Abstract
The allospecifc T cell population responding to a transplanted organ consists of both naïve and memory lymphocytes. Although it is established that naive T cells are activated by antigen within the organized structures of secondary lymphoid organs (the spleen, lymph nodes, and mucosal lympoid tissues), it is not clear whether memory T cell activation and propagation depend on homing to these organs. To answer this question, we investigated whether allospecific naïve or memory T cells can mediate acute cardiac allograft rejection in mutant mice that lack all of their secondary lymphoid tissues. The results of our experiments demonstrated that antigen-experienced memory T cells have two advantages over naïve T cells: (i) memory T cells mount a vigorous immune response that leads to allograft rejection independent of secondary lymphoid organs; and (ii) memory T cells generate more memory T cells without homing to secondary lymphoid organs. These unique properties of memory T cells were further confirmed by showing that memory-like T cells that arise from the homeostatic proliferation of naive T cells in the absence of antigenic stimulation are suboptimal at rejecting allografts and do not generate memory T cells in mice devoid of secondary lymphoid tissues.
A cardinal feature of the adaptive immune response is its ability to generate long-lived populations of memory T lymphocytes (1). Memory T cells are specific to the antigen encountered during the primary immune response and react rapidly and vigorously on re-encounter with the same antigen. Memory T cells that recognize microbial antigens provide the organism with long-lasting protection against potentially fatal infections. On the other hand, memory T cells that recognize donor alloantigens could jeopardize the survival of life-saving organ transplants (2). Therefore, understanding the fundamental mechanisms that govern memory T cell activation is important for vaccine development as well as the design of effective strategies for preventing allograft rejection.
The adaptive immune response is initiated within secondary lymphoid organs where naïve T cells encounter foreign antigen, are activated, and differentiate into effector cells (3–5). The majority of T cells participating in a primary immune response rapidly undergo apoptosis after the foreign antigen is eliminated, and only a small proportion of these cells survive to become memory T cells. Unlike naïve T cells whose homing is restricted to secondary lymphoid organs, memory T cells migrate to nonlymphoid tissues in the periphery (6–8). Recent studies have shown that memory T cells isolated from the nonlymphoid tissues of immunized mice have immediate ex vivo effector functions (6, 7). However, it remains unclear whether memory T cells can mount a productive immune response that leads to antigen clearance and the generation of more memory T cells in vivo independent of secondary lymphoid organs. Here we addressed these questions by examining whether allospecific memory T cells can mediate the rejection of cardiac allografts and generate more memory T cells in mutant mice that lack secondary lymphoid organs. We demonstrate that, unlike naïve T cells, antigen-experienced memory T cells mount a productive alloimmune response and beget more memory T cells independent of secondary lymphoid organs. We also show that these unique properties of memory T cells are not shared by memory-like T cells that arise from the homeostatic proliferation of naive T lymphocytes in the absence of antigenic stimulation.
Methods
Mouse Strains.
B6 mice homozygous for the mutation that leads to alymphoplasia (aly/aly) (CLEA Japan, Tokyo) (9), recombination activating gene-2 knockout (Rag2−/−) B6 mice (The Jackson Laboratory), and 2C T cell receptor-transgenic (TCR-tg) B6 mice were housed in specific pathogen-free environment. Wild-type (wt) BALB/c and B6 mice were purchased from The Jackson Laboratory.
Mouse Surgery and Tissue Analysis.
Heart donors were 6- to 8-week-old BALB/c (H-2d), and recipients were 6- to 8-week-old splenectomized B6 aly/aly (H-2b) mice. Splenectomy was performed 2 weeks before heart transplantation as follows: A 1.5-cm skin incision was made under anesthesia midway between the last rib and the hip, the peritoneal membrane was opened, and the entire spleen was removed intact after ligating the splenic vein and artery at the hilum. The peritoneal membrane and the skin were then closed separately. This procedure ensures that the spleen is removed in total and that no splenic fragments are left behind as confirmed by examining the mice at the time of death. Fully vascularized heterotopic heart transplantation was performed as described (5, 10). Transplanted mice were monitored daily, and rejection was defined as cessation of palpable cardiac contractions. Grafts were harvested at the time of rejection or at 100 days after transplantation in mice that did not reject their allografts. Graft tissue was fixed in B5 solution, embedded in paraffin, sectioned, and stained with hematoxylin/eosin or anti-mouse CD3 (PharMingen) followed by peroxidase-conjugated secondary antibody.
