Abstract
Several ion channels and pumps are regulated by syntaxin 1A, a component of the synaptic vesicle docking and fusion apparatus. One such regulated protein is the rat brain γ-aminobutyric acid (GABA) transporter GAT1. The N-terminal cytoplasmic domain of GAT1 directly interacts with syntaxin 1A; this interaction induces a decrease in the rate at which GABA and associated ions are transported. GAT1 function also is regulated by transporter substrates, raising the possibility that substrates mediate at least some of their effects by regulating the interaction between GAT1 and syntaxin 1A. In oocytes expressing GAT1 and syntaxin 1A, superfusion of transporter substrates increases the GAT1 transport rate. The substrate-induced rate change (i) is prevented by coapplication of GAT1 antagonists, (ii) does not occur in oocytes expressing GAT1 alone, and (iii) does not occur in oocytes expressing interaction-deficient syntaxin 1A mutants. In oocytes, and in hippocampal neurons that endogenously express both GAT1 and syntaxin 1A, substrate application results in a decrease in the fraction of syntaxin 1A that is bound to GAT1 on a time-scale comparable to the substrate-induced change in transport rates. These data suggest that substrate translocation regulates GAT1–syntaxin 1A interactions and provide a mechanism by which GABA transport can be increased during times of rising synaptic GABA concentrations.
Syntaxin 1A was originally characterized as a component of the machinery involved in transmitter release (1, 2), and is a key player in vesicle trafficking and fusion (3, 4). Syntaxin 1A directly interacts and functionally regulates several excitability proteins, including Ca2+ channels (5–10), cystic fibrosis Cl− channels (11, 12), K+ channels (13), and epithelial Na+ channels (14, 15). Mechanisms of the effects of syntaxin 1A include changes in protein trafficking and in channel properties such as gating. Syntaxin 1A also regulates Na+ and Cl−-dependent neurotransmitter transporters (16–19). For example, glycine transporter trafficking is altered by syntaxin 1A coexpression (19). Syntaxin 1A alters not only trafficking of the rat brain γ-aminobutyric acid (GABA) transporter GAT1 but also its rate of substrate translocation. The GAT1 N-terminal cytoplasmic tail binds the H3 domain of syntaxin 1A; the interaction causes a 4-fold decrease in substrate transport rates (18). These data suggest that protein–protein interactions regulate substrate translocation and identify a link between the machinery involved in transmitter release and uptake.
Syntaxin 1A interactions are not the only mechanism by which GAT1 and other transporters are regulated (20, 21). For example, protein kinase C activation correlates with both decreases in GABA transport and decreases in surface GAT1 expression (17, 22, 23). Tyrosine phosphorylation of GAT1 increases GAT1 surface expression by decreasing the transporter internalization rate (24). Additionally, as with the transporters for serotonin (25), dopamine (26), and norepinephrine (27), transporter substrates and antagonists also regulate GAT1. Within minutes, extracellularly applied GAT1 agonists increase GAT1 function in hippocampal neurons and in expression systems (28). These results suggest that cells regulate transporter function in a manner that correlates with extracellular transmitter levels. If this is true, then GABA may increase GAT1 function in part by regulating the association between syntaxin 1A and GAT1.
Materials and Methods
Reagents.
Cell culture reagents were obtained from Life Technologies. Papain was obtained from Worthington. Immunoblotting reagents and [3H]GABA were obtained from Amersham Pharmacia. Botulinum toxin C1 (BONT/C1) was obtained from Boehringer Mannheim. GAT1 Ab 346J was obtained from Nicholas Brecha (Univ. of California, Los Angeles; ref. 29); isoform-specific polyclonal syntaxin Abs were generated as described (11). Syntaxin mutants were described previously (18). All other reagents were obtained from Sigma.
Cell Culture.
Primary hippocampal cultures were prepared from postnatal day 0–3 rats by mincing tissue in α minimal essential medium (αMEM) supplemented with cysteine, glucose, and 100 units of papain. Tissue was incubated for 20 min at 37°C followed by gentle trituration, dilution, and plating. To obtain pure neuronal cultures, mixed cultures were treated for 48 h with 10 μM cytosine arabinonucleoside. Oocyte culture was performed as described (30).
Electrophysiology.
Two-electrode voltage-clamp procedures were performed as described (16). Drug applications were performed by using gravity flow; bath exchange was complete in 2 s. Measurements of GAT1 charge movements were as described (16, 31). Charge movements were measured during a 500-ms voltage step from −40 mV to −100 mV. Each oocyte was tested in the presence and absence of SKF89976A to isolate (by subtraction) the charge movements associated with the presence of GAT1 in the plasma membrane. Capacitative transients generated by these jumps were integrated to yield the amount of charge movement in and out of the membrane field of the oocyte. Surface transporter number was calculated from the equation N = Qmax/qzδ, where N is the number of transporters per oocyte, Qmax is the total charge movement, q is the elementary charge, and zδ is the sum total of the distance that all charges move within the membrane field. The empirical value of zδ for GAT1 is ≈1.0 (31). Surface transporter number was divided into peak steady-state current, measured at saturating GABA concentrations, to yield the transport rate. This rate also was assessed by measuring the time constant (estimated by single exponential fits) of the transient relaxations of voltage jumps (from −40 mV to −100 mV) performed in the presence of 100 μM GABA (31). For all experiments requiring GAT1 agonist pretreatment, the time interval between the end of the agonist pretreatment and the application of the agonist test pulse was 5 s; the time interval between the end of the agonist test pulse and further chronic agonist treatment was also 5 s.
Biotinylation and Immunoprecipitation.
