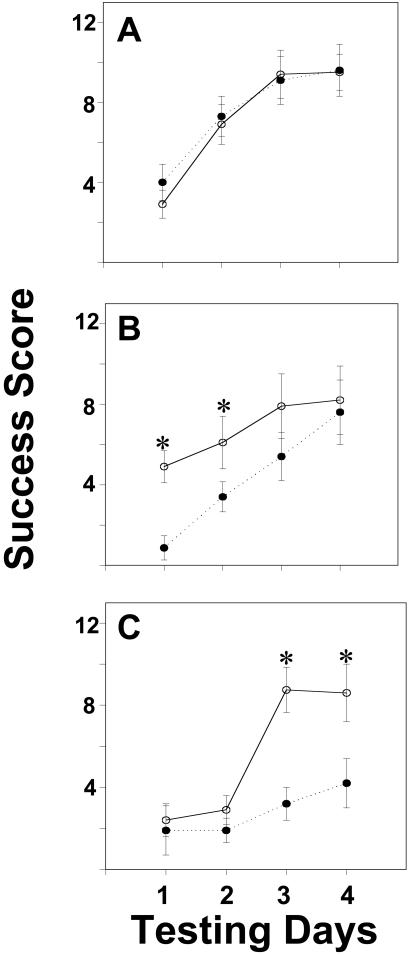
Analysis of success scores yielded a pattern of results that mirrors the escape latency findings. The ANOVA revealed significant effects of hormone dose [F(2,220) = 6.65, P < 0.003] and genotype [F(1,220) = 5.49, P < 0.025], and no interaction between these variables was detected. When scores for OVX control mice were examined, improvement was noted over time [F(3,67) = 26.60, P < 0.00001], but there were no effects of genotype [F(1,67) = 0.10]. Scores from females treated with E2 were influenced by genotype [F(1,152) = 9.15, P < 0.005]. A three-way interaction between hormone dose, genotype, and testing day [F(3,152) = 5.81, P < 0.001; Fig. 3] was noted.
Figure 3.
Changes in success scores (group means ± SEM) in the Morris water maze over the 4-day training period. All mice were OVX. Data from WT mice are represented by open circles and solid lines. ERβKO mouse data are represented by closed circles and dashed lines. In A, data from OVX mice are compared. B features data from OVX mice receiving a low dose of E2. Finally, in C, data from females receiving a high dose of E2 are shown. *, significantly different from ERβKO females (comparisons on the same day).
On days 1 and 2 of testing, ERβKO mice treated with the low E2 dose were less successful at locating the escape platform than WT littermates treated with the same dose [F(1,17) = 11.40, 4,92 respectively; P < 0.043, at least]. No differences were present on days 3 and 4 [F(1,17) = 1.97 and 0.75, respectively]. On the last 2 testing days, OVX ERβKO mice treated with the high dose of E2 were less successful than WT littermates given the same E2 dose [F(1,21) = 12.74, 5.35, respectively; P < 0.034 at least; Fig. 3].
In WT females, an interaction between hormone dose and test day was noted [F(6,100) = 3.19, P < 0.01]. On test day 2, WT females treated with the high E2 dose had lower success scores than OVX females. Both hormone dose effects [F(2,120) = 11.53, P < 0.00025] and an interaction between test day and dose [F(6,120) = 2.27, P < 0.045] were noted in ERβKO females. On test days 2, 3, and 4, females in the high E2 group had significantly lower success scores than OVX control females (P < 0.05).
E2 Does Not Inhibit Escape in the Cued Water Maze Task.
To assess motor ability and general motivation to exit the water maze, we conducted a cued test with five ERβKO individuals. All were OVX and treated with the high E2 dose, as described in Methods. Females displayed a significant decrease in latency to find the platform [F(2,15) = 21.83, P < 0.0006] and an increase in success scores [F(2,15) = 11.17, P < 0.005] over the 3 testing days. In both measures, the differences lie between the first test day and the other 2 days (P < 0.05). By the second day of testing, females found the platform in less than 20 sec and were successful in their escape attempts 11 of 12 times.
Estrogen Treatment Affects Uterine Weights and Concentrations of E2 in Plasma.