T Cell Preparation, Phenotyping, and Adoptive Transfer.
An adoptive transfer model in which a small proportion of allospecific CD8+ TCR-tg (2C) lymphocytes are parked in syngeneic wt hosts was used to prepare naïve and memory T cell populations. 2C lymphocytes recognize the Ld BALB/c alloantigen and therefore serve as a marker of allospecific T cells (11). Spleen and lymph node cells from naïve 2C mice (B6 background) were pooled and enriched for T cells by nonadherence to nylon wool. A population of enriched T cells containing 5 × 106 transgenic (CD8+1B2+) lymphocytes (1B2 is the clonotypic antibody that recognizes the transgenic TCR) was then injected i.v. into syngeneic wt B6 mice on day 0. The mice were immunized with 3 × 107 BALB/c splenocytes in PBS i.p. on days 2 and 10 to generate allospecific memory T cells or were left unimmunized to obtain naive T cells. Ten weeks later, spleen and lymph node cells were pooled and enriched for 2C T cells by magnetic cell separation (MACS) (Miltenyi Biotec, Auburn, CA). MACS was performed by depleting MHCII+ cells followed by positive selection of 1B2+ cells. The CD8+1B2+ (2C) cells present in the enriched naïve and memory populations were then quantitated before adoptive transfer by flow analysis using an anti-mouse CD8 antibody conjugated to phycoerythrin (PharMingen) and 1B2 IgG1 mAb followed by anti-mouse IgG1 antibody conjugated to FITC (Zymed) (12). The naïve and memory CD8+1B2+ cell populations were also phenotyped by flow analysis using anti-CD44-biotin/streptavidin-PerCP, anti-CD62L-APC, anti-CD25-APC, anti-1B11 followed by biotin-IgG2a/streptavidin-PerCP, and appropriate isotype antibody controls (all antibodies were purchased from PharMingen). Finally, a population of 5–10 × 106 enriched naïve or memory T cells containing a constant number of CD8+1B2+ (2C) T cells (1.5 × 105) was adoptively transferred to splenectomized aly/aly recipients of BALB/c hearts 2 days after transplantation.
An adoptive transfer model in which 2C lymphocytes are parked in syngeneic, lymphocyte-deficient Rag2−/− hosts was used to prepare allospecific memory-like T cell populations that arise in the absence of antigenic stimulation (naïveRag2−/−) (13–16). A total of 1 × 106 CD8+1B2+ T cells mixed with 2 × 107 wt B6 T cells were injected i.v. into syngeneic Rag2−/− mice on day 0. Ten weeks later, spleen and lymph node cells were pooled and enriched for 2C T cells as described in the previous paragraph. As positive control, another group of Rag2−/− recipients was immunized with 3 × 107 BALB/c splenocytes i.p. on days 2 and 10 after adoptive transfer to generate memory T cells (memoryRag2−/−). CD8+1B2+ cells present in the enriched naïveRag2−/− and memoryRag2−/− populations were then quantitated and phenotyped before adoptive transfer as described in the previous paragraph. Finally, a population of 5–10 × 106 enriched memoryRag2−/− or naiveRag2−/− T cells containing a constant number of allospecific 2C T cells (1.5 × 105) was adoptively transferred to splenectomized aly/aly recipients of BALB/c hearts 2 days after transplantation. As an additional control, a group of transplant recipients received a mixture of freshly isolated naive 2C (1.5 × 105) and naive wt B6 T cells (total number = 5 × 106 T cells).
Phenotyping and Quantitation of 2C T Cells Recovered from Transplanted Mice.