Surface biotinylation experiments were performed as described (28). For immunoprecipitations, oocytes were homogenized as described previously (29). Hippocampal neurons were lysed in buffer (9.1 mM dibasic sodium phosphate/1.7 mM monobasic sodium phosphate, pH 7.4/150 mM NaCl/0.5% sodium deoxycholate/0.1% SDS/250 μM PMSF/1 μg/ml aprotinin/1.0 mM activated sodium orthovanadate/5.0 mM sodium pyrophosphate) for 1 h at 4°C. Lysates were precleared with 10 μl of protein G-agarose conjugate, followed by immunoprecipitation of GAT1 by using anti-GAT1 Ab and protein G-agarose. The product was washed in buffer and run on a 10% acrylamide gel. Protein was transferred to a nitrocellulose membrane and visualized by using enhanced chemiluminescence reagents.
Results
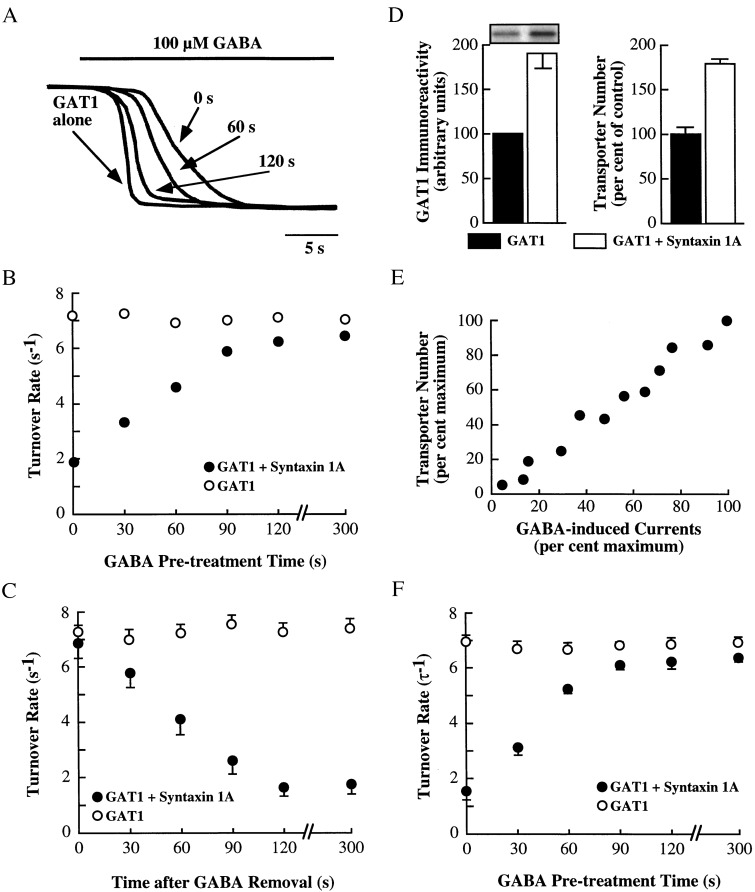
Application of GABA to oocytes expressing GAT1 and voltage-clamped at negative potentials induces an inward current that reflects the movement of one net charge into the oocyte per transport cycle (31, 32). In oocytes expressing GAT1 and syntaxin 1A, the rise time of the current is slowed, and this slowing is reversed by acute injection of BONT/C1, which functionally inactivates syntaxin 1A (18). Although the molecular mechanism that causes the change in transport kinetics in the presence of syntaxin 1A is unknown at present, the phenomenon suggests potential changes in GAT1 turnover rates (the rate of one substrate translocation cycle). To determine whether substrates of the transporter alter GAT1 transport kinetics, GABA was superfused for various lengths of time upon oocytes expressing GAT1 and syntaxin 1A (Fig. 1A). Measurement of the GABA-induced current in the absence of GABA pretreatment (0 s; right-most trace) revealed a slowed time-to-peak compared with oocytes expressing GAT1 alone (left-most trace). (For ease of comparison, all currents were scaled to the same peak.) Pretreatment of the same oocyte coexpressing GAT1 and syntaxin 1A with GABA for 60 s or 120 s caused a progressive decrease in the time-to-peak of the subsequently measured GABA-induced current. This decrease did not occur in oocytes expressing GAT1 alone that were then subjected to GABA pretreatment (data not shown).
Figure 1.
GABA up-regulates GAT1 turnover rates in oocytes expressing GAT1 and syntaxin 1A. (A) Raw traces of GABA-induced currents in one oocyte injected with 10 ng of GAT1 cRNA alone (left-most trace) or one oocyte injected with 10 ng of GAT1 cRNA and 20 ng of syntaxin 1A cRNA (three right-most traces). The oocyte coexpressing GAT1 and syntaxin 1A was pretreated with 100 μM GABA for the time period indicated (either 0, 60, or 120 s) before the recording of the GABA-induced current. The GAT1-alone trace was recorded without any previous GABA superfusion. The traces are scaled to the same peak current for comparison of the kinetics of the response. Range of measured currents was between 153 and 221 nA. (B) Changes in transporter turnover rate after GABA superfusion. Oocytes were injected with GAT1 cRNA alone (○) or with syntaxin 1A cRNA (●). Turnover number was calculated as peak whole-cell current elicited by 100 μM GABA divided by the number of functional transporters (see Materials and Methods). Values along the abscissa indicate the amount of time the oocyte was superfused with 100 μM GABA before measurement. Data are from 33–47 oocytes per data point, measured from five oocyte batches. SEMs are within the symbol size. (C) Time course of changes in GAT1 turnover rates after GABA wash-out. Oocytes expressing GAT1 alone (○) or with syntaxin 1A (●) were voltage clamped and superfused with 100 μM GABA for 5 min. Turnover rates were then measured as in B after GABA wash-out for various times as indicated on the abscissa. Data are from 9–14 oocytes per data point, measured from three oocyte batches. (D) Syntaxin 1A increases the surface expression of GABA transporters. Oocytes expressing GAT1 alone (filled bars) or with syntaxin 1A (open bars) were examined for surface GAT1 expression by biotinylation (Left) or by charge movement measurements (Right). The immunoblot shows surface fraction immunoreactivity when a GAT1 Ab for oocytes expressing GAT1 alone (left lane) or with syntaxin 1A (right lane) is used. Data are from two experiments, six oocytes per experiment, measured individually by electrophysiology and then processed as a batch for biotinylation. (E) Correlation of GABA uptake and transporter number. Individual oocytes expressing GAT1 alone were voltage clamped at −80 mV and assayed for currents by using 100 μM GABA. Transporter number was estimated in the same oocyte. Data are plotted relative to the oocyte expressing the largest currents (390 nA) and largest transporter number estimate (3.3 × 1011). The correlation coefficient was 0.94. (F) Same as B, except transporter turnover rates were calculated based on the inverse of the time constant (τ) of voltage-jump relaxations (see Materials and Methods).
