Abstract
Histone post-translational modifications (PTMs) interact in complex ways to regulate chromatin structure and gene expression. To investigate this interplay, we analyze ChIP-seq and RNA-seq data from knock-out mutants lacking enzymes responsible for H3K4me2/3, H3K9me3, or H3K27me3 in the phytopathogenic fungus Pyricularia oryzae. Loss of specific PTMs alters other PTMs and gene expression in a compartment-specific manner, with distinct effects across H3K4me2-rich euchromatin (EC), H3K27me3-rich facultative heterochromatin (fHC), H3K9me3-rich constitutive heterochromatin (cHC), and centromeres. We identify two distinct fHC subcompartments: K4-fHC, adjacent to EC, and K9-fHC, adjacent to cHC. Both contain poorly conserved genes, but K9-fHC harbors more transposable elements, while K4-fHC is more enriched for genes upregulated during infection, including effector-like genes. H3K27me3 levels in K4-fHC respond to changes in other PTMs, especially H3K9me3, and to environmental conditions. These findings suggest that K4-fHC functions as a reservoir of genes highly responsive to chromatin context and environmental cues.
Subject terms: Epigenetics, Fungal genomics
The genome of the fungus Pyricularia oryzae contains two distinct types of facultative heterochromatin, each characterized by different patterns of gene content, expression, and chromatin regulation.
Introduction
Posttranslational modifications (PTMs) of histone are essential epigenetic mechanisms that regulate gene expression and chromatin structure in eukaryotes, playing pivotal roles in various cellular processes such as transcriptional regulation, DNA repair, and genome stability. Specific histone PTMs act as binding sites for downstream proteins containing corresponding domains, such as bromodomains, chromodomains, and Tudor domains1. These proteins can recruit chromatin remodeling complexes, transcriptional activators or repressors, and DNA repair factors to modulate gene expression and cellular processes. It is known that histone PTMs, whether proximal or distal, can interact with each other, serving as a code for subsequent responses, a phenomenon known as histone PTM crosstalk2,3. For example, histone acetylation can promote the recruitment of histone demethylase or disrupt the binding of histone deacetylases, leading to changes in PTM patterns and transcriptional outcomes4.
One of the most well characterized PTMs, histone methylation, occurs on lysine and arginine residues and can have either activating or repressive effects on gene expression, depending on the position of the methylated residues in histone proteins and the degree of methylation5. For instance, di- and tri-methylation on histone 3 lysine 4 (H3K4me2/3) are generally associated with active transcription and are considered histone marks for euchromatic regions in the genome. Meanwhile, trimethylation on histone 3 lysine 9 and 27 (H3K9me3 and H3K27me3) are representative repressive marks against gene expression and are considered histone marks for constitutive and facultative heterochromatin, respectively.
Histone methylation on lysine is facilitated by a group of enzymes known as histone lysine methyltransferases (KMTs). Each histone modification is primarily catalyzed by a specific family of KMTs6. For instance, H3K4me2/3 is catalyzed by the KMT2 family, which includes Set1 in yeast. H3K9me3 and H3K27me3, on the other hand, are catalyzed by the KMT1 and KMT6 families, respectively. Genetic and biochemical analyses have revealed that KMTs function as a protein complex, such as COMPASS (COMplex of Proteins ASsociated with Set1) for KMT27, and PRC2 (Polycomb Repressive Complex 2) for KMT68–10. In the fungus Neurospora crassa, DIM5, belonging to the KMT1 family, also forms a protein complex, referred to as DCDC [DIM5/7/9, CUL4/DDB1 Complex]11.
The loss of KMT gene(s) often results in the redistribution of specific histone modifications that are not directly catalyzed by the respective KMTs. In mammals, knockout cells of two KMT1 genes, SUV3-9H1/H2, showed a loss of H3K9me3 and a subsequent gain of an alternative repressive mark, H3K27me3, at the pericentric heterochromatin12. Similarly, in N. crassa, the loss of any component of DCDC, which catalyzes H3K9me3, resulted in the relocation of H3K27me2/3 to the constitutive heterochromatin regions marked with H3K9me3 in the wild type13. Furthermore, the loss of the MES-4 KMT in Caenorhabditis elegans caused a global loss of the active histone mark H3K36me3 and a redistribution of H3K27me3 to germline genes14. A similar interplay between H3K27me3 and H3K36me3 has also been reported in the fungus Pyricularia oryzae15.
P. oryzae (syn. Magnaporthe oryzae) is a fungal pathogen that causes blast diseases in various gramineous plants such as rice, wheat, oat, and finger millet16. Rice blast typically causes 10–30% yield losses, although regional epidemics can lead to almost 100% loss17,18. Wheat blast is also an emerging threat to global wheat production, spreading from South America to Eurasia and Africa19,20.
Recently, it has been shown that histone PTMs play crucial roles in interactions between plants and fungi, including both parasitic and symbiotic interactions21. Microorganisms associated with plants produce small secreted molecules, termed effectors, which affect host cell structure and metabolism, thereby facilitating infection, symbiosis and/or triggering a defense response. In fungi, genes encoding effectors are often located in so-called lineage-specific (LS) regions, adaptive genomic regions (AGRs), or collectively in the accessory genome22,23. These regions are typically gene-sparse, rich in transposable elements (TE), exhibit low genetic conservation, and are marked by suppressive PTMs such as H3K9me3 and H3K27me3. In P. oryzae, H3K27me3 has been shown to contribute to the transcriptional regulation of effector genes upon infection15. Similarly, in Verticillium dahliae, the loss of H3K27me3 led to the activation of in planta-induced genes containing putative effector genes, suggesting the involvement of H3K27me3 in effector production during infection24. Additionally, gene clusters for the production of fungal secondary metabolites (SM) such as mycotoxins and phytohormones, some of which function as effectors, are also transcriptionally regulated by PTMs such as H3K9ac25,26 and H3K27me327, as well-documented in Aspergillus and Fusarium fungi. These accumulating data strongly support the idea that chromosomal regulation through PTMs contributes to the pathogenicity of fungal pathogens.
Here, we address the interplay among PTMs by performing chromatin immunoprecipitation sequencing (ChIP-seq) analysis alongside RNA sequencing (RNA-seq) analysis using P. oryzae mutants of three KMT genes: MoSet1, MoKmt1, and MoKmt6, which encode catalyzing enzymes for H3K4me2/3, H3K9me3, and H3K27me3, respectively. Through this analysis, we identified two distinct domains of facultative heterochromatin in the P. oryzae genome, which may have slightly different roles in genome function and regulation.
Results
Genomic compartments characterized by histone modifications in the Br48 genome
To explore the interplay among histone modifications in P. oryzae, ChIP-seq analysis was performed using deletion mutants in KMT genes. The mutants employed were Δmoset1, Δmokmt1, and Δmokmt6, which lack the enzyme responsible for H3K4me2/3, H3K9me3, and H3K27me3 modifications, respectively, in the wheat-infecting strain of P. oryzae, Br48 (WT)28. Our previous studies on the KMT mutants have shown that the active mark H3K4me2/3 plays a crucial role in pathogenicity on host plants, and the repressive marks H3K9me3 and H3K27me3 are also required for full virulence28.