Blood was collected from a splenectomized aly/aly transplant recipient at the time of rejection or at 100 days after transplantation in mice that did not reject their grafts. After RBC lysis, CD8+1B2+ T cells present in the blood were quantitated and phenotyped by flow analysis as described above. The number of retrieved 2C T cells was extrapolated according to the following formula: % CD8+1B2+ cells × number of cells per ml blood × 2 ml (estimated volume of blood per mouse). In each mouse studied, the number and phenotype of recovered 2C T cells were compared with the number and phenotype of the 2C T cells that were injected 2 days after transplantation.
Cytotoxic T Lymphocyte (CTL) Activity.
Spleen and lymph node T cells were harvested from wt B6 mice harboring either naïve or memory T cells (same as donor mice used in the adoptive experiments described above) were pooled, enriched for CD8+ T cells by magnetic cell separation (MACS), and immediately assayed for ex vivo CTL activity against BALB/c target splenocytes. Another group of wt B6 mice harboring memory T cells was rechallenged with 2 × 107 BALB/c splenocytes i.p. and s.c. 2 days before ex vivo CTL assay. Allospecific CTL activity was measured by incubating the CD8-enriched T cells with either Con A-activated (H-2d) BALB/c target cells or third-party cells, LK35.2 (H-2k) (American Type Culture Collection) for 3 h. Target cells were labeled with calcein-AM (Molecular Probes), and calcein release was measured in a LS50B luminescence spectrometer (Perkin–Elmer) (17). Experiments in which spontaneous calcein release was more than 25% of maximal release were excluded. Antigen-specific cytotoxic activity was calculated according to the following formula: % specific lysis = 100 × [(sample release − spontaneous release)/(maximum release − spontaneous release)]. The ex vivo CTL activity of RBC-lysed peripheral blood cells from splenectomized aly/aly recipients of BALB/c cardiac allografts was measured by the same method.
Results
Memory T Cells Mediate Allograft Rejection Independent of Secondary Lymphoid Organs.
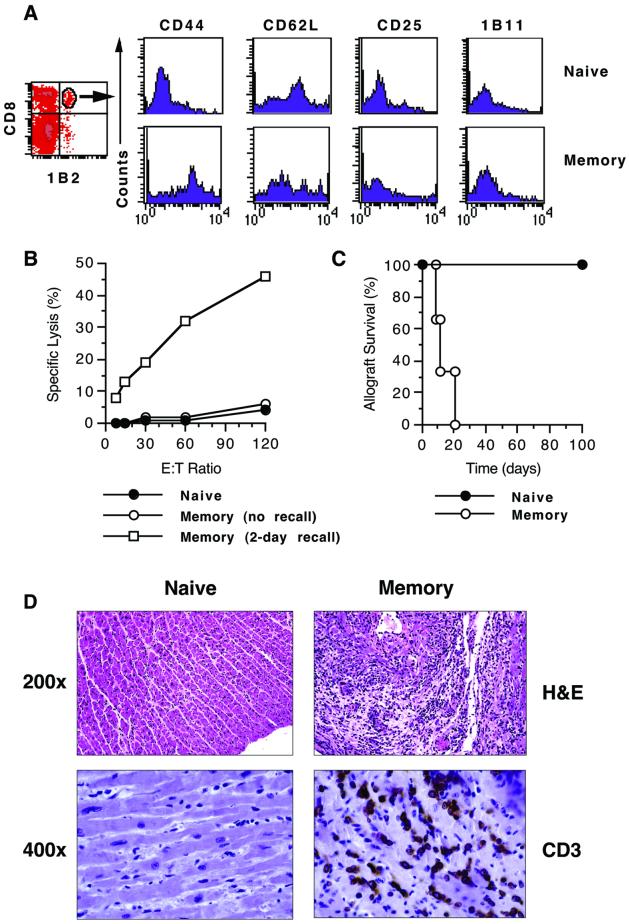
To investigate the role of secondary lymphoid organs in the in vivo recall of memory T cells, we tested whether alloreactive memory T cells mediate allograft rejection in mice devoid of secondary lymphoid tissues [splenectomized alymphoplastic (aly/aly) mice] (5, 9). These mice do not mount a primary alloimmune response and accept cardiac allografts permanently (5). Allospecific memory T cells were generated in vivo by immunizing B6 mice harboring 2C (CD8 TCR-tg) T cells with allogeneic BALB/c splenocytes as described in Methods. Ten weeks after immunization, spleen and lymph node cells were pooled, enriched for T cells, and adoptively transferred to splenectomized aly/aly mice transplanted with BALB/c hearts. To compare memory T cell function to that of naive T cells, a second group of transplanted, splenectomized aly/aly mice received T cells from unimmunized B6 mice harboring naive 2C lymphocytes for 10 weeks. The number of T cells