The rise-time data suggested that GABA pretreatment was altering the GAT1 turnover rate of oocytes expressing GAT1 and syntaxin 1A. Turnover rate was calculated (18) by measuring the total number of charges translocated per second (calculated from GABA-induced peak currents) and dividing by the number of functional transporters (calculated from GAT1-specific charge movements) in the same oocyte (Fig. 1B). In oocytes expressing GAT1 alone, the turnover rate was ≈7 at 22°C and saturating GABA concentrations, similar to previous estimates (31). GABA pretreatment had no effect on this rate. In oocytes expressing GAT1 and syntaxin 1A, the turnover rate was reduced to ≈2, as described (18). However, pretreatment with GABA caused a progressive increase in transporter turnover rate, approaching the levels of GAT1 alone-injected oocytes after ≈2 min. With GABA wash-out, the turnover rates returned to initial levels with a similar time course (Fig. 1C).
Additional experiments were performed to rule out alternative explanations. First, the method above of using charge movements to assess transporter number assumes that the charge movement measurements are not affected by syntaxin 1A coexpression. For example, if syntaxin 1A alters the distance within the membrane field that charges bind, estimates of transporter number based on these charge movements will be in error. To see whether such measurements were altered because of syntaxin 1A, estimates of changes in transporter number were compared with results from surface biotinylation experiments in the same oocytes (Fig. 1D). The coexpression of GAT1 and syntaxin 1A caused an ≈2-fold increase in the amount of GAT1 on the surface when assessed biochemically, as previously shown (33). Estimates of transporter number based on charge movements showed a similar 2-fold increase, suggesting that charge movements per se are not affected by syntaxin 1A coexpression. Second, the change in expression levels in the presence of syntaxin 1A raised the additional concern that charge movement measurements were skewed by expression levels. To test this hypothesis, transporter number estimates were plotted, in the same oocyte, as a function of peak GABA-induced currents at saturating GABA concentrations (Fig. 1E). Over a 20-fold range of currents, transporter number estimates were highly correlated, suggesting that expression levels were not altering charge movements per se. A third concern was that the interpreted change in turnover rate was actually an alteration in the charge-flux ratio, i.e., because currents and not GABA uptake was being monitored, substrates may be changing not the rate of GABA turnover, but rather the stoichiometry of 2 Na+ to 1 GABA in each cycle (34). To examine this possibility, the charge-flux ratio was examined in oocytes coexpressing GAT1 and syntaxin 1A by simultaneous measurement of [3H]GABA uptake (10 μM for 5 min) and [3H]GABA-induced currents. For five oocytes not pretreated with GABA, the charge flux ratio was 1.8 ± 0.5; for five oocytes pretreated with GABA for 5 min, the charge flux ratio was 2.1 ± 0.4. Fourth, turnover rates were recomputed by using voltage-jump relaxations in the presence of substrate; the relaxation rate is interpreted as being the time course of a single transporter cycle (31, 35, 36). This procedure, which is independent of charge movement measurements, yielded results that were almost identical to that obtained by using charge movements (Fig. 1F).