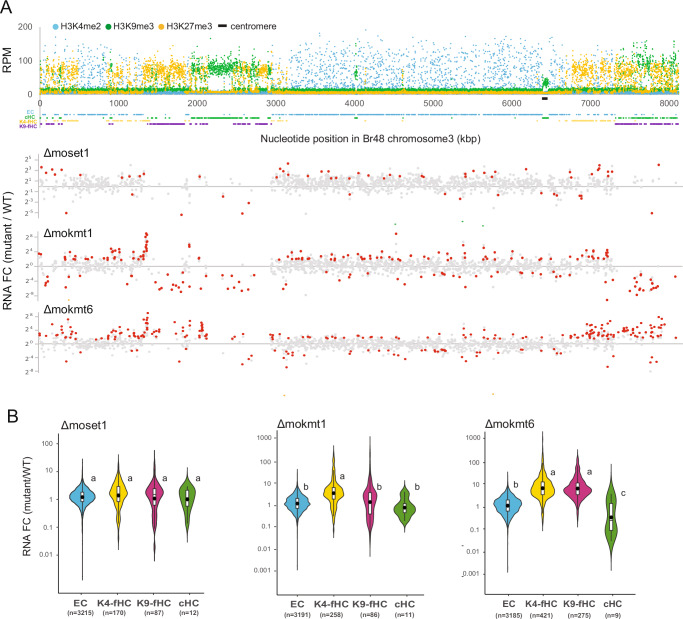
For each analysis, ChIP-seq reads were mapped to the telomere-to-telomere genome sequence of Br4829, and the reads per million mapped reads (RPM) was calculated using a 1 kb window across the chromosomes. The mapping data on the entire chromosome 1 in WT and the mutants are given in Fig. 1A and Supplementary Fig. 1, respectively. We first defined the genomic compartments: euchromatin (EC), constitutive heterochromatin (cHC), and facultative heterochromatin (fHC) using the ChIP-seq data. Based on the assumption that genomic domains composed of consecutive H3K4me2-rich, H3K9me3-rich, and H3K27me3-rich segments corresponded to EC, cHC, and fHC, respectively, we filtered 42,502 1 kb segments in the Br48 genome using the HOMER peak-calling software30 as described in Methods. Consequently, 8183 segments (19.3%) with 3285 genes (26.9%), 3417 segments (8.0%) with 68 genes (0.6%), and 7541 segments (17.7%) with 2313 genes (20.2%) were assigned to EC-, cHC-, and fHC-compartments, respectively. Additionally, 23,361 segments (55.0%) with 6558 genes (53.6%) were left as unassigned (UA) segments (Table 1). To be noted, the ratio of each compartment varied significantly among chromosomes. For instance, the cHC compartment comprises only 3.3% on chromosome 5, whereas it constitutes 13.3% on chromosome 3 (Table 1 and Supplementary Fig. 2). Similarly, the fHC compartment represents 23.6% on chromosome 3 but only 8.4% on chromosome 2.
Fig. 1. Gene deletion of a histone lysine methyltransferase in Pyricularia oryzae causes changes in the levels of histone modifications for which the enzyme itself is not primarily responsible.
A Read mapping data from ChIP-seq analysis of H3K4me2 (blue dots), H3K9me3 (green dots), and H3K27me3 (yellow dots) on chromosome 1 in the P. oryzae Br48 strain (WT). Each dot represents an RPM value of a 1 kb segment in the ChIP-seq analysis. The X-axis represents the genomic location, and the Y-axis represents the RPM value. Genomic compartments, EC, cHC, K4-fHC, and K9-fHC (see details in text) are indicated by blue, green, yellow, and purple lines, respectively, below the X-axis. B Changes in the levels of H3K4me2, H3K9me3, and H3K27me3 on chromosome 1 in the gene deletion mutants MoSet1 (Δmoset1), MoKmt1 (Δmokmt1), and MoKmt6 (Δmokmt6). Each dot represents an RPM fold change (FC) value of a 1 kb segment in the Br48 genome. Red, blue, and yellow dots represent RPM FC values of Δmoset1/WT, Δmokmt1/WT, and Δmokmt6/WT, respectively. The X-axis corresponds to the genome location shown in (A).
Table 1.
Number of segments in five genomic compartments across chromosomes of Pyricularia oryzae Br48
| Chromosome | EC | K4-fHC | K9-fHC | cHC | UA |
|---|---|---|---|---|---|
| Chr 1 | 1028 | 516 | 905 | 794 | 2997 |
| Chr 2 | 1988 | 310 | 358 | 356 | 4957 |
| Chr 3 | 1214 | 780 | 1139 | 1084 | 3904 |
| Chr 4 | 1268 | 566 | 223 | 196 | 3164 |
| Chr 5 | 978 | 208 | 215 | 147 | 2888 |
| Chr 6 | 1070 | 815 | 362 | 580 | 3450 |
| Chr 7 | 637 | 662 | 482 | 260 | 2001 |
| Total | 8183 | 3857 | 3684 | 3417 | 23361 |
EC euchromatin, K4-fHC H3K4me2-associated facultative heterochromatin, K9-fHC H3K9me3-associated facultative heterochromatin, cHC constitutive heterochromatin, UA unassigned segments.
To obtain a global view of the relationship among the histone PTMs and their alterations by the kmt mutations, fold changes (kmt mutant/WT) of the RPM values for each histone PTM on the entire chromosome 1 are shown in Fig. 1B. Additionally, correlation coefficients of the segment RPM values among histone PTMs in the WT strain and mutants were calculated (Supplementary Data 1).
As global tendencies, the following features were noted. Firstly, each kmt mutant displayed significant alterations in levels of histone PTMs that the enzyme itself is not primarily responsible for. The alterations were remarkable in specific genomic regions rather than being widespread across the entire genome. Secondly, the changes in histone PTMs, possibly influenced by their interplay, were particularly pronounced in fHC and cHC. In contrast, EC exhibited comparatively fewer alterations. Thirdly, the alterations in H3K4me2 levels were relatively moderate, while H3K27me3 levels were considerably affected by the loss of the other PTMs. Lastly, overall levels of H3K27me3 were positively and negatively correlated with those of H3K9me3 and H3K4me2, respectively, as judged by the correlation coefficient values (Supplementary Data 1).
Analysis of the effect of the Δmokmt1 mutation on H3K4me2 and H3K27me3 reveals two sub-compartments in facultative heterochromatin
To assess the impact of the Δmokmt1 mutation on H3K4me2 and H3K27me3, the fold change (Δmokmt1/WT) of their RPM values was calculated for each 1 kb segment. The fold changes of H3K4me2 and H3K27me3 in the genomic compartments are presented in violin plots (Supplementary Fig. 3A,B). In Δmokmt1, levels of H3K4me2 were slightly but significantly elevated in suppressive genome domains such as the cHC and fHC compartments. The Δmokmt1 mutation caused more pronounced alterations in H3K27me3 levels in a genomic compartment-dependent manner. While the average level of H3K27me3 remained largely unchanged in EC and UA, it was markedly elevated in cHC and decreased in fHC (Supplementary Fig. 3B). To further explore this, we examined the relationship between the fold changes of H3K27me3 and its levels in WT using scatter plots (Supplementary Fig. 3C). Overall, fold change values tended to decrease as H3K27me3 levels in WT increased. It appeared that there were two separated trend lines: one comprising cHC and fHC segments, and the other consisting of EC and fHC segments. Interestingly, H3K27me3 levels were generally elevated in the former group but mostly reduced in the latter by the Δmokmt1 mutation, suggesting that MoKmt1 could affect H3K27me3 levels in opposite directions depending on genomic context. Based on this observation, we hypothesized that two distinct H3K27me3-rich compartments exist: one associated with EC (H3K4me2/3) and the other with cHC (H3K9me3). To test this hypothesis and define these compartments, we conducted an association analysis with various parameters and found that adjacent regions significantly influence their delineation. Accordingly, the fHC segments were classified into two sub-compartments, K4-fHC and K9-fHC, depending on whether the segment is more closely adjacent to EC or cHC, respectively (see Methods for details). A simplified diagram illustrating the assignment of the segments to K4-fHC or K9-fHC is provided in Supplementary Fig. 3D.
When the assigned segments were plotted by compartment in scatter plots (Supplementary Fig. 3E), segments from each compartment generally exhibited distinct distribution patterns, although some overlap was observed between compartments. However, UA data were excluded from subsequent analyses, unless otherwise noted, because UA segments include those with differing properties due to our categorization rules (see Methods for details). These include segments with no called peak, those only partially overlapping a called peak, and those spanning the boundary between two different modification peaks.
Figure 2A–D are revised versions of Supplementary Fig. 3A–C, including the genomic compartments, K4-fHC and K9-fHC. The results indicated that the absence of MoKmt1 and, consequently, H3K9me3, often led to a decrease in H3K27me3 deposition in K4-fHC but rarely in K9-fHC, and a drastic increase in cHC. Furthermore, as mentioned above, the fold change values generally decreased as the levels of H3K27me3 in WT increased (Fig. 2C). This suggests that MoKmt1, and possibly H3K9me3, can influence H3K27me3 levels even in genomic regions with low levels of H3K9me3 and/or H3K27me3.
Fig. 2. Analysis of the effect of the Δmokmt1 mutation on H3K4me2 and H3K27me3 reveals two sub-compartments in facultative heterochromatin.
Violin plots depicting fold change (FC) values of H3K4me2 (A) and H3K27me3 (B) RPM in Δmokmt1 relative to WT across different genomic compartments. Based on adjacent genomic regions (see Methods for details), the facultative heterochromatic (fHC) compartment was subdivided into two groups: K4-fHC and K9-fHC. Statistically significant differences are indicated by different letters (Tukey’s HSD test on logarithmically transformed data, P ≤ 0.01). Scatter plot showing RPM FC values of H3K27me3 (Δmokmt1/WT) (Y-axis) relative to H3K27me3 (C) or H3K9me3 (D) levels in WT (X-axis). Each dot represents an RPM FC value for a 1 kb segment in the Br48 genome. Genomic segments are color-coded as follows: EC (blue), K4-fHC (yellow), K9-fHC (purple), and cHC (green).