was adjusted such that the transferred population contained an equal number of allospecific 2C lymphocytes (1.5 × 105 CD8+1B2+ cells) in each case. As shown in Fig. 1A, 2C lymphocytes in unimmunized B6 mice retained a naive phenotype (CD44lowCD62LhighCD25low1B11low) whereas those in immunized mice exhibited a shift toward the memory phenotype (CD44highCD62LlowCD25low1B11low). Moreover, CD8 T cells from immunized mice lacked immediate, allospecific, ex vivo CTL activity but rapidly acquired such activity upon in vivo rechallenge with BALB/c splenocytes (Fig. 1B), suggesting that the harvested population contained predominantly central memory T cells. No cytolytic activity was observed against third-party target cells. After transfer to transplanted, splenectomized aly/aly hosts, the naive T cell population failed to induce cardiac allograft rejection (Fig. 1C). This finding is consistent with the dependence of naïve T cells on secondary lymphoid organs to mount a primary alloimmune response (5). In contrast, the T cell population that contains memory T cells induced prompt acute rejection of cardiac allografts upon transfer to splenectomized aly/aly hosts (Fig. 1B). Histopathologic examination confirmed that allograft loss in mice that received memory T cells was caused by high-grade acute rejection characterized by heavy infiltration of the myocardium with CD3+ cells (Fig. 1D). On the other hand, cardiac allografts accepted by mice that received naive T cells did not exhibit histopathologic findings of rejection and were free of CD3+ cell infiltrates (Fig. 1D). These results establish that memory, but not naive, T cells mount a productive immune response in vivo that leads to allograft rejection independent of secondary lymphoid organs.
Figure 1.
Memory T cells mediate allograft rejection independent of secondary lymphoid organs. Naïve and memory T cells were generated in wt hosts and adoptively transferred to splenectomized aly/aly heart transplant recipients as described in Methods. Identical enrichment methods were applied to naïve and memory T cells. (A) Phenotype of naive and memory allospecific T cells determined before adoptive transfer by four-color flow analysis after gating on the CD8+1B2+ (2C TCR-tg) cell population. Histograms shown are representative of three separate experiments. (B) CTL activity of naïve and memory T cells against target BALB/c splenocytes before adoptive transfer. CD8-enriched T cells from memory (immunized) mice were assayed for ex vivo CTL activity either immediately or 2 days after in vivo recall with BALB/c splenocytes. One representative assay is shown. (C) Cardiac allograft survival in splenectomized aly/aly mice that received either naive (n = 3) or memory (n = 3) T cell populations. (D) Histological (hematoxylin/eosin, H&E) and immunohistochemical (CD3) analysis of cardiac allograft tissue harvested 100 days after transplantation in the naive group and on the day of rejection in the memory group.
Memory-Like T Cells Have Intermediate in Vivo Function in the Absence of Secondary Lymphoid Organs.
