Abstract
TH1 memory T cells derived from T cell receptor transgenic mice, in which the T cell antigen receptor is specific for a cytochrome C peptide in association with I-Ek, were transferred into normal B10.A mice and allowed to adopt a resting phenotype. When challenged, 30–60 days after transfer, with i.v. cytochrome C, the transgenic cells rapidly became activated, expressed mRNA for IFNγ, and began to divide. However, after 48 h, the frequency of the cells fell progressively, reaching levels only slightly above the limit of detection by day 8 and thereafter remain depressed for up to 90 days. The remaining cells were anergic as shown by limitation in proliferation and IFNγ production in response to in vitro antigen stimulation. Even if challenged with antigen emulsified in complete Freund's adjuvant, the overall pattern was similar, except that in the draining lymph nodes, the surviving antigen-specific cells were not anergic, although spleen cells were still strikingly anergic. Thus, antigenic challenge of mice possessing resting memory TH1 CD4 T cells leads to the unanticipated loss of most of the specific cells and an apparent depletion rather than enhancement of immunologic memory.
Keywords: tolerance‖TH1 cells‖anergy‖immunologic memory‖apoptosis
Priming naive T cells leads to rapid expansion and the adoption of an activated phenotype. Many of these cells then undergo apoptotic cell death (1–4). In primary responses that result in a state of immunologic memory, the frequency of specific cells increases as a result of priming and, for CD4 cells, they may differentiate, adopting a polarized phenotype in which they are principally IFNγ or IL-4 producers and are designated TH1 or TH2 cells. Activated CD4 T cells generally leave the recirculating pool, acquire the capacity to enter tissues, and usually produce large amounts of cytokine promptly upon antigenic challenge (3).
CD4 memory cells survive for extended periods (5) but many adopt a quiescent character and some reacquire the expression of CD62L (6) and CCR7 (7), suggesting they have reentered the recirculating pool. The in vivo requirements for reactivation of these cells are not clear and, although it would be anticipated that antigenic challenge should lead to their expansion, little is actually known regarding their fate upon activation.
Here we test the capacity of a population of T cell antigen receptor (TCR) transgenic cells primed either in vitro or in vivo and allowed to return to a resting memory state in normal B10.A mice to respond to antigenic challenge. After an initial burst of activation, cytokine production, and proliferation, these cells were largely eliminated. The few remaining cells entered an anergic state that persisted for at least 35 days.
Methods
Mice.
5C.C7 TCR transgenic Rag2−/− B10.A mice were obtained from Taconic Farms (Germantown, NY). Female B10.A mice were purchased from the Division of Cancer Treatment, National Cancer Institute (Frederick, MD).
Culture Medium.
Complete RPMI (cRPMI) consisted of RPMI 1640 medium (Biofluids/BioSource International, Rockville, MD) supplemented with 10% FBS (Life Technologies, Rockville, MD), 2 mM L-glutamine, 0.05 mM 2-mercaptoethanol, 100 units/ml penicillin, and 100 μg/ml streptomycin.
Antibodies and Cytokines.
Allophycocyanin-anti-CD4 (RM4–5), FITC and biotin-anti-Vα11 (RR8–1), PE and biotin-anti-Vβ3 (KJ25), PE-anti-CD62L (Mel14), PE-anti-CD69 (H1.2F3), CyChrom-anti-CD44 (IM7), Allophycocyanin-anti-IFNγ (XMG1.2), FITC-anti-I-Ak (11–5.2), FITC-anti-I-Ad (AMS-32.1), FITC-anti-CD8 (53–6.7), FITC-anti B220 (RA3–6B2), Streptavidin-CyChrom and Streptavidin-Allophycocyanin were all from PharMingen. Anti CD3 (2C11), anti-IL-4 (11B11), anti-CD28 (37.51), and anti Fcγ receptor II/III (2.4G2) were prepared by Harlan Bioproducts for Science (Indianapolis, IN). Human IL-2 was a gift from Cetus. Mouse IL-12 was purchased from R&D Systems.
Priming of CD4+ Cells in Vitro.