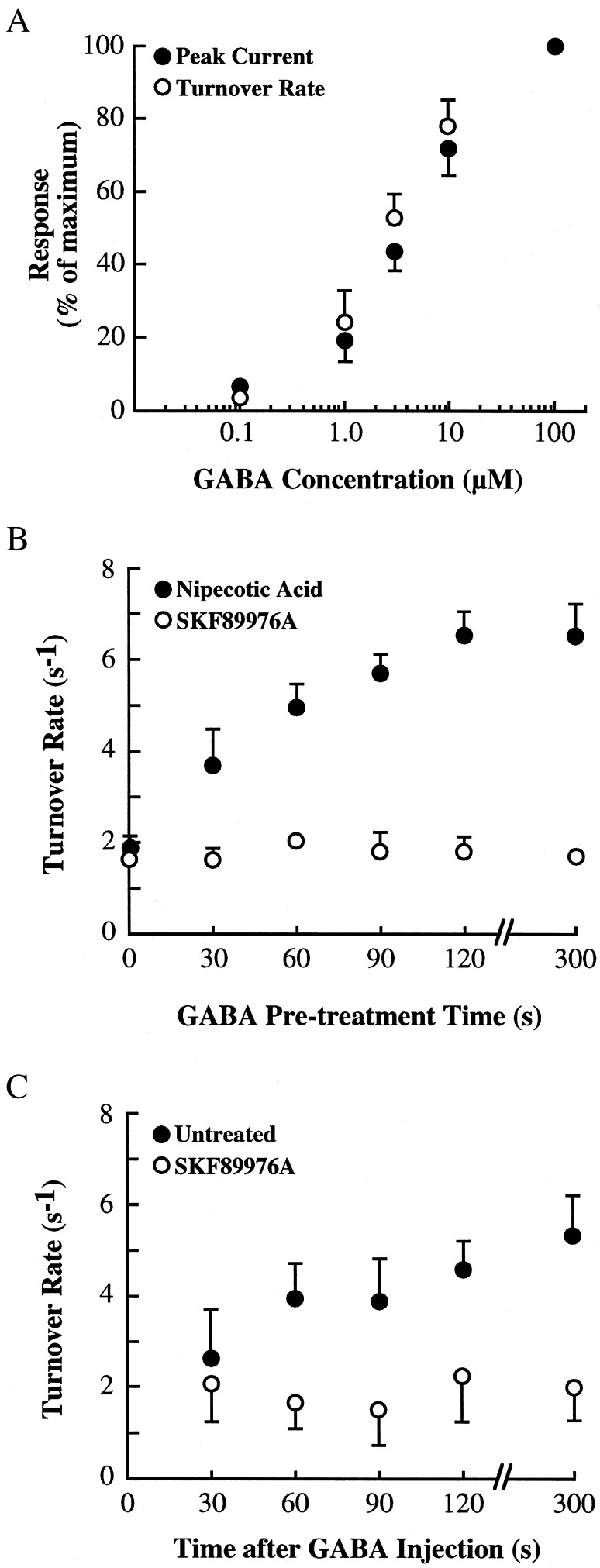
The ability of extracellular GABA to regulate turnover rates of oocytes expressing GAT1 and syntaxin 1A suggested that this effect might be due to direct action of GABA on the transporter. Three experiments were performed to evaluate this possibility. First, the concentration of GABA necessary to induce the change in turnover rates was compared with the concentration of GABA to induce transport-mediated currents (Fig. 2A). In oocytes expressing GAT1 and syntaxin 1A, measurement of GABA-induced peak currents across various GABA concentrations revealed a half-maximal effective GABA concentration of ≈5 μM, similar to previous estimates (31). Next, turnover rates were calculated before and after 5-min treatment with GABA at various concentrations. The half-maximal effective concentration for eliciting changes in GAT1 turnover rates was similar to the values for transporter activation. Second, pretreatment with a saturating concentration of the GAT1 substrate nipecotic acid resulted in an increase in turnover rates that was very similar to the effect of GABA (Fig. 2B). The half-maximal effective nipecotic acid concentration for eliciting changes in GAT1 turnover rates was 28 μM (data not shown), which is comparable to values for transport (15 μM; ref. 37). However, pretreatment with the nontransportable GAT1 antagonist SKF89976A failed to alter turnover rates, suggesting that substrates of GAT1 mediate this regulation. Pretreatment of oocytes expressing GAT1 alone with either nipecotic acid or SKF89976A had no effect on turnover rates (data not shown). Third, injection of a high concentration of GABA into the oocyte and its effects on turnover rates were examined (Fig. 2C). Intracellular GABA caused an increase in turnover rates, suggesting either efflux or intracellular GABA itself was responsible for this effect. Application of SKF89976A, which blocks efflux, prevented the turnover rate change, suggesting that efflux of GABA and not intracellular GABA per se was required. These data support the idea that GABA regulates transporter rates through its activity on the transporter.
Figure 2.
Up-regulation of GAT1 turnover number is related to substrate translocation. (A) Half-maximal effective GABA concentrations necessary to elicit peak currents and alter turnover rates are similar. Oocytes were expressing both GAT1 and syntaxin 1A. Peak GABA-induced currents (●) were calculated at the GABA concentration shown along the abscissa (12 oocytes). Turnover rate (○) was calculated before and immediately after 5-min incubation with the GABA concentration shown along the abscissa (at least 13 oocytes per data point). Peak current data are plotted as the percentage of peak current measured at 100 μM GABA; turnover rate data are plotted as the percent of the change in turnover rate induced by 5-min superfusion of 100 μM GABA. (B) Effects of GAT1 substrates and antagonists on GAT1 turnover rates. Turnover rates were measured by using 300 μM nipecotic acid (●) or 10 μM SKF89976A (○). Data are from 14–18 oocytes per data point, measured from four oocyte batches. (C) Turnover rates are changed by GABA efflux but not by intracellular GABA. Thirty seconds before measurement, oocytes expressing GAT1 and syntaxin 1A were injected with 10 mM GABA. Turnover rates were then measured for oocytes superfused in the absence (●) or presence (○) of 10 μM SKF89976A. Data are from 6–9 oocytes per data point, measured from two oocyte batches.