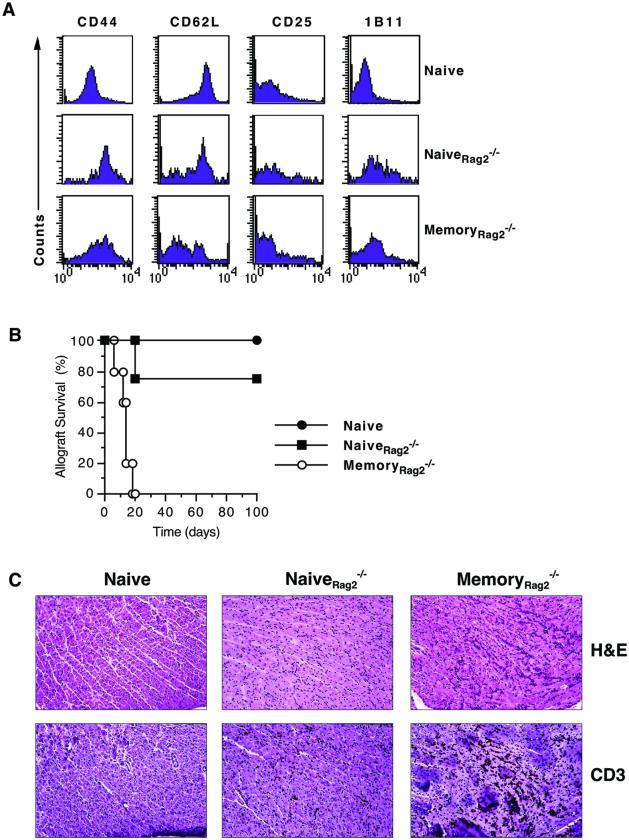
Naive T cells transferred into a lymphopenic host undergo homeostatic proliferation and acquire memory-like markers in the absence of antigen-specific stimulation (13–16). However, it is not known whether these cells function in vivo as antigen-experienced, “true” memory T lymphocytes or as naïve T cells. To address this question, we transferred a mixture of naive B6 and 2C T cells that were parked for 10 weeks in lymphocyte-deficient Rag2−/− mice (naiveRag2−/−) to splenectomized aly/aly mice transplanted with BALB/c hearts. Control mice received an equal number of either unparked naive B6 and 2C T cells (naive) or memory B6 and 2C T cells generated in immunized Rag2−/− hosts (memoryRag2−/−). The majority of 2C T lymphocytes in the naiveRag2−/− population were CD44high (Fig. 2A), but unlike antigen-experienced memoryRag2−/− T cells, the naiveRag2−/− population did not consistently induce allograft rejection in splenectomized aly/aly recipients (Fig. 2B). As shown in Fig. 2B, acute allograft rejection occurred in only one of four mice that received naiveRag2−/− T cells. NaiveRag2−/− T cells also behaved differently from the unparked naive population in that they infiltrated cardiac allografts that were still functioning at 100 days after transplantation whereas the naive T cells did not (Fig. 2C). Therefore, memory-like T cells arising from the homeostatic proliferation of naive T cells in the absence of antigenic stimulation have intermediate in vivo function in mice that lack secondary lymphoid organs and thus are distinct from either naive or true memory T cells.
Figure 2.
Memory-like T cells have intermediate in vivo function in the absence of secondary lymphoid organs. Memory-like (naiveRag2−/−) and antigen-experienced memory (memoryRag2−/−) T cells were generated in Rag2−/− hosts and adoptively transferred to splenectomized aly/aly heart transplant recipients. The naïve group received T cells harvested from naïve wt and 2C mice as described in Methods. (A) Phenotype of naive, naiveRag2−/−, and memoryRag2−/− allospecific T cells determined before adoptive transfer by four-color flow analysis after gating on the CD8+1B2+ (2C TCR-tg) cell population. Histograms shown are representative of 4–5 experiments. (B) Cardiac allograft survival in splenectomized aly/aly mice that received naïve (●, n = 4), naiveRag2−/− (■, n = 4), or memoryRag2−/− (○, n = 5) T cell populations. (C) Histological (hematoxylin/eosin, H&E) and immunohistochemical (CD3) analysis of cardiac allograft tissue harvested 100 days after transplantation in the naive and naiveRag2−/− group and on the day of rejection in the memoryRag2−/− group. (Magnification = ×200.)
CD8 Memory T Cells Generate More Memory T Cells in the Absence of Secondary Lymphoid Organs.