Hormone treatment, but not genotype, had a significant effect on uterine weights [F(2,64) = 47.99, P < 0.000001]. Uterine weights in each treatment group differed significantly from the other two groups (P < 0.05). The means (in milligrams) and SEM for uteri collected from females in each hormone group were as follows: OVX = 24.56 ± 2.37; OVX + E2 oil-diluted implant = 88.12 ± 13.30; OVX + crystalline E2 implant = 197.33 ± 12.44.
In addition, hormone treatment had the desired effect of creating different concentrations of E2 in plasma [F(2,51) = 46.62, P < 0.000001]. The crystalline E2-17β-packed implant yielded a blood concentration that averaged 98.05 ± 6.00 (SEM) pg/ml. This concentration is similar to that measured in plasma of C57BL/6J mice during proestrous (ref. 32; E.F.R., unpublished data). This E2 concentration differs significantly from the measured plasma levels in OVX controls (16.1 ± 9.3 pg/ml) and in OVX mice that received the oil-diluted dose of E2 (13.4 ± 3.6 pg/ml). It is clear from behavioral data and uterine weights that control OVX females and OVX mice treated with the low dose of E2 were experiencing different levels of E2 in the blood. However, because the low E2 dose yields plasma E2 concentrations close to the bottom end of the physiological range, we are not surprised that the assay did not show a significant difference in plasma E2 levels among females in these groups. Moreover, this concentration (13.4 pg/ml) is similar to that measured during diestrus in ovary-intact mice (32).
ERβ Affects Expression of ERα-ir in Hippocampus.
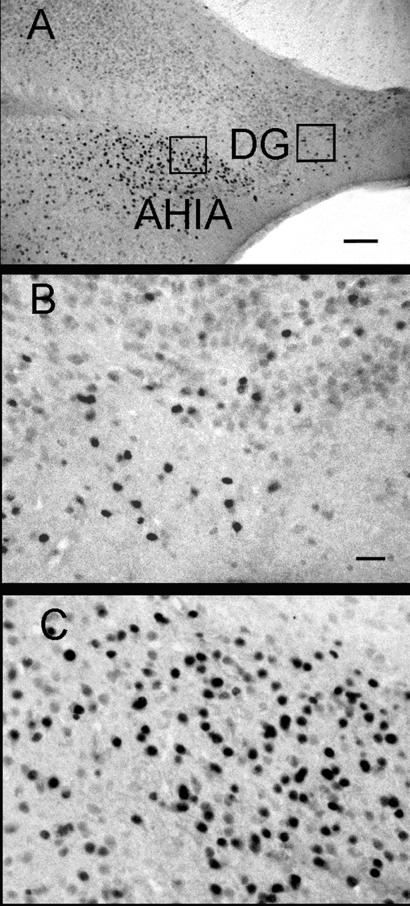
Decreased numbers of ERα-ir cells were noted in hippocampi of estrogen-treated ERβKO females compared with untreated OVX ERβKO mice [F(1,32) = 5.82, P < 0.025] or any other treatment group tested. In the hippocampus, an effect of hormone treatment was noted on ERα-ir cell numbers. There was no interaction between the effects of hormone treatment and immunocytochemical run [F(1,32) = 0.01], nor was there an effect of genotype [(F1,32) = 1.44]. The effect of hormone treatment on ERα-ir can be attributed to significantly fewer ERα-ir cell numbers in brains of ERβKO females that received the high E2 dose as compared with OVX ERβKO mice (P < 0.05; Table 1). In the AHIA, no significant effects of immunocytochemical run, hormones, or genotype were noted [F(1,36) = 0.01, 2.10, 0.00, respectively]. Intensely stained ERα-ir cells were present in the AHIA (Fig. 4). In the hippocampus, many lightly stained cells were present throughout the region, but only the darkly immunoreactive cells, similar in intensity to those counted in the AHIA, were counted (Fig. 4). Most of these ERα-ir cells were noted in the ventral portion of the granular layer of the dentate gyrus.
Table 1.