Characterization and functional validation of K4‑fHC and K9‑fHC, two sub‑compartments of facultative heterochromatin
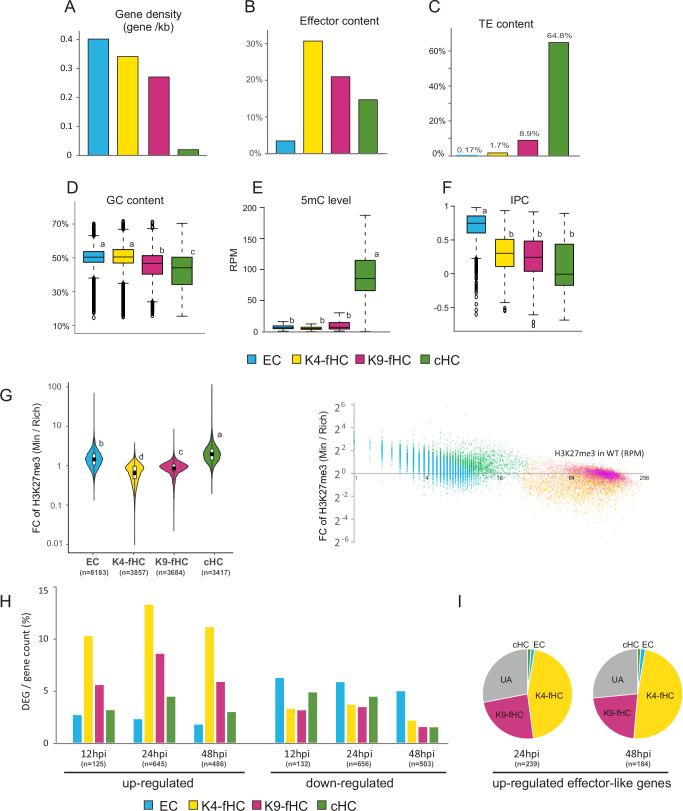
To characterize the sub-compartments K4-fHC and K9-fHC, we examined gene density, effector content, TE content, GC content, and 5-methylcytosin (5mC) levels. K4-fHC and K9-fHC consist of 3857 and 3684 segments, containing 1316 and 997 genes, respectively (Table 1). Gene density is slightly higher in K4-fHC (0.34 genes/kb) compared to K9-fHC (0.27 genes/kb) (Fig. 3A). An EffectorP search identified 1439 effector candidate proteins in the Br48 genome, with 404 and 209 candidates located in K4-fHC and K9-fHC, respectively. Interestingly, the proportion of effector candidates relative to total genes within each compartment is higher in K4-fHC (30.7%) than in K9-fHC (21.0%), and both are much higher than in EC (3.4%) (Fig. 3B).
Fig. 3. Characterization of two sub‑compartments of facultative heterochromatin, K4‑fHC and K9‑fHC.
Genomic features of each compartment: gene density (A), effector content (B), transposable element (TE) content (C), and GC content (D), 5-methylcytosine (5mC) level (E), and index of phylogenetic concordance (IPC)(F). Box plots show the GC content of segments (D), RPM values of segments from MeDIP analysis (E), and IPC values of genes (F) in each genomic compartment. Different letters indicated statistically significant differences (Tukey’s HSD test, P ≤ 0.01). G Changes in H3K27me3 levels under two culture conditions (minimal vs. rich media). Fold change values (Min/Rich) are displayed as violin plots (left) and scatter plots (right) relative to H3K27me3 levels in the wild-type (WT). Scatter plots showing the data for compartment separately are provided in Supplementary Fig. 4. Different letters indicated statistically significant differences (Tukey’s HSD test on logarithmically transformed data, P ≤ 0.01). H Differentially expressed genes (DEGs, FDR P < 0.05) during infection at 12, 24, and 48 h post-inoculation (hpi) compared to expression in rich media. DEGs were classified as up- or down-regulated and grouped by genomic compartment. DEG counts were normalized to the total number of genes in each compartment. I Pie charts showing the genomic compartments of effector-like genes up-regulated during infection.
In contrast, the proportion of TE-containing segments is higher in K9-fHC (8.9%) than in K4-fHC (1.7%) (Fig. 3C). Consistently, the average GC content in K9-fHC (45.7%) is significantly lower than that in K4-fHC (50.1%) (Fig. 3D), possibly due to repeat-induced point mutation (RIP)31, which causes C:G to T:A transitions often in TE sequences. However, no significant difference was detected in 5mC levels between K4-fHC and K9-fHC (Fig. 3E).
We also assess IPC (Index of Phylogenetic Concordance) values29 of resident genes in these genomic compartments. IPC is an index assigned to each gene as a correlation coefficient between gene similarity and species similarity within ascomycete fungi, and thus indicates how conserved the gene is within the population. The IPC values across the four genome compartments are shown in box plots (Fig. 3F). The average IPC value in EC is significantly higher than those in the suppressive compartments, K4-fHC, K9-fHC, and cHC, indicating that genes in EC are more conserved than those in K4-fHC, K9-fHC, and cHC. No significant difference in average IPC values was observed among K4-fHC, K9-fHC, and cHC. These results demonstrate that although K4-fHC and K9-fHC share some features, they differ considerably in genomic composition.
To address whether the sub-compartments K4-fHC and K9-fHC are functionally distinct in a biological context, we investigated changes in their H3K27me3 levels under different culture conditions and gene expression levels during infection. ChIP-seq analysis was performed using mycelia of the WT strain cultured in rich and minimal media. The fold changes (minimal/rich media) in H3K27me3 levels across the four genomic compartments are presented as violin and scatter plots (Fig. 3G and Supplementary Fig. 4). The average fold change of H3K27me3 in K4-fHC was significantly lower than that in K9-fHC. These results suggest that H3K27me3 levels are more dynamic in K4-fHC than in K9-fHC, consistent with observations in the Δmokmt1 mutant.
We also examined the genomic locations of differentially expressed genes (DEGs; FDR p value < 0.05, fold change > 3) in infected leaves. RNA-seq analysis of Br48-infected leaves at three time points post-inoculation revealed that K4-fHC was consistently the most enriched compartment for up-regulated DEGs (Fig. 3H). Up-regulated DEGs were also more frequently detected in K9-fHC and cHC than in EC. Conversely, down-regulated DEGs were depleted in both K4-fHC and K9-fHC (Fig. 3G). When focusing on effector-like genes upregulated during infection, approximately half of them were located in the K4-FH compartment (Fig. 3I). Overall, these results support the presence of two distinct fHC compartments in the P. oryzae genome and suggest that K4-fHC serves as a reservoir of genes highly responsive to cellular and environmental stimuli.
Impact of the Δmoset1 and Δmokmt6 mutations on non-target histone PTMs
Figures 4A and B show violin plots showing the fold changes (Δmoset1/WT) of H3K9me3 and H3K27me3, respectively, across the four genomic compartments. The most striking change induced by the moset1 mutation was a decrease in the average H3K9me3 level in cHC. This reduction was observed regardless of the H3K9me3 level in WT, except for segments with H3K9me3 levels exceeding 170 RPM (Fig. 4C).
Fig. 4. The deletion of the MoSet1 gene primary induces alterations in H3K9me3 and H3K27me3 levels in the cHC compartment.
Fold change (FC) values of H3K9me3 (A) and H3K27me3 (B) RPMs in Δmoset1 relative to WT are presented in violin plots across four genomic compartments, euchromatin (EC), H3K4me2-associated facultative heterochromatin (K4-fHC), H3K9me3-associated facultative heterochromatin (K9-fHC), and constitutive heterochromatin (cHC) segments. Different letters indicate statistically significant differences (Tukey’s HSD test on logarithmic transformed data, P ≤ 0.01). RPM FC values (Δmoset1/WT) of H3K9me3 (C) and H3K27me3 (D) are presented in a scatter plot with reference to H3K9me3 levels in WT. Each dot represents an RPM FC value of a 1 kb segment in the Br48 genome. Segments in EC, K4-fHC, K9-fHC, and cHC are shown in blue, yellow, purple, and green, respectively.
Conversely, in Δmoset1, the average level of H3K27me3 was elevated in cHC (Fig. 4B, D), suggesting a potential compensatory mechanism by H3K27me3 for the decreased H3K9me3 levels. Interestingly, the decrease in H3K27me3 levels caused by the moset1 mutation was more pronounced in one of the H3K27me3-rich compartments, K4-fHC, than in K9-fHC (Fig. 4B, D), supporting the idea that these two fHC compartments are regulated in somewhat distinct ways.