CD4 T cells were isolated from lymph nodes of transgenic mice by negative selection, as described (8), and primed under TH1-inducing conditions [T-depleted, irradiated spleen cells from normal B10.A as antigen-presenting cells, 1 μM cytochrome C peptide 88–104, IL-12 (10 ng/ml), anti IL-4 (10 μg/ml), and IL-2 (10 units/ml) for two rounds of 6 days each]. After incubation in IL-2-containing cRPMI for 2 days, 2–5 × 107 TH1 cells were transferred to normal B10.A mice. In some experiments, stimulated cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes) before transfer.
Antigen Challenge in Vivo.
Four to eight weeks after transfer, 1–100 μg of cytochrome C (Sigma) in 200 μl of PBS were injected i.v. or 100 μg of cytochrome C in 100 μl of PBS emulsified with an equal volume of complete Freund's adjuvant (CFA) was injected s.c. Blood, spleen cells, lymph node cells, and liver lymphocytes were obtained after cell transfer and at various times after antigen challenge. When unlabeled cells had been transferred, cells were stained with FITC-anti Vα11, PE-anti Vβ3, and allophycocyanin-anti CD4. When CFSE-labeled cells had been transferred, PE-anti Vβ3, allophycocyanin-anti CD4, biotin-anti Vα11, and CyChrome-streptavidin were used. Events (20,000–30,000) were collected on a FACS Calibur and analyzed by using CELL QUEST software (Becton Dickinson). Transferred cells were identified by either CFSE fluorescence or as Vα11+, Vβ3+ CD4+ T cells. The frequency of transgenic cells was normalized by taking account of both the percent of Vα11, Vβ3+ cells in the blood before challenge and the frequency of Vα11, Vβ3 double-positive cells in normal B10.A mice (≤0.5%). The normalized value was referred to as the Index. Index = [Recipient Vα11, Vβ3 in CD4 (%) − B10A Vα11, Vβ3 in CD4 (%)]/[Pre Ag challenge RecipientVα11, Vβ3 in CD4 (%) − B10A Vα11, Vβ3 in CD4 (%)]. Cells from antigen-challenged or unchallenged mice were stained for expression of IFNγ as described (5).
Detection of Apoptosis.
Cells ware incubated with PE-Annexin-V (PharMingen) in propidium iodide (PI) staining solution (PharMingen) and analyzed for Annexin-V staining by flow cytometry. When CFSE-labeled cells were used, CFSE+ and PI- cells were gated, and the percent of Annexin V+ cells determined. When unlabeled cells were used, cells were stained initially with FITC-anti Vα11, biotin-anti Vβ3, and streptavidin-allophycocyanin, and then with PE-Annexin-V and PI. Vα11+, Vβ3+, PI- cells were gated and the percent of Annexin V+ cells measured.
Reverse Transcription–PCR.
Total RNA from spleen cells of mice at various times after antigen challenge or from unchallenged mice was isolated by using TRIzol (GIBCO/BRL, Gaithersburg, MD). First-strand cDNAs were made by using the Super Script Preamplification System (GIBCO/BRL) according to the manufacturer's protocol. PCR was carried out in a Gene Amp PCR system 9700 (Perkin-Elmer) by using Platinum PCR SuperMix (GIBCO/BRL) with cDNA templates. The PCR conditions were 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min for 28 cycles. The sense primer for IFNγ was 5′-GAAAAGGAGTCGCTGCTG-AT-3′ and the antisense primer was 5′-AGATACAACCCCGCAATCAC-3′. The sense primer for β-actin was 5′-GATGACGATATCGCTGCGCTG-3′ and the antisense primer was 5′-TACGACCAGAGGCATACAGG-3′.
Restimulation of Memory Cells in Vitro.
Cells (2 × 106) were cultured in individual wells of a 24-well plate in 1 ml cRPMI and IL-2 (10 units/ml) with cytochrome C peptide (0.001–10 μM). Four days later, cells were harvested and total cell numbers were counted. Before and after culture, cells were stained with FITC anti-Vα11, PE anti-Vβ3 and allophycocyanin anti-CD4.
Relative expansion of transgenic cell number was calculated as follows. Preculture transgenic cell number = (% Vα11,Vβ3 cells in CD4 cells in recipient − % Vα11,Vβ3 cells in CD4 cells in normal B10.A) × %CD4 cells in recipient × 2 × 102. Postculture transgenic cell number = (% Vα11,Vβ3 cells in postculture of recipient cells × 10−2 × postculture cell number) − [(% Vα11,Vβ3 cells in CD4 cells in normal B10.A) × %CD4 cells in recipient × 2 × 102] × [(% Vα11,Vβ3 cells in postculture normal B10.A × postculture normal B10.A cell number)/% Vα11,Vβ3 cells in preculture normal B10.A × 2 × 106)]. Relative expansion = postculture transgenic cell number/preculture transgenic cell number.