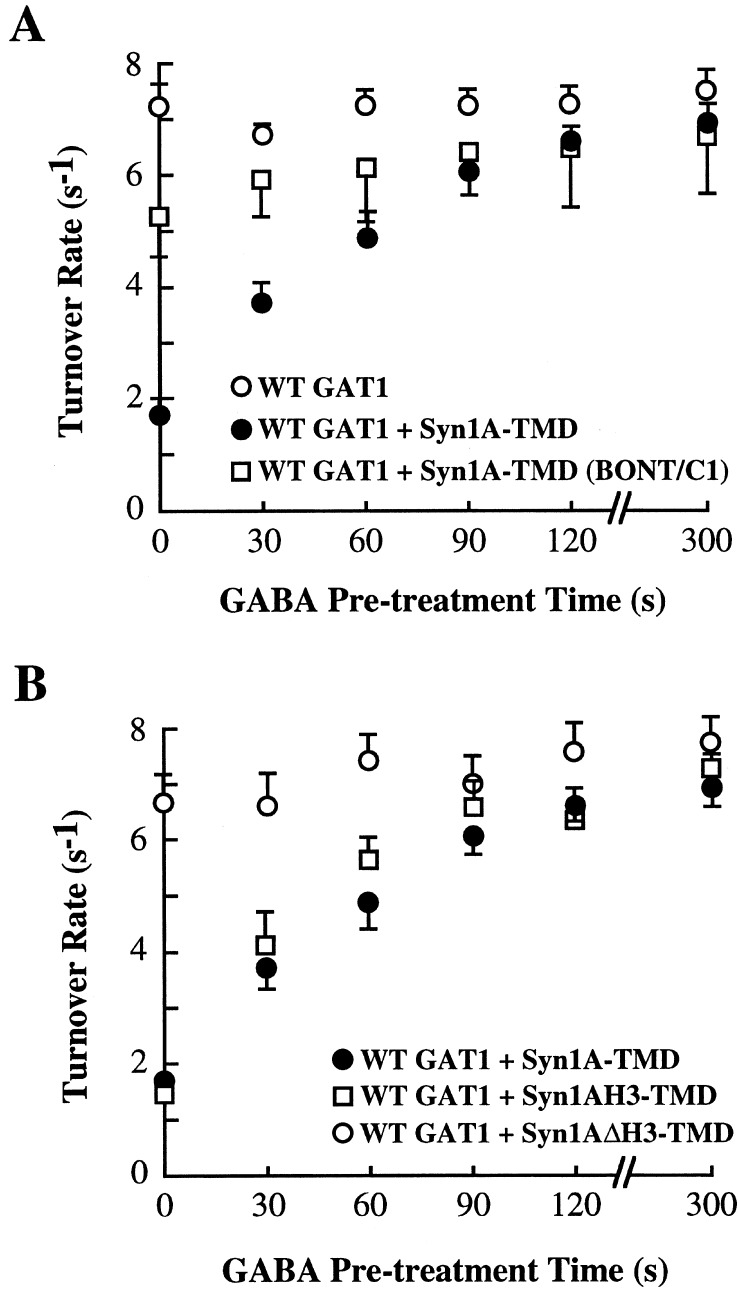
The above data showing that substrates of the transporter can up-regulate GAT1 turnover rates, but only in oocytes expressing GAT1 and syntaxin 1A, suggested that these substrates might be mediating their regulatory effects by affecting the ability of syntaxin 1A to negatively regulate GAT1 turnover rates. To test this hypothesis further, turnover rates were measured in oocytes in which the syntaxin 1A interaction with GAT1 was altered. First, oocytes were acutely injected with BONT/C1 before GABA pretreatment (Fig. 3A). BONT/C1 injection caused an increase in GAT1 turnover rates; in the presence of GABA, this turnover rate increased slightly, suggesting that uncleaved syntaxin 1A is necessary for the GABA-induced regulation. The slight increase in turnover rates seen in BONT/C1-treated oocytes after GABA pretreatment is consistent with the idea that some uncleaved syntaxin 1A is present to mediate its inhibitory effects on GAT1 function. Second, oocytes were injected with GAT1 and either of two syntaxin 1A mutants, one (Syn1AH3-TMD) that contains the H3 domain and functionally interacts with GAT1 and one (Syn1AΔH3-TMD) that lacks the H3 domain and does not functionally interact with GAT1 (18). With respect to substrate-mediated regulation of turnover rates, oocytes expressing the interacting syntaxin isoform resembled oocytes expressing full-length syntaxin 1A; oocytes expressing the noninteracting syntaxin 1A resembled oocytes expressing wild-type GAT1 alone (Fig. 3B).
Figure 3.
Regulation of GAT1 turnover rates depends on GAT1 interactions with syntaxin 1A. (A) BONT/C1 alters syntaxin 1A's regulation of GAT1 turnover rates. Oocytes were injected with GAT1 cRNA alone (○) or with full-length syntaxin 1A cRNA (Syn1A-TMD; ●). Some oocytes were injected 20 min before assay with 1 ng of BONT/C1 (□). Data are from 12–14 oocytes per data point from the same oocyte batch. (B) A syntaxin 1A mutant that does not interact with GAT1 is not regulated by GABA. Oocytes were injected with wild-type GAT1 cRNA in combination with full-length syntaxin 1A cRNA (Syn1A-TMD; ●; data replotted from A), a syntaxin 1A mutant containing the H3 and transmembrane domains (Syn1AH3-TMD; □), and a syntaxin 1A mutant encoding all but the H3 domain (Syn1AΔH3-TMD; ○). Data are from 8–14 oocytes per data point from three oocyte batches.