Based on the violin plots showing the fold changes (Δmokmt6/WT) of H3K4me2 and H3K9me3 across the four genomic compartments (Fig. 5A, B), an increase in the average levels of H3K4me2 was observed primarily in K4-fHC and K9-fHC, with a slight elevation in cHC. In particular segments within K4-fHC and K9-fHC, a strikingly high elevation in H3K4me2 deposition was observed (Fig. 5C, E), which was associated with subsequent upregulation of gene expression, as detailed later.
Fig. 5. The deletion of the MoKmt6 gene causes more global alterations in H3K9me3 levels than in H3K4me2 levels.
Fold change (FC) values of H3K4me2 (A) and H3K9me3 (B) RPMs in Δmokmt6 relative to WT are presented in violin plots with respect to five different genomic compartments. Different letters indicate statistically significant differences (Tukey’s HSD test on logarithmic transformed data, P ≤ 0.01). RPM FC values (Δmokmt6/WT) of H3K4me2 (C, E) and H3K9me3 (D, F) are presented in a scatter plot with reference to H3K9me3 (C, D) or H3K27me3 (E, F) levels in WT. Each dot represents an RPM FC value of a 1 kb segment in the Br48 genome. Segments in EC, K4-fHC, K9-fHC, and cHC are shown in blue, yellow, purple, and green, respectively.
The mokmt6 mutation also exerted a significant impact on H3K9me3 levels, largely in a compartment-specific manner. H3K9me3 deposition generally increased within cHC (Fig. 5B), suggesting compensatory mechanisms for the loss of H3K27me3 by H3K9me3 in Δmokmt6. Conversely, H3K9me3 levels generally decreased in the other genomic compartments (Fig. 5B), suggesting a role of H3K27me3 to maintain H3K9me3 levels, especially in fHC. However, certain segments within K9-fHC, but very few within K4-fHC, showed significant enrichment of H3K9me3 in Δmokmt6 (Fig. 5D, F).
Comparison of the effects of KMT mutations across functional genome elements
The impact of KMT mutations across functional genome elements, such as centromeres, coding sequences (CDs), and TEs, was examined. To identify centromeres in Br48, ChIP-seq data of the CenpA-like protein (Cse-4, MGG_06445) were used for peak calling with the HOMER program, identifying seven centromeres, one per chromosome, ranging from 71 kb to 101 kb in span32. Consequently, 564 segments were assigned to centromeres (CEN), all belonged to the cHC compartment with high H3K9me3 levels (Fig. 1A), except 5 segments to UA with high levels of both H3K9me3 and H3K27me3. For TE segments, sequences homologous to those identified in Br4829 were selected via BLAST search (E-value < 1e-10), resulting in 2965 TE-containing segments. A CD segment was defined as one fully contained within a gene body. For a clear comparison, 564 segments, the same number as the CEN segments, were randomly selected from both the TE and CD segment groups and used for further analysis. The TE segments mostly resided within the repressive cHC (76.9%) and fHC (12.5%) compartments. The CD segments consisted of 28.5% EC, 10.8% K4-fHC, 6.3% K9-fHC, 0.7% cHC, and 54.5% UA.
Figures 6A and B present the fold change values of H3K27me3 in the Δmokmt1 mutant using scatter and box plots, respectively. Overall, the fold change values were more dependent on genomic compartments than on element types. For instance, the H3K27me3 fold change values of CD segments did not change significantly in EC but were decreased in K4-fHC (Fig. 6B). Similarly, the H3K27me3 fold change values of TE segments also showed compartment-dependent changes (Fig. 6B). Consistently, the CEN segments showed elevated fold change values, similar to other segments in cHC. However, the H3K9me3 levels of the CEN segments in WT were confined to a relatively narrow range, mostly between 30 and 100 RPM, whereas other cHC segments, such as TE segments, ranged more broadly, up to 250 RPM (Figs. 2A and 6A).
Fig. 6. Histone PTM interplay is more dependent on genomic compartments than on element types.
A Scatter plots showing fold-change (FC) values of H3K27me3 RPM in Δmokmt1 relative to WT, with reference to H3K27me3 levels in WT. Each gray dot represents the FC value of a 1 kb segment in the Br48 genome. A total of 564 genome segments were selected for each of the following categories: coding sequences (CD), centromeres (CEN), and transposable elements (TE), and are shown in blue, red, and yellow, respectively (see details in the text). B Box plots displaying FC values of H3K27me3 RPM in Δmokmt1 relative to WT across different genomic compartments, focusing on genomic elements such as CD (blue), CEN (red), and TE (yellow). Different letters indicate statistically significant differences (Tukey’s HSD test on log-transformed data, P ≤ 0.01). Scatter plots showing FC values of H3K9me3 RPM in Δmokmt6 (C) and Δmoset1 (D) relative to WT, with reference to H3K9me3 levels in WT. Color coding in B–D is as described in (A).
Figure 6C, D show the H3K9me3 fold change values of the CD, CEN, and TE segments in the Δmokmt6 and Δmoset1 mutants, respectively, in scatter plots. While H3K9me3 levels tended to rise in cHC (Fig. 5B), the levels in CEN segments were not significantly affected by the mutation in Δmokmt6 (Fig. 6C), suggesting that centromeres are regulated slightly in a different manner form other cHC segments. Similarly, H3K9me3 fold change values in Δmoset1 were only slightly decreased in CEN segments, whereas they were more drastically reduced in TE segments (Fig. 6D). These results indicate that centromeres are regulated within a relatively narrow range and behave somewhat differently from other regions in cHC, likely forming a distinct sub-compartment in the genome.
Relationship between histone PTMs and gene expression in the kmt mutants
To gain insight into the impact of kmt mutations on gene regulation in P. oryzae, we conducted RNA-seq analysis of the Δmoset1, Δmokmt1, and Δmokmt6 mutants, along with the WT strain. RNA fold change values were calculated by dividing the average transcripts per million (TPM) from each mutant by the corresponding average from WT. These fold change values were then mapped onto the Br48 genome to examine the relationship between genomic location and changes in gene expression. An example from the entire chromosome 3 is shown in Fig. 7A. Genes with fewer than 5 TPM in both the kmt mutant and WT were excluded to minimize bias from dividing small numbers.
Fig. 7. The loss of MoKmt6 leads to remarkable compartment-specific changes in gene expression.
A Read mapping data from ChIP-seq analysis of H3K4me2 (blue dots), H3K9me3 (green dots), and H3K27me3 (yellow dots) on chromosome 3 in the P. oryzae Br48 strain. Each dot represents an RPM value of a 1 kb segment in the ChIP-seq analysis. Fold change (FC) values of gene expression in three kmt mutants relative to WT are plotted at the genomic locations of the genes below the ChIP-seq mapping data. Differentially expressed genes (DEGs, p < 0.01) are highlighted in red, and non-DEGs are shown as gray dots. The X-axis represents the genomic location, and the Y-axis represents the RPM value (upper panel) or FC values (lower panel). B RNA FC values in each kmt mutant relative to WT are presented in violin plots across four different genomic compartments. Different letters indicate statistically significant differences (Tukey’s HSD test on logarithmic transformed data, P < 0.01). Segments in EC, K4-fHC, K9-fHC, and cHC are shown in blue, yellow, purple, and green, respectively.
In Δmoset1, slightly and moderately upregulated genes were prevalent throughout the genome (Fig. 7A, B). MoSet1 catalyzes H3K4me2/3, an active histone mark and a hallmark of EC. However, the genomic distribution of both up- and down-regulated DEGs showed no bias toward EC (Fig. 7A), which is further supported by the average RNA fold change across compartments (Fig. 7B). These results do not support a model in which H3K4me2/3 directly activates gene expression.
In Δmokmt1, upregulated genes were predominantly enriched in fHC, particularly within the K4-fHC compartment (Fig. 7A, B). As previous data showed a significant reduction in H3K27me3 levels within K4-fHC in Δmokmt1 (Fig. 2B), this gene upregulation is likely driven by decreased H3K27me3 deposition. Notably, despite the drastic loss of the repressive mark H3K9me3 in cHC, down-regulated genes were rather predominant in this compartment. These findings suggest that the changes in gene expression caused by MoKmt1 deletion are largely attributable to altered H3K27me3 deposition.
In contrast to Δmoset1 and Δmokmt1, Δmokmt6 showed a marked increase in gene expression within its associated compartments, K4-fHC and K9-fHC (Fig. 7A, B). This supports a simple model in which loss of the repressive histone mark H3K27me3 leads to increased gene expression in the fHC compartment. Thus, H3K27me3 likely acts as a molecular signal for directly repressing gene expression.