Numbers (mean ± SEM) of immunoreactive ERα cells in the AHIA and hippocampus
| AHIA | Hippocampus | |
|---|---|---|
| WTOVX | 235.5 ± 30.0 | 150.0 ± 18.0 |
| WTOVX + E2 | 235.0 ± 20.5 | 158.0 ± 17.6 |
| ERβKO OVX | 202.0 ± 21.0 | 149.0 ± 24.0 |
| ERβKO OVX + E2 | 211 ± 17.7 | 93.4 ± 13.0* |
Significantly different from hippocampus ERα-ir cell numbers in ERβKO OVX females (P < 0.05).
Figure 4.
Photomicrographs of ERα-ir in the hippocampus and adjacent AHIA. A low-magnification view is presented in A (20×) and even higher (×40) examination of ERα-ir cells in the hippocampus (B) and AHIA (C). Boxed regions in A represent the areas shown in B and C. (Bar in A = 100 μm; in B = 10 μm.) DG, dentate gyrus; AHTA, amygdalohippocampal area.
Discussion
Our data show that two doses of E2, which yield plasma concentrations similar to those experienced during the mouse estrous cycle, either completely blocked (high dose) or delayed (low dose) learning acquisition in the spatial water maze task in ERβKO but not in WT littermates. Thus, the lack of ERβ severely impairs spatial learning in mice. Because the doses of E2 were within the physiological range, we infer that ERβ is actively involved in reference memory formation during the normal estrous cycle. In contrast, our previous work with an even higher dose of E2 than used here showed that water maze learning was impaired in WT but not in ERαKO females (8). Together, these data suggest that the inhibitory effect of E2 on learning is mediated by ERα, and the lack of ERβ increases sensitivity to the negative consequences of E2 on reference memory, particularly spatial learning. Thus, when ERβ is not functional, the actions of E2 on ERα are “unmasked” and are more pronounced than under conditions where both ERs are responsive.
One way to describe this relationship is to examine E2 dose-dependent effects on behavior in WT and ERβKO females. In WT females, there was only a single time point when a difference in behavior could be attributed to concentrations of E2 in plasma. On day 2, success scores for OVX WT mice were significantly higher than scores for females in the high E2 dose group. In contrast, ERβKO females treated with the high E2 dose had lower success scores than their OVX counterparts on all except the first day of the task. In addition, in ERβKO but not in WT females, there were significant effects of E2 dose on latencies to escape from the maze. Slower escape latencies accompanied poor success scores in females receiving the high E2 dose. The low E2 dose also had behavioral effects in ERβKOs; however, these effects were transient. On the first 2 days of testing, ERβKO females in the low E2 group were slower to escape than untreated OVX mice. On the last day, ERβKOs in the low E2 group were faster to escape than their counterparts in the high E2 dose group. Thus, in the absence of ERβ, treatment with a low dose of E2 delayed spatial learning, whereas exposure to the high E2 dose blocked learning acquisition during the 4-day test. Importantly, these dosage effects of E2 within the physiological range are apparent only in the absence of functional ERβ.
ERβ may also serve to protect ERα protein from down-regulation by E2, perhaps by binding available ligand. Here we show that the numbers of ERα-ir-positive nuclei after estrogen treatment were significantly reduced only in ERβKO mice. Likewise, in regions of the hypothalamus such as the ventromedial nucleus, preoptic area, and the arcuate nucleus in ERβKO, but not in WT females, this same down-regulation of E2 on ERα-ir cells has been reported (33). In the hippocampus, reduced ERα could influence the levels or activity of many growth factors and/or neurotransmitters known or suspected to be involved in learning, including acetylcholine, neural growth factors, and their receptors (34–37).