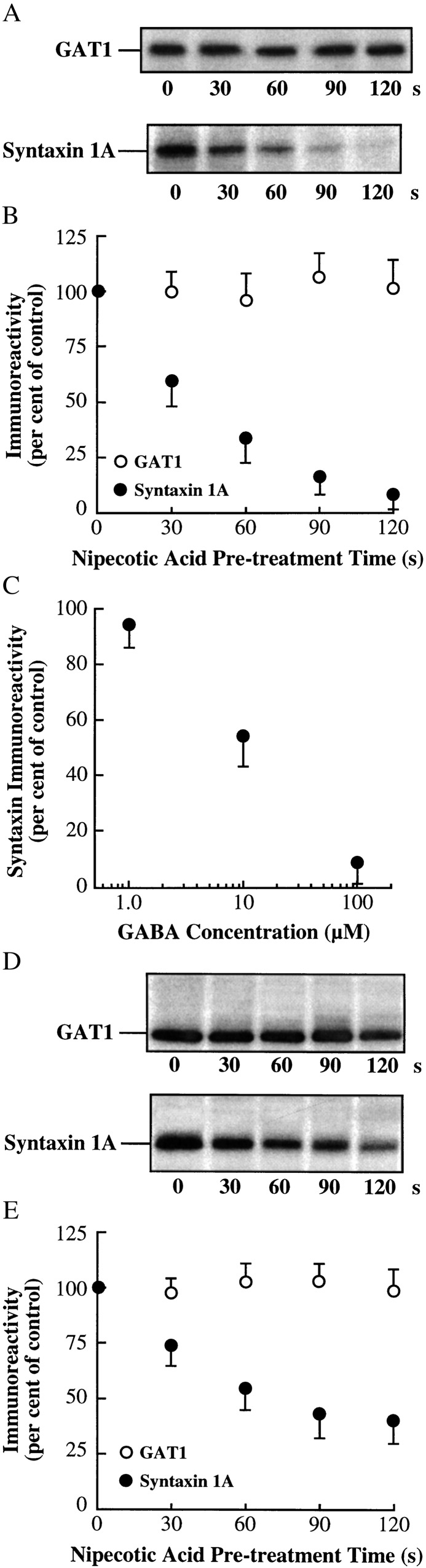
One explanation for the increase in GAT1 turnover rates by substrate pretreatment is that substrate dissociates the interaction between GAT1 and syntaxin 1A. To test this hypothesis, and to examine endogenous regulation by transporter substrates, the GAT1–syntaxin 1A interaction was examined biochemically in both oocytes and dissociated hippocampal neurons (Fig. 4). Oocytes were treated with nipecotic acid for various times and subjected to rapid freezing and homogenization. Nipecotic acid pretreatment did not alter the amount of GAT1 immunoprecipitated from these lysates (Fig. 4A Upper). However, the amount of syntaxin 1A that precipitated with GAT1 decreased as the nipecotic acid pretreatment time increased (Fig. 4A Lower). After 2 min, the amount of syntaxin 1A present in the GAT1 precipitates was negligible (Fig. 4B); these data are consistent with the almost complete recovery of turnover rates to GAT1-alone levels (see Fig. 1B). The dissociation also occurs when GABA is used (Fig. 4C), with a half-maximal efficacy similar to that seen for changes in turnover rates (see Fig. 2A). A similar result of substrate dissociation is seen in hippocampal neurons that endogenously express GAT1 and syntaxin 1A (Fig. 4 D and E), although at saturating substrate concentrations, approximately one-third of the syntaxin 1A remains in a complex with GAT1. Whether this represents a functional complex that is refractory to substrate modification, or a nonfunctional complex (e.g., an intracellular association), is not known.
Figure 4.
GAT1 substrates cause a dissociation of GAT1 and syntaxin 1A in oocytes and hippocampal neurons. (A) Representative immunoblots of coimmunoprecipitation experiments from oocytes (six oocytes per lane). Oocytes expressing GAT1 and syntaxin 1A were treated with 300 μM nipecotic acid for the time period indicated under each blot. Oocytes were then placed on ice, homogenized, and subjected to precipitation using GAT1 Ab; the precipitates were then immunoblotted for the presence of GAT1 (Upper) and syntaxin 1A (Lower). (B) Quantification of experiments performed as in A. GAT1 immunoreactivity (○) is plotted as a percentage of that obtained before application of 300 μM nipecotic acid (time = 0). Syntaxin 1A immunoreactivity (●) is plotted as a percentage of the ratio of syntaxin 1A to GAT1 immunoreactivity at a given time point compared with the ratio of syntaxin 1A to GAT1 immunoreactivity at time = 0. Data are from three experiments, six oocytes per data point. (C) Syntaxin dissociation from GAT1 is dependent on substrate concentration. Experiments are as in A and B, except that oocytes were superfused with various GABA concentrations (as shown on the abscissa) for 2 min. The amount of syntaxin immunoreactivity at each GABA concentration is plotted relative to oocytes superfused with saline alone. Data are from three experiments, six oocytes per data point. (D) Representative immunoblots of coimmunoprecipitation experiments from hippocampal neurons. Cultures were treated as described in A. (E) Quantification of experiments performed as in D. Data are plotted as described for B. Data are from four separate experiments.
Discussion
GABA transporters are found on neurons and glia (38) and function to regulate extracellular GABA concentrations through cotransport of ions down their electrochemical gradient. GABA uptake inhibitors affect both GABAA and GABAB receptor-mediated synaptic transmission (39–41), and depolarization can induce GABA efflux that activates postsynaptic receptors (42). These data demonstrate a physiological role for GABA transporters and suggest that regulation of GAT1 function is important in neuronal signaling. One regulator of GAT1 function is syntaxin 1A, which acts in part by decreasing transporter turnover rates through interactions with the N-terminal tail of GAT1 (18). The present data showing that transporter substrates increase GAT1 turnover rates only in the presence of syntaxin 1A constructs that interact with GAT1 and that GAT1 substrates reduce the amount of syntaxin 1A in complex with GAT1 are consistent with the hypothesis that transporter substrates negatively regulate protein–protein interactions between syntaxin 1A and GAT1. Thus, transporter turnover rates will increase in parallel with increasing extracellular substrate concentrations.