To further address the direct and indirect effects of histone PTM loss on gene expression in the kmt mutants, we categorized up- and down-regulated DEGs into six groups based on their fold change values. These groups are as follows: extremely-up (Ex-up), fold change ≧ 10; highly-up (H-up), 10 > fold change ≧ 5; moderately-up (M-up), 5 > fold change ≧ 2; moderately-down (M-down) 1/2 ≧ fold change > 1/5), highly-down (H-down), 1/5 ≧ fold change > 1/10; and extremely-down (Ex-down), 1/10 ≧ fold change. Subsequently, these gene groups were represented with different colors in graphs showing fold changes of a histone PTM in the kmt mutants. In this analysis, we included genes in the UA compartment since the data is mainly presented in a compartment independent manner.
Figure 8A shows the fold changes of H3K27me3 in Δmoset1 relative to the H3K4me2 and H3K27me3 levels in WT. Interestingly, genes with greater RNA fold changes, either up- or down-regulated, in Δmoset1 were predominantly located in genomic segments with low H3K4me2 levels in WT. If H3K4me2 directly regulated gene expression, its loss would be expected to have a more pronounced effect on genes with high H3K4me2 levels than on those with low levels. Therefore, these data do not support the model that H3K4me2/3 directly regulates gene expression, as previously suggested. Notably, these genes were also typically found in genomic segments with low H3K9me3 and H3K27me3 levels (Fig. 8A lower panels, Supplementary Fig. 5), indicating they mostly lie outside repressive chromatin regions. Indeed, 66.9% of DEGs in Δmoset1 were found in UA segments (Supplementary Fig. 6), which are often adjacent to EC compartments but not encompassed by any histone PTM peaks. Thus, these UA segments may represent a subset of euchromatin that remains under some level of control by MoSet1.
Fig. 8. Gene upregulation in facultative heterochromatin is often accompanied by consistent alterations in histone modifications.
FC values of H3K4me2 or H3K27me3 RPM in Δmoset1 (A), Δmokmt1 (B), and Δmokmt6 (C) relative to WT are presented in a scatter plot, with reference to H3K4me2 or H3K27me3 levels in WT. Each dot represents an FC value of a 1 kb segment in the Br48 genome. The genomic segments containing genes that were up- or down-regulated in the kmt mutants are shown in different colors according to the levels of up- or down-regulation as follows: Red, FC ≧ 10; purple, 10 > FC ≧ 5; light pink, 5 > FC ≧ 2; pale gray, 2 > FC > 1/2; light green, 1/2 ≧ FC > 1/5; light blue, 1/5 ≧ FC > 1/10; dark blue, 1/10 ≧ FC.
We also examined the role of the suppressive mark H3K27me3 in gene expression changes in Δmoset1. A small subset of up-regulated and down-regulated genes showed lower and higher H3K27me3 levels, respectively, in Δmoset1. However, such consistent changes in H3K27me3 levels with gene expression changes were not broadly observed. Thus, gene regulation by the alteration of H3K27me3 levels in Δmoset1 appears to be limited.
In Δmokmt1, Ex-up genes were predominantly observed in genomic segments with low H3K4me2 levels (Fig. 8B). These genes often, though not always, exhibited histone PTMs alteration consistent with gene up-regulation, such as an increase in H3K4me2 and a decrease in H3K27me3 (Fig. 8B). Interestingly, the difference between subsets of these up-regulated genes can be explained, at least partly, by the H3K27me3 levels at their genomic locations in WT (Fig. 8B, right panels). Specifically, such consistent changes in H3K4me2 and H3K27me3 levels were prominent in up-regulated genes located in genomic segments with high H3K27me3 levels but were less evident in those with low H3K27me3. These results suggest that gene expression in high H3K27me3 compartments, such as fHC, especially K4-fHC, is regulated through dynamic changes in histone PTMs, whereas in low H3K27me3 compartments, such as EC and UA, gene expression may be controlled by other mechanisms or histone PTMs not examined here.
For downregulated genes, a decrease in H3K4me2 and an increase in H3K27me3, both of which are consistent with gene downregulation, were detected with some genes (Supplementary Fig. 5), but the degree of the association with gene expression levels was not as pronounced as in the upregulated genes. Additionally, the compartment-specific gene regulation as observed with the upregulated genes in Δmokmt1 was not evident with the downregulated genes.
In Δmokmt6, high fold changes in H3K4me2 were predominantly observed with up-regulated genes in genomic segments with low H3K4me2 and high H3K27me3 levels, and thus, in fHC compartments (Fig. 8C). Over 80% of genes belonging to Ex-up were detected within fHC (Supplementary Fig. 6), with some showing a drastic increase in H3K4me2 levels (Fig. 8C). Meanwhile, upregulated genes in segments with low H3K27me3 levels remained mostly within the H- and M-up groups (Fig. 8C and Supplementary Fig. 6). Some of these genes were also associated with a notable increase in H3K4me2 levels. These results suggest that the loss of the suppressive mark H3K27me3 could induce extensive gene upregulation in fHC and moderate gene upregulation in EC and UA. In contrast, downregulated genes were predominantly located in genomic regions with low H3K4me2 and H3K27me3 levels and exhibited mostly low fold changes of H3K4me2 (Fig. 8C). Thus, gene downregulation in Δmokmt6 seemed to be regulated not directly by alterations in the histone PTMs examined here.
Discussion
Two distinct compartments in facultative heterochromatin of the P. oryzae Br48 genome
It is proposed that genomes of phytopathogenic fungi have a bipartite architecture, often referred to as the core genome and the accessory genome33,34. The core genome is gene-rich, contains fewer TEs, and is highly conserved, whereas the accessory genome is gene-sparse, repeat-rich, often lineage-specific, and exhibits low genetic conservation33,34. The accessory genome contains genes encoding effector proteins, gene clusters for production of secondary metabolites, and possible horizontally transferred genes29,35, and thus appears to account for rapid evolution, allowing adaptation to specific niches in the ecosystem.
H3K27me3 is a hallmark of fHC and often associated with the accessory genome and the regulation of genes within it in response to environmental signals15,27. In this study, we identified two distinct H3K27me3-rich genomic compartments, K4-fHC and K9-fHC, in the Br48 genome. It seems unlikely that these compartments are formed solely due to the influence of adjacent genomic regions, as H3K27me3 levels in Δmokmt1 decreased in K4-fHC regions adjacent to EC but remained largely unchanged in K9-fHC regions adjacent to cHC, where the Δmokmt1 deletion caused the most drastic changes in H3K9me3 levels (Fig. 2B–D). Notably, H3K27me3 levels also slightly decreased in K4-fHC but not in K9-fHC, following the loss of MoSet1 (Fig. 4B, D). In addition, H3K27me3 levels were more dynamic in K4-fHC than in K9-fHC under different culture conditions (Fig. 3G). These data indicate that the H3K27me3 modification in K4-fHC is more dynamic and sensitive to environmental or cellular changes than in K9-fHC.
Additionally, the K4-fHC and K9-fHC compartments differ in gene content, TE content, and GC content. Gene density is higher in K4-fHC (0.34 genes/kb) than in K9-fHC (0.27 genes/kb), whereas TE content is greater in K9-fHC (8.9%) than in K4-fHC (1.7%) (Fig. 3C). The lower GC content and higher 5mC average level in K9-fHC relative to K4-fHC likely reflect its elevated TE content. However, these differences are less pronounced when compared to the cHC compartment, which has a much lower gene density (0.03 genes/kb) and a much higher TE content (64.8%). Thus, although K9-fHC exhibits some characteristics of the accessory genome, these traits are relatively weak.
Meanwhile, based on IPC analysis, genetic conservation in both K4-fHC and K9-fHC is much lower than in EC (Fig. 3F), suggesting that both compartments exhibit accessory genome-like features. The ratio of effector candidates to total genes was higher in K9-fHC (21.0%) and particularly in K4-fHC (30.7%) compared to EC (3.4%) (Fig. 3B), aligning with previous findings that effectors are enriched in fHC of the P. oryzae genome15. This supports the idea that both K4-fHC and K9-fHC belong to the accessory genome. Thus, while K4-fHC shares core genome-like features in terms of gene density and TE content, both K4-fHC and K9-fHC exhibit accessory genome-like characteristics in gene conservation and content, indicating that the structure of the P. oryzae genome is not so simple as the core-accessory genome model.