Several studies have documented direct interactions between ERα and -β both in vitro (38–40) and in vivo (33, 41, 42), and colocalization of the two receptor forms occurs in specific subsets of neurons throughout the brain (43, 44). ERα and -β can form heterodimers in vitro and, in cell transfection studies, ERβ functionally suppresses ERα transcriptional activity (39, 45–47). ERβ has been postulated to act as a repressor to ERα in complicated processes such as cell growth or tumorigenesis, and changes in the ERα/-β ratio are associated with several types of cancer (48, 49). The mechanism of estrogen action on learning is unknown but could include both genomic and nongenomic effects as well as actions mediated via other proteins or receptors. For any of these pathways, ERβ might act as a repressor of ERα. For example, E2 activates the mitogen-activated protein kinase (MAPK) cascade within minutes in cortical tissue (20) and facilitates rapid initiation of kainate-induced current in individual hippocampal neurons (19). Neither effect was blocked by the potent antiestrogen ICI 182,780. Moreover, when brain tissues from WT and ERαKO mice were compared, extracellular signal-regulated kinases (ERK) phosphorylation was significantly enhanced in the absence of functional ERα (20). Thus, ERα may suppress overall MAPK activity, either directly or indirectly. Recently, a G protein-coupled receptor homolog, GPR30, has been show to affect ERK activation in the absence of either ERα or -β, perhaps via a nongenomic action of E2 on growth factors (49). It is possible that E2 actions on the MAPK cascade may have consequences for learning when both ERα and -β are present, and these actions may be disrupted when one or both of the ERs is not available.
Another profound manner in which estrogens could affect learning and memory is via neural remodeling and plasticity. E2 alters many aspects of hippocampal morphology, including synapse number, spine density, and astrocytic volume (50–52). In brain, astrocytes contain ERβ (53, 54). Moreover, ERβKO brains display fewer Nissl-stained neurons and increased glial fibrillary acidic protein immunoreactive cells in the medial amygdala and preoptic area, as compared with WT littermates (55). It is well known that adult neurogenesis can be triggered by estrogen (56), yet the ER responsible for these effects has not been identified. In addition, estrogen exposure can promote or deter apoptosis (57), with the identity of the ER responsible for neuroprotection under debate (58, 59). Regardless of whether ERβ acts to reduce estrogen-related synaptogenesis, glia concentrations, prevent cell death and/or stimulate neurogenesis, any or some of these effects may impact learning and memory.
Learning is a complex behavior, and it is possible that another behavioral phenotype of the ERβKO mice could affect their ability to learn. Sexual behaviors have been reported to be normal in male and female ERβKO mice (26). In addition, although ERαKO females required estrogen treatment to learn to avoid shock in a simple 24-h learning task, WT and ERβKO females performed well on the task regardless of estrogen status (60). Yet, one recent report suggests that female ERβKO mice have elevated levels of anxiety and reduced levels of activity in open field tests (61). We have collected similar data from OVX ERβKO and WT female littermates given no hormone or treated with the high E2 dose used in the current experiments (D. B. Imwalle and E.F.R., unpublished data). In our study, ERβKO mice displayed elevated anxiety, but E2 had no effect. Thus, although anxiety could be a contributing factor, it cannot completely explain the E2 dose-dependent learning impairment in ERβKOs.
Our results are compelling: WT females were able to learn a spatial task with or without E2 replacement, but ERβKOs could perform only when no E2 was given. Yet there are many issues that still need to be addressed. For example, in female rats, E2 influences learning strategies (62). Thus it is still possible that lack of ERβ specifically influences a type or component of learning in a spatial task. Given our previous findings that ERαKO females do not learn well in an inhibitory avoidance task (60), it is tempting to speculate that the two ERs affect different types of learning; perhaps ERα is needed for emotional learning that is likely to rely on the amygdala, whereas hippocampal-dependent spatial learning requires ERβ. Given the demographics of aging and increase in treatment of menopausal symptoms with estrogen replacement therapy, it would be useful to know more about the interactions and independent functions of the two ERs. These data can lead to the development of ER-specific agonists and antagonists that will be needed to ensure proper cognitive function in postmenopausal women.
Acknowledgments
We are grateful to Savera Shetty, Mary McCanna, and Aileen Wills for excellent technical assistance. We thank Dr. Nancy Desmond for insightful comments on these data. This work was supported by National Institutes of Health Grants R01 MH57759 and K02 MH01349 (to E.F.R.) and R01 DK57982 (to M.A.S.). In addition, this research was supported by National Institute of Child Health and Human Development/National Institutes of Health through cooperative agreement U54 HD28934, as part of the Specialized Cooperative Centers Program in Reproduction Research.