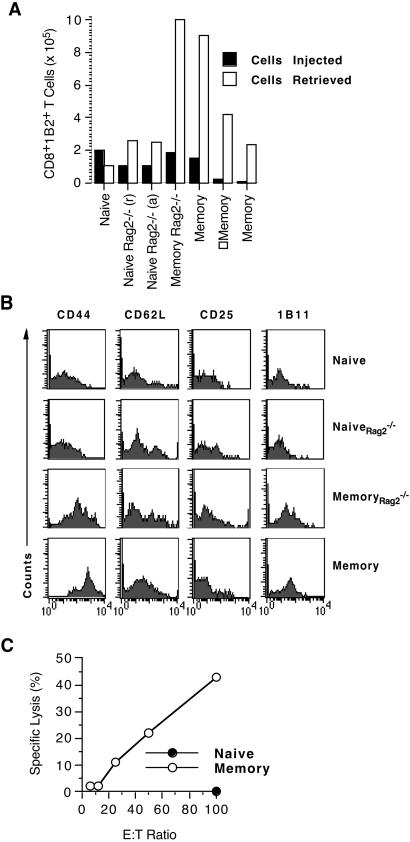
It has been proposed that central memory T cells that reside in secondary lymphoid organs are responsible for replenishing the memory pool whereas effector memory T cells that circulate through peripheral tissues exert effector functions upon reencounter with antigen but do not generate more memory T cells (6, 18). We addressed this hypothesis by testing whether secondary lymphoid organs are necessary for propagating T cell memory. Specifically, we asked whether the transferred memory T cell population that leads to cardiac allograft rejection in mice devoid of secondary lymphoid organs (as shown in Fig. 1) gave rise to more memory T cells. To answer this question, we quantitated and phenotyped 2C T cells present in the blood of splenectomized aly/aly hosts at the time of graft harvest and compared them to the number and phenotype of the 2C T cells that were injected 2 days after transplantation. Antigen-experienced memory 2C T cells generated in B6 mice (memory) or in Rag2−/− mice (memoryRag2−/−) increased significantly in number (5- to 40-fold increase) after allograft rejection and retained their memory phenotype (Fig. 3 A and B). In contrast, the memory-like 2C T cell population (naiveRag2−/−) generated in the absence of antigenic stimulation increased by only 2-fold and did not retain the CD44high phenotype irrespective of whether allograft rejection occurred or not. Naive 2C T cells, on the other hand, retained their naive phenotype and appeared to have decreased in number 100 days after transplantation. Moreover, the T cell population retrieved from the blood of the memory group exhibited immediate ex vivo CTL activity against BALB/c target splenocytes, suggesting that it contained functional memory T cells, predominantly of the effector subset (Fig. 3C). These findings indicate that antigen-experienced, true memory T cells beget more memory T cells in the absence of secondary lymphoid tissue.
Figure 3.
CD8 memory T cells generate more memory T cells in the absence of secondary lymphoid organs. Heart transplantation and adoptive transfer of T cells to splenectomized aly/aly mice were performed as in Figs. 1 and 2. CD8+1B2+ (2C TCR-tg) cells present in the blood of splenectomized aly/aly hosts at the time of cardiac allograft rejection (naiveRag2−/−, memoryRag2−/−, and memory) or at 100 days after transplantation in mice that accepted their allografts [naive and naiveRag2−/−(a)] were quantitated (A), phenotyped by flow analysis (B), and assayed for immediate ex vivo CTL activity (C). The bar graph compares the number of CD8+1B2+ T cells injected to the number of CD8+1B2+ cells retrieved in seven individual mice. The number of CD8+1B2+ cells retrieved was extrapolated based on the number of CD8+1B2+ T cells present per ml of blood.
Discussion
Allograft rejection is a T cell-dependent process mediated by either CD4 or CD8 T cells (19). Using cardiac allograft rejection as a readout for in vivo memory T cell function, we demonstrated here that an unfractionated population of antigen-experienced memory T cells mounts a productive immune response and generates more CD8 memory T cells independent of secondary lymphoid organs. These characteristics of antigen-experienced, true memory T cells were not shared by either naïve T cells or memory-like T cells generated in the absence of antigenic stimulation. Although our experimental approach does not distinguish between CD4 and CD8 memory recall, it approximates physiologic conditions wherein CD4 and CD8 T cells function in unison during alloimmune responses. Our observation that the number of memory CD8 TCR-tg lymphocytes (2C) increases after allograft rejection in splenectomized aly/aly hosts indicates that the propagation of CD8 memory T cells is also independent of secondary lymphoid organs. Whether CD4 memory T cells follow the same rules as their CD8 counterparts remains to be tested in a system where alloreactive CD4 T cells can be tracked and quantitated.