RNA-seq analysis of Δmokmt6 and Δmokmt1 revealed that genes in K4-fHC were remarkably overrepresented among the Ex-up and H-up genes, suggesting that these genes are highly responsive to changes in histone status and potentially to environmental cues (Supplementary Fig. 6). Indeed, genes located in K4-fHC were the most overrepresented among up-regulated DEGs in infected leaves across all examined time points (Fig. 3H). In addition, nearly half of the up-regulated effector-like genes during infection are located within K4-fHC, suggesting that this genomic compartment plays a pivotal role in the pathogenicity of P. oryzae.
In support of this, a recent study demonstrated that the number of H3K4me3-H3K27me3 co-marked genes increases markedly during Fusarium graminearum infection, suggesting that temporally coordinated bivalent histone modifications contribute to fungal invasion of host plants36. The increased levels of H3K4me2 at upregulated genes within the H3K27me3-rich fHC compartments in Δmokmt6 (Fig. 8C and Supplementary Fig. 6) may indicate that similar processes occur in P. oryzae. Overall, the two genomic compartments identified in this study, K4-fHC and K9-fHC, may both serve as repositories for niche-determining genes, though they appear to be regulated somewhat differently to support the pathogenicity of P. oryzae.
Interplay of histone PTMs in P. oryzae
While DNA serves as the repository of genomic information, multicellular organisms utilize only a subset of this information in a manner dependent on cell type and environmental conditions. Epigenetics encompasses the biochemical mechanisms that regulate the genome within this context. Particularly, histone PTMs play a fundamental role in defining and maintaining functionally distinct regions of the genome.
In this study, detailed bioinformatic analysis of ChIP-seq data from the three P. oryzae kmt mutants revealed that the potential interplay of histone PTMs operates differently, in most cases, across distinct genomic compartments. In Δmoset1, the most significant alteration was observed in the cHC compartment, characterized by a reduction in H3K9me3 and an increase in H3K27me3. It is well-established that H3K4me2/3, catalyzed by the KMT2 family, serves as an epigenetic mark for gene activation in various eukaryotes37–39. Interestingly, the representative gene of the KMT2 family, SET1 in Saccharomyces cerevisiae, was initially identified as essential for the transcriptional silencing of silent mating-type loci in the subtelomeric region40. Additionally, SET1 has been shown to play a role in silencing rDNA and the retrotransposon Ty1 in S. cerevisiae41,42. Since rDNA repeats and TEs are frequently targeted by H3K9me3 in various eukaryotes43–45, the observed downregulation of H3K9me3 in Δmoset1 aligns with previous reports. A plausible model to explain the relationship between MoSet1 and the cHC compartment involves the downregulation of a gene(s) necessary for H3K9me3 in Δmoset1, coupled with a compensatory enrichment of H3K27me3 in regions depleted of H3K9me3. However, this hypothesis warrants further investigation.
ChIP-seq analysis of H3K9me3 and H3K27me3 in Δmokmt1 and Δmokmt6 strongly suggests a profound mutual influence between these two histone modifications (Figs. 2 and 5). It has been shown that a loss of H3K9me3 leads to a subsequent gain of H3K27me3 in mammals12,46 and fungi47,48. Similarly, in plants, loss of heterochromatin components leads to similar redistribution of H3K27me349. Our findings in P. oryzae were consistent with these previous observations (Fig. 2). In contrast, a loss of H3K27me3 in the fHC compartment of Δmokmt6 did not result in a gain but rather a decrease in H3K9me3 (Fig. 5), indicating the absence of a compensatory mechanism for H3K9me3 in response to the loss of H3K27me3 in the H3K27me3-rich regions of the P. oryzae genome. Interestingly, however, a significant increase in H3K9me3 was observed in the cHC compartment of Δmokmt6, suggesting that the enhanced deposition of H3K9me3 may compensate for the loss of H3K27me3 within this compartment. In mammals, the PRC2 complex, which contains the enzyme catalyzing H3K27me3, has been found to crosstalk with G9a/GLP, members of the KMT1 family responsible for H3K9 methylation50. Such crosstalk between protein complexes containing KMT1 and KMT6 could also exist in fungi, leading to the interplay between H3K9me3 and H3K27me3 observed in this study.
Effects of histone PTMs on gene expression in P. oryzae
RNA-seq analysis of Δmoset1 and Δmokmt1 revealed that up- or downregulated genes were not predominantly located within the genomic compartments where the corresponding histone PTM was lost (Fig. 7B). In contrast, Δmokmt6 showed significant gene upregulation in its respective fHC compartment (Fig. 7B). These findings raise the question of whether H3K4me2/3 and H3K9me3 act as direct regulators of gene expression. Notably, in the EC compartment, the loss of the active marks H3K4me2/3 did not consistently lead to gene downregulation but more often resulted in gene upregulation in the RNA-seq analysis.
H3K4me2/3 are widely recognized as histone marks associated with gene activation across a broad range of eukaryotes. Indeed, the distribution patterns of H3K4me2/3 and H3K9me3 in the P. oryzae genome correlate well with active and silent gene expression, respectively29. Additionally, substrate-induced gene expression of GH6 and GH7 cellulases was associated with enrichment of H3K4me2 in P. oryzae51. Interestingly, however, expression levels of GH6 and GH7 cellulases under non-inducing conditions increased in the Δmoset1 mutant, suggesting a possible role of H3K4 methylation in gene repression51. This is consistent with the observations on the CclA gene, which encodes a component of the COMPASS complex catalyzing H3K4me2/3, in Aspergillus nidulans and A. fumigatus. Deletion of the CclA gene resulted in depletion of H3K4me2/3 and increased expression of cryptic SM gene clusters52,53, suggesting that CclA-mediated H3K4me2/3 contributes to gene silencing of SM clusters. Thus, H3K4me2 may not directly activate gene expression but rather serve as a mark for open chromatin, contributing to the regulation of gene expression there in either an activating or suppressive manner.
In Δmokmt1, gene upregulation was predominantly observed in fHC, whereas fewer instances were noted in cHC, where high levels of H3K9me3 were lost (Fig. 7B and Supplementary Fig. 6). It should be noted that the number of genes in cHC is relatively small since the gene density in cHC is significantly lower compared to other genomic compartments in the Br48 genome (Fig. 3A). H3K9me3 is known to play a crucial role in establishing constitutive heterochromatin, and the loss of its catalyzing enzymes is often accompanied by the activation of targets such as TEs. Consistently, we observed the activation of certain class I TEs, including Mg-SINE, MGL (a LINE-like element), and Retro5 (an LTR element), but not all TEs, in the Δmokmt1 mutant. However, the majority of genes in cHC were not upregulated (Fig. 7B). Thus, it is possible that gene activation in the kmt1 mutants may be attributed, at least partly, to a decrease in H3K27me3 due to possible interplay between H3K9me3 and H3K27me3.
In contrast to the observations in Δmokmt1 and Δmoset1, the loss of the repressive mark H3K27me3 in the Δmokmt6 mutant led to a substantial upregulation of genes within the associated compartment, fHC. This finding is consistent with the previous reports that knockout or knockdown of KMT6 genes in various fungal species resulted in the depletion of H3K27me3 and subsequent upregulation of a subset of genes normally suppressed by H3K27me315,24,27,54. However, the role of H3K27me3 in directly regulating gene expression is still a matter of debate. In Verticillium dahliae, an investigation into the relationship between gene activation and H3K27me3 levels under different culture conditions revealed that H3K27me3 depletion was not the primary event leading to transcriptional activation24. Kramer et al. proposed that gene activation in this context involves additional factors, with H3K27me3 functioning as a binding site or nucleation point to mediate transcriptional activation in response to specific environmental signals21. This study also showed that only a subset of genes in the fHC compartment were up-regulated in Δmokmt6 (Fig. 7B and Supplementary Fig. 6), indicating that the removal of H3K27me3 does not always result in gene activation. Additionally, H3K27me3 levels of genes up-regulated in the fHC compartment of Δmokmt1 were mostly reduced but remained higher than the basal level. These data contradict the hypothesis that H3K27me3 is the sole and absolute key to switching gene activation and suppression, but may support the idea that H3K27me3 is one of the crucial factors that directly regulate gene expression. In this context, our data suggest that the involvement of histone PTMs in gene regulation can differ across distinct genomic compartments. In the fHC compartments, dynamic changes in both H3K4me2 and H3K27me3 contribute to gene activation, at least to a certain extent, whereas none of the examined histone PTMs appear to contribute to gene regulation in the EC and UA compartments. Thus, the impact of histone PTMs on gene expression should be evaluated separately for each genomic compartment.