Abbreviations
- E2
steroid hormone estradiol
- ERβ
estrogen receptor β
- ERβKO
ERβ knockout
- WT
wild type
- ERα-ir
ERα immunoreactivity
- AHIA
amygdalohippocampus
- DG
dentate gyrus
- AHIA
amygdalohippocampal area
- OVX
ovariectomized
References
- 1.Galea L A, Kavaliers M, Ossenkopp K P, Hampson E. Horm Behav. 1995;29:106–125. doi: 10.1006/hbeh.1995.1008. [DOI] [PubMed] [Google Scholar]
- 2.Daniel J M, Fader A J, Spencer A L, Dohanich G P. Horm Behav. 1997;32:217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- 3.Chesler E J, Juraska J M. Horm Behav. 2000;38:234–242. doi: 10.1006/hbeh.2000.1626. [DOI] [PubMed] [Google Scholar]
- 4.Lacreuse A, Verreault M, Herndon J G. Psychoneuroendocrinology. 2001;26:623–639. doi: 10.1016/s0306-4530(01)00017-8. [DOI] [PubMed] [Google Scholar]
- 5.Rissanen A, Puolivali J, van Groen T, Riekkinen P., Jr NeuroReport. 1999;10:1369–1372. doi: 10.1097/00001756-199904260-00039. [DOI] [PubMed] [Google Scholar]
- 6.Wilson I A, Puolivali J, Heikkinen T, Riekkinen P., Jr Eur J Pharmacol. 1999;24:93–99. doi: 10.1016/s0014-2999(99)00583-x. [DOI] [PubMed] [Google Scholar]
- 7.Warren S G, Juraska J M. Behav Neurosci. 1997;111:259–266. doi: 10.1037//0735-7044.111.2.259. [DOI] [PubMed] [Google Scholar]
- 8.Fugger H N, Cunningham S G, Rissman E F, Foster T C. Horm Behav. 1998;34:163–170. doi: 10.1006/hbeh.1998.1475. [DOI] [PubMed] [Google Scholar]
- 9.Bimonte H A, Denenberg V H. Psychoneuroendocrinology. 1999;24:161–173. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- 10.Hampson E, Kimura D. Behav Neurosci. 1988;102:456–459. doi: 10.1037//0735-7044.102.3.456. [DOI] [PubMed] [Google Scholar]
- 11.Phillips S M, Sherwin B B. Psychoneuroendocrinology. 1992;17:497–506. doi: 10.1016/0306-4530(92)90008-u. [DOI] [PubMed] [Google Scholar]
- 12.Sherwin B B. Exp Gerontol. 1994;29:423–430. doi: 10.1016/0531-5565(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 13.Maki P, Zonderman A, Resnick S. Am J Psychiatry. 2001;158:227–233. doi: 10.1176/appi.ajp.158.2.227. [DOI] [PubMed] [Google Scholar]
- 14.Asthana S, Baker L D, Craft S, Stanczyk F Z, Veith R C, Raskind M A, Plymate S R. Neurology. 2001;57:605–612. doi: 10.1212/wnl.57.4.605. [DOI] [PubMed] [Google Scholar]
- 15.Yaffe K, Krueger K, Sarkar S, Grady D, Barrett-Connor E, Cox D A, Nickelsen T. N Engl J Med. 2001;344:1207–1213. doi: 10.1056/NEJM200104193441604. [DOI] [PubMed] [Google Scholar]
- 16.Shughrue P J, Merchenthaler I. Neuroscience. 2000;99:605–612. doi: 10.1016/s0306-4522(00)00242-6. [DOI] [PubMed] [Google Scholar]
- 17.Shughrue P J, Lane M V, Merchenthaler I. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Shughrue P J, Lane M V, Merchenthaler I. J Comp Neurol. 2001;436:64–81. [PubMed] [Google Scholar]
- 19.Gu Q, Korach K S, Moss R L. Endocrinology. 1999;140:660–666. doi: 10.1210/endo.140.2.6500. [DOI] [PubMed] [Google Scholar]
- 20.Singh M, Setalo G, Jr, Guan X, Frail D E, Toran-Allerand C D. J Neurosci. 2000;20:1694–1700. doi: 10.1523/JNEUROSCI.20-05-01694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rissman E F, Wersinger S R, Fugger H N, Foster T C. Brain Res. 1999;835:80–90. doi: 10.1016/s0006-8993(99)01452-3. [DOI] [PubMed] [Google Scholar]