Recent studies have suggested the existence of two subsets of memory T cells: one that circulates through secondary lymphoid organs (central memory) and another that circulates through nonlymphoid tissues (effector memory) (6, 7, 18). It has also been proposed that the extralymphoid effector memory T cells are poised for immediate response to foreign antigen in the periphery whereas central memory T cells are specialized to proliferate within secondary lymphoid organs to generate more memory T cells (20). The memory T cell population studied in our experiments was isolated from the spleens and lymph nodes of immunized mice and lacked immediate ex vivo cytolytic activity (but rapidly acquired such activity upon antigenic recall), suggesting that it contained predominantly central CD8 memory T cells. Interestingly, this memory population mounted a productive immune response and generated more effector memory T cells in mice that lack secondary lymphoid organs. Our results therefore suggest that a CD8 memory population consisting predominantly of central memory T cells is not only capable of mounting a productive immune response outside secondary lymphoid organs but also expands the effector memory pool without homing to secondary lymphoid tissues. It remains possible that the expansion of the central memory pool, on the other hand, depends on antigen re-encounter within secondary lymhoid tissues (6, 21).
Alymphoplasia in aly/aly mice is caused by a recessive mutation in the gene encoding Nf-κb-inducing kinase (NIK) (22). Although adoptively transferred memory T cells used in our experiments did not contain the NIK mutation, it is possible that the absence of functional NIK in host cells could have influenced the migration or function of the transferred T cells caused by dysregulated cytokine or chemokine production in extralymphoid tissues. We believe that this possibility is unlikely as the only significant immune defects in aly/aly mice that cannot be attributed to the absence of secondary lymphoid tissues are restricted primarily to B cells (23), and the expression of chemokine and chemokine receptors involved in lymphocyte homing appears to be normal in these mice (24).
The data presented here underscore that immunologic memory is not merely the consequence of increased frequency of antigen-specific naïve T cells but is caused by the presence of a qualitatively distinct population of memory T cells. These memory T cells differ from their naïve counterparts in that they respond to foreign antigen outside the context of secondary lymphoid organs. This property, which is shared by memory B cells (25), endows the host with the ability to rapidly eliminate dangerous microbes shortly after their entry into peripheral tissues. On the other hand, this same property of memory lymphocytes poses a formidable obstacle to the long-term survival of organ transplants as current immunosuppressive agents are designed to target the homing and activation of naïve lymphocytes.
Acknowledgments
We thank Drs. Rafi Ahmed (Emory University) and Kaja Murali-Krishna (University of Washington, Seattle) for their helpful comments. This work was supported by National Institutes of Health Grants AI41643, AI44644, and AI49466.