Additionally, it is noteworthy that the biological significance of KMT6 genes can differ among fungal species. For example, certain fungal species, such as S. cerevisiae and Schizosaccharomyces pombe, or even some genera, such as Aspergillus and Penicillium, completely lack KMT6 orthologs55. Moreover, KMT6 appears to be essential in Fusarium fujikuroi54, but not in F. graminearum27, suggesting that the roles of KMT6 genes can vary considerably even within a single genus. Thus, it is possible that the roles of KMT6 genes in gene regulation are different depending on the fungal species.
Methods
High-throughput sequencing of fungal RNA
The wheat blast fungus strain Br48, collected in Brazil in 199056, was stored on barley seed media at 4 °C for long-term preservation57. For working cultures, a barley grain from the stock was placed on potato dextrose agar (PDA) slant media and incubated at 25 °C. To prepare RNA for high-throughput sequencing, fungal plugs were transferred to flasks containing complete medium (CM) (0.5% sucrose, 0.3% casamino acids, and 0.3% yeast extract) and incubated on a shaker at 120 rpm and 25 °C for 4 days. Fungal RNA was extracted using Sepasol RNA I SuperG (Nacalai Tesque) as previously described in ref. 58 and further purified with the NucleoSpin RNA Clean-up Kit (Macherey–Nagel, Düren, Germany).
For RNA-seq analysis of infected leaves, conidia of Br48 were sprayed onto leaves of barley cultivar Nigrate with 0.01% Tween-20, and the inoculated plants were transferred to a plant growth chamber at 25 °C after keeping under moisture conditions for 24 h. Inoculated leaves were sampled at 12 (under moisture condition), 24, and 48 h post inoculation, and subjected to RNA extraction as described above.
Libraries for HiSeq sequencing (HiSeq 2500 and HiSeq X) were prepared using kits provided by the sequencing platform manufacturer at Macrogen Japan Co., Ltd (Kyoto, Japan).
Chromatin immunoprecipitation-seq analysis
A ChIP assay was conducted using the ChIP-IT™ Express kit (Active Motif, Carlsbad, USA) according to the manufacturer’s protocol as previously described29. Fungal mycelia were cultured in CM liquid medium for 4 days, or in minimal medium (0.17% yeast nitrogen base without amino acids; BD Difco) for 2 days following a 3-day preculture in CM medium, at 26 °C on an orbital shaker set to 120 rpm. A 50 mg portion of mycelia was harvested and incubated at room temperature for 15 min in 10 ml of phosphate-buffered saline (PBS) containing 1% formaldehyde. Chromatin was sheared by sonication using a Bioruptor apparatus (Cosmo Bio Co., Ltd, Japan) for three cycles of 1 min on at high intensity (200 W) and 30 s off, followed by five cycles of 1 min on at medium intensity (160 W) and 30 s off. The sheared chromatin fragments ranged from ~100 to 500 bp, as determined by agarose gel electrophoresis. Antibodies against demethylated H3 Lys4 (Active Motif, #39141), trimethylated H3 Lys9 (Active Motif, #39161), and trimethylated H3 Lys27 (Active Motif, #39156) were obtained from Active Motif. ChIP DNA was recovered using phenol-chloroform extraction and ethanol precipitation. Libraries for high-throughput sequencing were prepared using the NEBNext Ultra II DNA Library Prep Kit for Illumina and sequenced on the HiSeq X platform at Macrogen Japan Co., Ltd (Kyoto, Japan).
Bioinformatic analysis
Short reads obtained from ChIP-seq and RNA-seq analysis were mapped to the Br48 genome using CLC Genomics Workbench ver. 11.0.1. Differentially expressed genes (DEGs) were screened using an empirical analysis of DGE (Digital Gene Expression data) in CLC Genomics Workbench.
Three genomic compartments, euchromatin (EU), facultative heterochromatin (fHC), and constitutive heterochromatin (cHC), were detected using the HOMER “findPeaks” command30. Default parameter settings of the HOMER software package were used, except for “-style histone”. The locations of called peaks are provided in Supplementary Data 2–4. Each 1 kb segment was assigned to a compartment if more than 80% of its sequence falls within a called peak. If a single segment overlapped with peaks from two or more compartments, it was classified as unassigned (UA). Segments that were not classified by this procedure were also assigned to the UA category.
fHC segments were assigned to either the H3K4me2-associated fHC (K4-fHC) or the H3K9me3-associated fHC (K9-fHC) compartment based on their adjacent compartments, with UA excluded. If both neighboring compartments of an fHC region were EC or cHC, the region was assigned to K4-fHC or K9-fHC, respectively. If an fHC region was flanked by EC on one side and cHC on the other, it was divided into two sub-regions, with the EC-facing sub-region assigned to K4-fHC and the cHC-facing sub-region to K9-fHC. The boundary between K4-fHC and K9-fHC was set at the midpoint.
Statistics and reproducibility
The statistical methods used are described in the “Bioinformatic analysis” section or in the figure legends. Threshold values are also provided in the text. Sample sizes are indicated within the graphs or given in the text. ChIP-seq and RNA-seq analyses were performed with at least two biological replicates. For all boxplots in this work, the horizontal line and closed circle represent the median and the average, respectively.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science (#21H02195 and #24K01758).
Author contributions
H.N. designed the study and the main conceptual ideas. T.D., A.M., N.K. and K.P. collected the data. K.P. and K.I. aided in interpreting the results. H.N. and T.D. wrote the manuscript.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Chris Koon Ho Wong & Rosie Bunton-Stasyshyn.
Data availability
The telomere-to-telomere genome sequence of the Br48 strain was deposited at DDBJ/EMBL/GenBank under the accession numbers AP027063- AP027069. RNA-seq and ChIP-seq reads of the Br48 strain have been deposited on DDBJ Sequence Read Archive under the accession numbers PRJDB2912, PRJDB3851, PRJDB20151, and PRJDB15953. The source data underlying the figures in this manuscript can be found in Supplementary Data 5. All other data are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-025-08473-2.
References
- 1.Bannister, A. J. & Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res.21, 381–395 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fischle, W., Wang, Y. & Allis, C. D. Histone and chromatin cross-talk. Curr. Opin. Cell Biol.15, 172–183 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Lee, J.-S., Smith, E. & Shilatifard, A. The language of histone crosstalk. Cell142, 682–685 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang, T., Cooper, S. & Brockdorff, N. The interplay of histone modifications – writers that read. EMBO Rep.16, 1467–1481 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greer, E. L. & Shi, Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nat. Rev. Genet.13, 343–357 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allis, C. D. et al. New nomenclature for chromatin-modifying enzymes. Cell131, 633–636 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Miller, T. et al. COMPASS: a complex of proteins associated with a trithorax-related SET domain protein. Proc. Natl. Acad. Sci. USA98, 12902–12907 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao, R. et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Sci298, 1039–1043 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Czermin, B. et al. Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal polycomb sites. Cell111, 185–196 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Kuzmichev, A., Nishioka, K., Erdjument-Bromage, H., Tempst, P. & Reinberg, D. Histone methyltransferase activity associated with a human multiprotein complex containing the Enhancer of Zeste protein. Genes. Dev.16, 2893–2905 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis, Z. A. et al. DNA Methylation and normal chromosome behavior in Neurospora depend on five components of a histone methyltransferase complex, DCDC. PLoS Genet.6, e1001196 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters, A. H. F. M. et al. Partitioning and plasticity of repressive histone methylation states in mammalian chromatin. Mol. Cell.12, 1577–1589 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Jamieson, K. et al. Loss of HP1 causes depletion of H3K27me3 from facultative heterochromatin and gain of H3K27me2 at constitutive heterochromatin. Genome Res.26, 97–107 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaydos, L. J., Rechtsteiner, A., Egelhofer, T. A., Carroll, C. R. & Strome, S. Antagonism between MES-4 and polycomb repressive complex 2 promotes appropriate gene expression in C. elegans germ cells. Cell Rep.2, 1169–1177 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang, W., Huang, J. & Cook, D. E. Histone modification dynamics at H3K27 are associated with altered transcription of in planta induced genes in Magnaporthe oryzae. PLoS Genet.17, e1009376 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato, H. et al. Pathogenicity, mating ability and DNA restriction fragment length polymorphisms of Pyricularia populations isolated from Gramineae, Bambusideae and Zingiberaceae plants. J. Gen. Plant Pathol.66, 30–47 (2000). [Google Scholar]