- 22.Rissman E F, Early A E, Taylor J A, Korach K S, Lubahn D B. Endocrinology. 1997;138:507–510. doi: 10.1210/endo.138.1.4985. [DOI] [PubMed] [Google Scholar]
- 23.Wersinger S R, Haisenleder D J, Lubahn D B, Rissman E F. Endocrine. 1999;11:137–143. doi: 10.1385/ENDO:11:2:137. [DOI] [PubMed] [Google Scholar]
- 24.Wersinger S R, Rissman E F. J Neurosci. 2000;20:4248–4254. doi: 10.1523/JNEUROSCI.20-11-04248.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krege J H, Hogdin J B, Couse J F, Enmark E, Warner M, Mahler J F, Sar M, Korach K S, Gustafsson J-Å, Smithies O. Proc Natl Acad Sci USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogawa S, Chan J, Chester A E, Gustafsson J-Å, Korach K S, Pfaff D W. Proc Natl Acad Sci USA. 1999;96:12887–12892. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gustafsson J-Å. Eur J Cancer. 2000;36, Suppl. 4:S16. doi: 10.1016/s0959-8049(00)00206-9. [DOI] [PubMed] [Google Scholar]
- 28.Shughrue P J, Merchenthaler I. Front Neuroendocrinol. 2001;21:95–101. doi: 10.1006/frne.1999.0190. [DOI] [PubMed] [Google Scholar]
- 29.Friend K E, Resnick E M, Ang L W, Shupnik M A. Mol Cell Endocrinol. 1997;131:147–155. doi: 10.1016/s0303-7207(97)00098-1. [DOI] [PubMed] [Google Scholar]
- 30.Moffatt C A, Rissman E F, Shupnik M A, Blaustein J D. J Neurosci. 1998;18:9556–9563. doi: 10.1523/JNEUROSCI.18-22-09556.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franklin K B J, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic; 1997. [Google Scholar]
- 32.Nelson J F, Felicio L S, Osterburg H H, Finch C E. Endocrinology. 1992;130:805–810. doi: 10.1210/endo.130.2.1733727. [DOI] [PubMed] [Google Scholar]
- 33.Temple J L, Fugger H N, Li X, Shetty SJ, Gustafsson J-Å, Rissman E. Endocrinology. 2001;142:510–513. doi: 10.1210/endo.142.1.8054. [DOI] [PubMed] [Google Scholar]
- 34.Daniel J M, Dohanich G P. J Neurosci. 2001;21:6949–6956. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gibbs R B, Burke A M, Johnson D A. Horm Behav. 1998;34:112–125. doi: 10.1006/hbeh.1998.1452. [DOI] [PubMed] [Google Scholar]
- 36.Gibbs R B, Wu D, Hersh L B, Pfaff DW. Exp Neurol. 1994;129:70–80. doi: 10.1006/exnr.1994.1148. [DOI] [PubMed] [Google Scholar]
- 37.Sohrabji F, Miranda R C, Toran-Allerand C D. Proc Natl Acad Sci USA. 1995;21:11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hall J M, McDonnell D P. Endocrinology. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- 39.Paech K, Webb P, Kuiper G G, Nilsson S, Gustafsson J-Å, Kushner P J, Scanlan T S. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 40.Maruyama S, Fujimoto N, Asano K, Ito A. J Steroid Biochem Mol Biol. 2001;78:177–184. doi: 10.1016/s0960-0760(01)00083-8. [DOI] [PubMed] [Google Scholar]
- 41.Saville B, Wormke M, Wang F, Nguyen T, Enmark E, Kuiper G, Gustafsson J-Å, Safe S. J Biol Chem. 2000;275:5379–5387. doi: 10.1074/jbc.275.8.5379. [DOI] [PubMed] [Google Scholar]