A change in the transporter turnover rate is only one mechanism by which substrates may regulate transporter participation in neuronal signaling. Transporter function also is regulated by rapid redistribution of the transporter between intracellular locations and the plasma membrane; triggers for this form of regulation include transporter substrates. For example, psychostimulants that are either substrates or antagonists of the serotonin transporter regulate the ability or inability, respectively, of the transporter to be phosphorylated by protein kinase C, and the level of protein kinase C phosphorylation positively correlates with net transporter internalization (25). In GAT1, both transporter substrates (28) and syntaxin 1A (33) have been shown to up-regulate surface GAT1 expression. At present, the extent to which substrate-induced dissociation of GAT1 and syntaxin 1A influences GAT1 trafficking is not known. The time course of the effects on turnover rates and trafficking suggest that these regulatory events may be separate. Transporter substrates are not the only trigger for the dissociation of GAT1 from syntaxin 1A. Munc18, a syntaxin 1A-binding partner and component of the synaptic vesicle cycle (43, 44), regulates this interaction (17). One would predict that Munc18 would act in a manner similar to GAT1 substrates in the regulation of GAT1 turnover rates.
The inhibition by syntaxin 1A likely occurs because syntaxin 1A prevents the N-terminal tail of GAT1 to participate normally in the translocation process (18). The present data are consistent with the hypothesis that substrates place the transporter in a conformation in which the N-terminal tail is less likely to interact with syntaxin 1A, and therefore can presumably participate normally in substrate translocation. This state-dependent regulation by cell surface signaling molecules of protein–protein interactions is at least conceptually similar to that seen in G protein-coupled receptors, in which agonist occupation of the receptor regulates its binding to G proteins, kinases, and arrestins (45, 46). The net result of substrate occupation of the transporter would be to increase substrate translocation and thus reduce transmitter signaling. This negative regulation of signaling is consistent with that seen for the interaction of syntaxin 1A and certain calcium channels, where increased calcium levels lead to an increase in the affinity of the interaction (47); this interaction in turn reduces calcium channel function (6–10). Thus, via syntaxin 1A, both calcium channel and transmitter transporter substrates directly regulate their own permeation, providing an efficient mechanism by which to negatively regulate synaptic signaling.
Acknowledgments
I thank Randy Blakely for initial suggestions regarding this project. This work was supported by National Institutes of Health Grants DA10509 and MH61468 to M.W.Q. and HD38985 to the University of Alabama at Birmingham Mental Retardation Research Center.
Abbreviations
- GABA
γ-aminobutyric acid
- BONT/C1
botulinum toxin C1
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Bennett M K, García-Arrarás J E, Elferink L A, Peterson K, Fleming A M, Hazuka C D, Scheller R H. Cell. 1993;74:863–873. doi: 10.1016/0092-8674(93)90466-4. [DOI] [PubMed] [Google Scholar]
- 2.Söllner T, Whiteheart S W, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman J E. Nature (London) 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 3.Südhof T C. Nature (London) 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 4.Rothman J E. Protein Sci. 1996;5:185–194. doi: 10.1002/pro.5560050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheng Z-H, Rettig J, Takahashi M, Catterall W A. Neuron. 1994;13:1303–1313. doi: 10.1016/0896-6273(94)90417-0. [DOI] [PubMed] [Google Scholar]
- 6.Bezprozvanny I, Scheller R H, Tsien R W. Nature (London) 1995;378:623–626. doi: 10.1038/378623a0. [DOI] [PubMed] [Google Scholar]
- 7.Smirnova T, Fossier P, Stinnakre J, Mallet J, Baux G. Neuroscience. 1995;68:125–133. doi: 10.1016/0306-4522(95)00134-5. [DOI] [PubMed] [Google Scholar]
- 8.Wiser O, Bennett M K, Atlas D. EMBO J. 1996;15:4100–4110. [PMC free article] [PubMed] [Google Scholar]
- 9.Sutton K G, McRory J E, Guthrie H, Murphy T H, Snutch T P. Nature (London) 1999;401:800–804. doi: 10.1038/44586. [DOI] [PubMed] [Google Scholar]
- 10.Jarvis S E, Magga J M, Beedle A M, Braun J E, Zamponi G W. J Biol Chem. 2000;275:6388–6394. doi: 10.1074/jbc.275.9.6388. [DOI] [PubMed] [Google Scholar]
- 11.Naren A P, Nelson D J, Xie W, Jovov B, Pevsner J, Bennett M K, Benos D J, Quick M W, Kirk K L. Nature (London) 1997;390:302–305. doi: 10.1038/36882. [DOI] [PubMed] [Google Scholar]