- 17.Dean, R. et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol.13, 414–430 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khatun, M., Nessa, B., Salam, M. & Kabir, M. Strategy for rice disease management in Bangladesh. Bangladesh Rice J.25, 23–36 (2021). [Google Scholar]
- 19.Islam, M. T. et al. Emergence of wheat blast in Bangladesh was caused by a South American lineage of Magnaporthe oryzae. BMC Biol.14, 84 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tembo, B. et al. Detection and characterization of fungus (Magnaporthe oryzae pathotype Triticum) causing wheat blast disease on rain-fed grown wheat (Triticum aestivum L.) in Zambia. PLoS ONE15, e0238724 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kramer, H. M., Cook, D. E., Seidl, M. F. & Thomma, B. P. H. J. Epigenetic regulation of nuclear processes in fungal plant pathogens. PLoS Pathog.19, e1011525 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cook, D. E., Kramer, H. M., Torres, D. E., Seidl, M. F. & Thomma, B. P. H. J. A unique chromatin profile defines adaptive genomic regions in a fungal plant pathogen. Elife9, e62208 (2020). [DOI] [PMC free article] [PubMed]
- 23.de Jonge, R. et al. Extensive chromosomal reshuffling drives evolution of virulence in an asexual pathogen. Genome Res.23, 1271–1282 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kramer, H. M., Seidl, M. F., Thomma, B. P. H. J. & Cook, D. E. Local rather than global H3K27me3 dynamics are associated with differential gene expression in Verticillium dahliae. mBio13, e0356621 (2022). [DOI] [PMC free article] [PubMed]
- 25.Nützmann, H.-W., Fischer, J., Scherlach, K., Hertweck, C. & Brakhage, A. A. Distinct amino acids of histone H3 control secondary metabolism in Aspergillus nidulans. Appl. Environ. Microbiol.79, 6102–6109 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Studt, L. et al. Two histone deacetylases, FfHda1 and FfHda2, are important for Fusarium fujikuroi secondary metabolism and virulence. Appl. Environ. Microbiol.79, 7719–7734 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Connolly, L. R., Smith, K. M. & Freitag, M. The Fusarium graminearum histone H3 K27 methyltransferase KMT6 regulates development and expression of secondary metabolite gene clusters. PLoS Genet.9, e1003916 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pham, K. T. M. et al. MoSET1 (histone H3K4 methyltransferase in Magnaporthe oryzae) regulates global gene expression during infection-related morphogenesis. PLoS Genet.11, e1005385 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi, N. et al. Horizontally transferred DNA in the genome of the fungus Pyricularia oryzae is associated with repressive histone modifications. Mol. Biol. Evol.40, msad186 (2023). [DOI] [PMC free article] [PubMed]
- 30.Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell38, 576–589 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cambareri, E. B., Jensen, B. C., Schabtach, E. & Selker, E. U. Repeat-induced G-C to A-T mutations in Neurospora. Science244, 1571–1575 (1989). [DOI] [PubMed] [Google Scholar]
- 32.Morimoto, A., Dang, T. A., Ikeda, K. I. & Nakayashiki, H. Asynchronous evolution of centromeric sequences across chromosomes in Pyricularia oryzae. Genes Genet. Syst.100, 24–00208 (2025). ggs. [DOI] [PubMed] [Google Scholar]
- 33.Croll, D. & McDonald, B. A. The accessory genome as a cradle for adaptive evolution in pathogens. PLoS Pathog.8, e1002608 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong, S., Raffaele, S. & Kamoun, S. The two-speed genomes of filamentous pathogens: waltz with plants. Curr. Opin. Genet. Dev.35, 57–65 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Freitag, M. Histone methylation by SET domain proteins in fungi. Annu. Rev. Microbiol.71, 413–439 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Zhao, X. et al. Temporally-coordinated bivalent histone modifications of BCG1 enable fungal invasion and immune evasion. Nat. Commun.15, 231 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pokholok, D. K. et al. Genome-wide map of nucleosome acetylation and methylation in yeast. Cell122, 517–527 (2005). [DOI] [PubMed] [Google Scholar]
- 38.Barski, A. et al. High-resolution profiling of histone methylations in the human genome. Cell129, 823–837 (2007). [DOI] [PubMed] [Google Scholar]
- 39.van Dijk, K. et al. Dynamic changes in genome-wide histone H3 lysine 4 methylation patterns in response to dehydration stress in Arabidopsis thaliana. BMC Plant Biol.10, 238 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nislow, C., Ray, E. & Pillus, L. SET1, A yeast member of the Trithorax family, functions in transcriptional silencing and diverse cellular processes. Mol. Biol. Cell8, 2421–2436 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Briggs, S. D. et al. Histone H3 lysine 4 methylation is mediated by Set1 and required for cell growth and rDNA silencing in Saccharomyces cerevisiae. Genes Dev.15, 3286–3295 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berretta, J., Pinskaya, M. & Morillon, A. A cryptic, unstable transcript mediates transcriptional trans -silencing of the Ty1 retrotransposon in S. cerevisiae. Genes Dev.22, 615–626 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng, J. C. & Karpen, G. H. H3K9 methylation and RNA interference regulate nucleolar organization and repeated DNA stability. Nat. Cell Biol.9, 25–35 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murayama, A. et al. Epigenetic control of rDNA loci in response to intracellular energy status. Cell133, 627–639 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Zeller, P. et al. Histone H3K9 methylation is dispensable for Caenorhabditis elegans development but suppresses RNA:DNA hybrid-associated repeat instability. Nat. Genet.48, 1385–1395 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Cooper, S. et al. Targeting polycomb to pericentric heterochromatin in embryonic stem cells reveals a role for H2AK119u1 in PRC2 recruitment. Cell Rep.7, 1456–1470 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Basenko, E. Y. et al. Genome-wide redistribution of H3K27me3 is linked to genotoxic stress and defective growth. Proc. Natl. Acad. Sci. USA.112, E6339–48 (2015). [DOI] [PMC free article] [PubMed]
- 48.Studt-Reinhold, L. et al. H3K27me3 is vital for fungal development and secondary metabolite gene silencing, and substitutes for the loss of H3K9me3 in the plant pathogen Fusarium proliferatum. PLoS Genet.20, e1011075 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deleris, A. et al. Loss of the DNA methyltransferase MET1 induces H3K9 hypermethylation at PcG target genes and redistribution of H3K27 trimethylation to transposons in Arabidopsis thaliana. PLoS Genet.8, e1003062 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mozzetta, C. et al. The histone H3 lysine 9 methyltransferases G9a and GLP regulate polycomb repressive complex 2-mediated gene silencing. Mol. Cell.53, 277–289 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Van, B. V., Pham, K. T. M. & Nakayashiki, H. Substrate-induced transcriptional activation of the MoCel7C cellulase gene is associated with methylation of histone H3 at lysine 4 in the rice blast fungus Magnaporthe oryzae. Appl. Environ. Microbiol.79, 6823–6832 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bok, J. W. et al. Chromatin-level regulation of biosynthetic gene clusters. Nat. Chem. Biol.5, 462–464 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palmer, J. M. et al. Loss of CclA, required for histone 3 lysine 4 methylation, decreases growth but increases secondary metabolite production in Aspergillus fumigatus. PeerJ1, e4 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Studt, L. et al. Knock-down of the methyltransferase Kmt6 relieves H3K27me3 and results in induction of cryptic and otherwise silent secondary metabolite gene clusters in Fusarium fujikuroi. Environ. Microbiol.18, 4037–4054 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang, X., Noberini, R., Bonaldi, T., Collemare, J. & Seidl, M. F. The histone code of the fungal genus Aspergillus uncovered by evolutionary and proteomic analyses. Microb. Genom.8, mgen000856 (2022). [DOI] [PMC free article] [PubMed]
- 56.Urashima, A. S., Igarashi, S. & Kato, H. Host range, mating type, and fertility of Pyricularia grisea from wheat in Brazil. Plant Dis.77, 1211 (1993). [Google Scholar]
- 57.Nakayashiki, H., Kiyotomi, K., Tosa, Y. & Mayama, S. Transposition of the retrotransposon MAGGY in heterologous species of filamentous fungi. Genetics153, 693–703 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nguyen, Q. B. et al. Systematic functional analysis of calcium-signalling proteins in the genome of the rice-blast fungus, Magnaporthe oryzae, using a high-throughput RNA-silencing system. Mol. Microbiol.68, 1348–1365 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The telomere-to-telomere genome sequence of the Br48 strain was deposited at DDBJ/EMBL/GenBank under the accession numbers AP027063- AP027069. RNA-seq and ChIP-seq reads of the Br48 strain have been deposited on DDBJ Sequence Read Archive under the accession numbers PRJDB2912, PRJDB3851, PRJDB20151, and PRJDB15953. The source data underlying the figures in this manuscript can be found in Supplementary Data 5. All other data are available from the corresponding author on reasonable request.