- 42.Weihua Z, Saji S, Makinen S, Cheng G, Jensen E V, Warner M, Gustafsson J-Å. Proc Natl Acad Sci USA. 2000;97:5936–5941. doi: 10.1073/pnas.97.11.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gréco B, Allegretto E A, Tetel M J, Blaustein J D. Endocrinology. 2001;142:5172–5181. doi: 10.1210/endo.142.12.8560. [DOI] [PubMed] [Google Scholar]
- 44.Shughrue P J, Scrimo P J, Merchenthaler I. Endocrinology. 1998;139:5267–5270. doi: 10.1210/endo.139.12.6525. [DOI] [PubMed] [Google Scholar]
- 45.Windahl S H, Vidal O, Andersson G, Gustafsson J-Å, Ohlsson C. J Clin Invest. 1999;104:895–901. doi: 10.1172/JCI6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pettersson K, Grandien K, Kuiper G G J M, Gustafsson J-Å. Mol Endocrinol. 1997;11:1486–1496. doi: 10.1210/mend.11.10.9989. [DOI] [PubMed] [Google Scholar]
- 47.Pettersson K, Delaunay F, Gustafsson J-Å. Oncogene. 2000;12:4970–4978. doi: 10.1038/sj.onc.1203828. [DOI] [PubMed] [Google Scholar]
- 48.Foley E F, Jazaeri A A, Shupnik M A, Hazaeri O, Rice LW. Cancer Res. 2000;60:245–248. [PubMed] [Google Scholar]
- 49.Filardo E J, Quinn J A, Bland K I, Frackelton A R. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 50.Woolley C S, Gould E, Frankfurt M, McEwen B S. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klintsova A, Levy W B, Desmond N L. Brain Res. 1995;690:269–274. doi: 10.1016/0006-8993(95)00642-4. [DOI] [PubMed] [Google Scholar]
- 52.Miranda P, Williams C L, Einstein G. J Neurosci. 1999;19:3316–3325. doi: 10.1523/JNEUROSCI.19-09-03316.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jung-Testas I, Renoir M, Bugnard H, Greene G L, Baulieu E E. J Steroid Biochem Mol Biol. 1992;41:621–631. doi: 10.1016/0960-0760(92)90394-x. [DOI] [PubMed] [Google Scholar]
- 54.Santagati S, Melcangi R C, Celotti F, Martini L, Maggi A. J Neurochem. 1994;63:2058–2064. doi: 10.1046/j.1471-4159.1994.63062058.x. [DOI] [PubMed] [Google Scholar]
- 55.Wang L, Andersson S, Warner M, Gustafsson J-Å. Proc Natl Acad Sci USA. 2001;98:2792–2796. doi: 10.1073/pnas.041617498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tanapat P, Hastings N B, Reeves A J, Gould E. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harms C, Lautenschlager M, Bergk A, Katchanov J, Freyer D, Kapinya K, Herwig U, Megow D, Dirnagl U, Weber J R, Hortnagl H. J Neurosci. 2001;21:2600–2609. doi: 10.1523/JNEUROSCI.21-08-02600.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dubal D B, Zhu H, Yu J, Rau S W, Shughrue P J, Merchenthaler I, Kindy M S, Wise P M. Proc Natl Acad Sci USA. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sampei K, Goto S, Alkayed N J, Crain B J, Korach K S, Traystman R J, Demas G E, Nelson R J, Hurn P D. Stroke. 2000;31:738–743. doi: 10.1161/01.str.31.3.738. [DOI] [PubMed] [Google Scholar]
- 60.Fugger H N, Foster T C, Gustafsson J-Å, Rissman E F. Brain Res. 2000;883:258–264. doi: 10.1016/s0006-8993(00)02993-0. [DOI] [PubMed] [Google Scholar]
- 61.Krezel W, Dupont S, Krust A, Chambon P, Chapman P F. Proc Natl Acad Sci USA. 2001;98:12278–12282. doi: 10.1073/pnas.221451898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Korol, D. L. & Kolo, L. L. (2002) Behav. Neurosci., in press. [DOI] [PubMed]