- 12.Naren A P, Quick M W, Collawn J, Nelson D J, Kirk K L. Proc Natl Acad Sci USA. 1998;95:10972–10977. doi: 10.1073/pnas.95.18.10972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fili O, Michaelevski I, Bledi Y, Chikvashvili D, Singer-Lahat D, Boshwitz H, Linial M, Lotan I. J Neurosci. 2001;21:1964–1974. doi: 10.1523/JNEUROSCI.21-06-01964.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qi J, Peters K W, Liu C, Wang J M, Edinger R S, Johnson J P, Watkins S C, Frizzell R A. J Biol Chem. 1999;274:30345–30348. doi: 10.1074/jbc.274.43.30345. [DOI] [PubMed] [Google Scholar]
- 15.Saxena S, Quick M W, Tousson A, Oh Y, Warnock D G. J Biol Chem. 1999;274:20812–20817. doi: 10.1074/jbc.274.30.20812. [DOI] [PubMed] [Google Scholar]
- 16.Quick M W, Corey J L, Davidson N, Lester H A. J Neurosci. 1997;17:2967–2979. doi: 10.1523/JNEUROSCI.17-09-02967.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beckman M L, Bernstein E M, Quick M W. J Neurosci. 1998;18:6103–6112. doi: 10.1523/JNEUROSCI.18-16-06103.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deken S, Beckman M, Boos L, Quick M W. Nat Neurosci. 2000;3:998–1003. doi: 10.1038/79939. [DOI] [PubMed] [Google Scholar]
- 19.Geerlings A, Lopez-Corcuera B, Aragon C. FEBS Lett. 2000;470:51–54. doi: 10.1016/s0014-5793(00)01297-7. [DOI] [PubMed] [Google Scholar]
- 20.Beckman M L, Quick M W. J Membr Biol. 1998;164:1–10. doi: 10.1007/s002329900388. [DOI] [PubMed] [Google Scholar]
- 21.Blakely R D, Bauman A L. Curr Opin Neurobiol. 2000;10:328–336. doi: 10.1016/s0959-4388(00)00088-x. [DOI] [PubMed] [Google Scholar]
- 22.Osawa I, Saito N, Koga T, Tanaka C. Neurosci Res. 1994;19:287–293. doi: 10.1016/0168-0102(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 23.Sato K, Betz H, Schloss P. FEBS Lett. 1995;375:99–102. doi: 10.1016/0014-5793(95)01191-g. [DOI] [PubMed] [Google Scholar]
- 24.Law R M, Stafford A, Quick M W. J Biol Chem. 2000;275:23986–23991. doi: 10.1074/jbc.M910283199. [DOI] [PubMed] [Google Scholar]
- 25.Ramamoorthy S, Blakely R D. Science. 1999;285:763–766. doi: 10.1126/science.285.5428.763. [DOI] [PubMed] [Google Scholar]
- 26.Saunders C, Ferrer J V, Shi L, Chen J, Merrill G, Lamb M E, Leeb-Lundberg L M, Carvelli L, Javitch J A, Galli A. Proc Natl Acad Sci USA. 2000;97:6850–6855. doi: 10.1073/pnas.110035297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu M Y, Shamburger S, Li J, Ordway G A. J Pharmacol Exp Ther. 2000;295:951–959. [PubMed] [Google Scholar]
- 28.Bernstein E M, Quick M W. J Biol Chem. 1999;274:889–895. doi: 10.1074/jbc.274.2.889. [DOI] [PubMed] [Google Scholar]
- 29.Corey J L, Davidson N, Lester H A, Brecha N, Quick M W. J Biol Chem. 1994;269:14759–14767. [PubMed] [Google Scholar]
- 30.Quick M W, Lester H A. In: Ion Channels of Excitable Cells, Methods in Neurosciences. Narahashi T, Conn P M, editors. Vol. 19. San Diego: Academic; 1994. pp. 261–279. [Google Scholar]
- 31.Mager S, Naeve J, Quick M W, Labarca C, Davidson N, Lester H A. Neuron. 1993;10:177–188. doi: 10.1016/0896-6273(93)90309-f. [DOI] [PubMed] [Google Scholar]
- 32.Kavanaugh M P, Arriza J L, North R A, Amara S G. J Biol Chem. 1992;267:22007–22009. [PubMed] [Google Scholar]
- 33.Horton N, Quick M W. Mol Membr Biol. 2001;18:39–44. [PubMed] [Google Scholar]
- 34.Loo D D, Eskandari S, Boorer K J, Sarkar H K, Wright E M. J Biol Chem. 2000;275:37414–37422. doi: 10.1074/jbc.M007241200. [DOI] [PubMed] [Google Scholar]
- 35.Hilgemann D W, Nicoll D A, Philipson K D. Nature (London) 1991;352:715–718. doi: 10.1038/352715a0. [DOI] [PubMed] [Google Scholar]
- 36.Parent L, Supplisson S, Loo D D, Wright E M. J Membr Biol. 1992;125:49–62. doi: 10.1007/BF00235797. [DOI] [PubMed] [Google Scholar]
- 37.Larsson O M, Krogsgaard-Larsen P, Schousboe A. Neurochem Int. 1985;7:853–860. doi: 10.1016/0197-0186(85)90041-5. [DOI] [PubMed] [Google Scholar]
- 38.Ribak C E, Tong W M Y, Brecha N C. J Comp Neurol. 1996;367:595–606. doi: 10.1002/(SICI)1096-9861(19960415)367:4<595::AID-CNE9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 39.Dingledine R, Korn S J. J Physiol (London) 1985;366:387–409. doi: 10.1113/jphysiol.1985.sp015804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Isaacson J S, Solis J M, Nicoll R A. Neuron. 1993;10:165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- 41.Solis J M, Nicoll R A. J Neurosci. 1992;12:3466–3472. doi: 10.1523/JNEUROSCI.12-09-03466.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaspary H L, Wang W, Richerson G B. J Neurophysiol. 1998;80:270–281. doi: 10.1152/jn.1998.80.1.270. [DOI] [PubMed] [Google Scholar]
- 43.Hata Y, Slaughter C A, Südhof T C. Nature (London) 1993;366:347–351. doi: 10.1038/366347a0. [DOI] [PubMed] [Google Scholar]
- 44.Pevsner J, Hsu S-C, Scheller R H. Proc Natl Acad Sci USA. 1994;91:1445–1449. doi: 10.1073/pnas.91.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferguson S S, Barak L S, Zhang J, Caron M G. Can J Physiol Pharmacol. 1996;74:1095–1110. doi: 10.1139/cjpp-74-10-1095. [DOI] [PubMed] [Google Scholar]
- 46.Lefkowitz R J. J Biol Chem. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- 47.Kim D K, Catterall W A. Proc Natl Acad Sci USA. 1997;94:14782–14786. doi: 10.1073/pnas.94.26.14782. [DOI] [PMC free article] [PubMed] [Google Scholar]