Abbreviations
- aly
alymphoplasia gene
- Rag2
recombination activating gene-2
- TCR-tg
T cell receptor-transgenic
- wt
wild type
- CTL
cytotoxic T lymphocyte
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Sprent J, Surh C D. Curr Opin Immunol. 2001;13:248–254. doi: 10.1016/s0952-7915(00)00211-9. [DOI] [PubMed] [Google Scholar]
- 2.Heeger P S, Greenspan N S, Kuhlenschmidt S, Dejelo C, Hricik D E, Schulak J A, Tary-Lehmann M. J Immunol. 1999;163:2267–2275. [PubMed] [Google Scholar]
- 3.Karrer U, Althage A, Odermatt B, Roberts C W M, Korsmeyer S J, Miyawaki S, Hengartner H, Zinkernagel R M. J Exp Med. 1997;185:2157–2170. doi: 10.1084/jem.185.12.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ochsenbein A F, Klenerman P, Karrer U, Ludewig B, Pericin M, Hengartner H, Zinkernagel R M. Proc Natl Acad Sci USA. 1999;96:2233–2238. doi: 10.1073/pnas.96.5.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lakkis F G, Arakelov A, Konieczny B T, Inoue Y. Nat Med. 2000;6:686–688. doi: 10.1038/76267. [DOI] [PubMed] [Google Scholar]
- 6.Reinhardt R L, Khoruts A, Merica R, Zell T, Jenkins M K. Nature (London) 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 7.Masopust D, Vezys V, Marzo A L, Lefrancois L. Science. 2001;291:2413–2417. [PubMed] [Google Scholar]
- 8.Weninger W, Crowley M A, Manjunath N, von Andrian U H. J Exp Med. 2001;194:953–966. doi: 10.1084/jem.194.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyawaki S, Nakamura Y, Suzuka H, Koba M, Yasumizu R, Ikehara S, Shibata Y. Eur J Immunol. 1994;24:429–434. doi: 10.1002/eji.1830240224. [DOI] [PubMed] [Google Scholar]
- 10.Niimi M. J Heart Lung Transplant. 2001;20:1123–1128. doi: 10.1016/s1053-2498(01)00309-6. [DOI] [PubMed] [Google Scholar]
- 11.Sha W C, Nelson C A, Newberry R D, Kranz D M, Russel J H, Loh D Y. Nature (London) 1988;335:271–274. doi: 10.1038/335271a0. [DOI] [PubMed] [Google Scholar]
- 12.Dai Z, Arakelov A, Wagener M, Konieczny B T, Lakkis F G. J Immunol. 1999;163:3131–3137. [PubMed] [Google Scholar]
- 13.Kieper W C, Jameson S C. Proc Natl Acad Sci USA. 1999;96:13306–13311. doi: 10.1073/pnas.96.23.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murali-Krishna K, Ahmed R. J Immunol. 2000;165:1733–1737. doi: 10.4049/jimmunol.165.4.1733. [DOI] [PubMed] [Google Scholar]
- 15.Cho B K, Rao V P, Ge Q, Eisen H N, Chen J. J Exp Med. 2000;192:549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldrath A W, Bogatzki L Y, Bevan M J. J Exp Med. 2000;192:557–564. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai Z, Konieczny B T, Lakkis F G. J Immunol. 2000;165:3031–3036. doi: 10.4049/jimmunol.165.6.3031. [DOI] [PubMed] [Google Scholar]
- 18.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Nature (London) 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 19.Auchincloss H J, Sykes M, Sachs D H. In: Fundamental Immunology. Paul W E, editor. Philadelphia: Lippincott–Raven; 1999. pp. 1175–1235. [Google Scholar]
- 20.Mackay C R, von Andrian U H. Science. 2001;291:2323–2324. doi: 10.1126/science.1059984. [DOI] [PubMed] [Google Scholar]
- 21.Sallusto F, Lanzavecchia F. J Clin Invest. 2001;108:805–806. doi: 10.1172/JCI14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shinkura R, Kitada K, Matsuda F, Tashiro K, Ikuta K, Suzuki M, Kogishi K, Serikawa T, Honjo T. Nat Genet. 1999;22:74–77. doi: 10.1038/8780. [DOI] [PubMed] [Google Scholar]
- 23.Karrer U, Althage A, Odermatt B, Hengartner H, Zinkernagel R M. Eur J Immunol. 2000;30:2799–2807. doi: 10.1002/1521-4141(200010)30:10<2799::AID-IMMU2799>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 24.Fagarasan S, Shinkura R, Kamata T, Nogaki F, Ikuta K, Tashiro K, Honjo T. J Exp Med. 2000;191:1477–1486. doi: 10.1084/jem.191.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ochsenbein A F, Pinschewer D D, Sierro S, Horvath E, Hengartner H, Zinkernagel R M. Proc Natl Acad Sci USA. 2000;97:13263–13268. doi: 10.1073/pnas.230417497. [DOI] [PMC free article] [PubMed] [Google Scholar]