It should be noted that the correction factor for the endogenous Vα11,Vβ3 cells in the postculture of recipient spleen cells is usually negligible.
Results
Characterization of Transferred Cells.
CD4+ T cells were purified from B10.A Rag2−/− mice transgenic for TCR α- and β-chains encoding a receptor specific for pigeon cytochrome C amino acids 88–104 in association with I-Ek (9). The cells were primed in vitro under TH1 conditions for two rounds of 6 days each. When stimulated with immobilized anti-CD3 and anti-CD28 for 6 h, more than 90% of the cells were IFNγ+. Primed cells (20–50 × 106) were injected i.v. into normal B10.A recipients.
The frequency of transferred cells in the blood declined during the initial period after transfer at a rate of ≈2.6% per day (setting the frequency of transgenic cells at days 7–10 as 100%), but stabilized by 30 days; thereafter, the rate of diminution of cells was 0.33% per day. At 30 days after transfer, the transferred cells were CD44 bright and contained both CD62L bright and dull cells. Few of the transferred cells were CD69+ (≈3.5%) or CD25+ (≈2.5%). Based on CFSE staining, most of the cells had not divided; variable proportions of the cells had divided once, and very few cells had divided twice. Thus, by 30 days after transfer, the transgenic cells seem to have adopted a resting-memory phenotype and their frequency was at a quasi-steady-state level (10, 11).
Antigen Challenge Results in Expansion Followed by Loss of Transferred Cells.
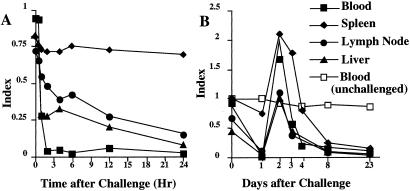
Mice that had received primed cells 30 days earlier were challenged with 100 μg of cytochrome C protein i.v. Transferred cells diminished in the blood within 1 h after challenge and had left the blood completely within 2 h. In the lymph node and liver, a rapid decline also occurred in the frequency of transferred cells, but this rapid decline was followed by a slower phase of diminution (Fig. 1A). Cell numbers were initially maintained in the spleen.
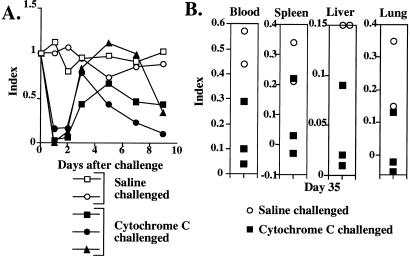
Figure 1.
Memory T cells are eliminated by i.v. challenge with antigen. Thirty days after TH1 cell transfer, recipients were challenged with 100 μg of cytochrome C i.v. The frequency of transgenic cells in blood, spleen, pooled lymph node, and liver was measured at various times after challenge and is reported as an “index” (see Materials and Methods). (A) Frequency of transgenic cells in the first 24 h after challenge; (B) frequency of transgenic cells for a 23-day period after challenge.
At 2 days after challenge, transferred cells were found at levels equivalent to or above those present before challenge but their frequency fell thereafter reaching levels at or barely above background by 8 days (Fig. 1B). Transferred cell numbers did not increase for the length of the period of observation (in some experiments, 90 days). Challenge with 1 μg of cytochrome C resulted in loss of transferred cells from blood, spleen and lymph node, although at a slightly slower pace than was observed with 100 μg.
Diminution upon antigen challenge was also observed with TH2-primed 5C.C7 cells and with ovalbumin-specific DO.11.10 cells primed to be TH1 cells and then transferred to BALB/c recipients (data not shown).
The diminution of cells in response to challenge with cytochrome C did not seem to be caused by the presence of very high numbers of transgenic cells in the recipient. When we plotted the frequency of transgenic cells in the blood at the time of the transfer against the degree of diminution at day 5 after challenge, we found no correlation (r2 = 0). This finding was obtained with initial transgenic cell frequencies between 1% and 20%.
Memory Cells Respond to Antigen Challenge with a Burst of Activation and Cytokine Production Before Elimination.
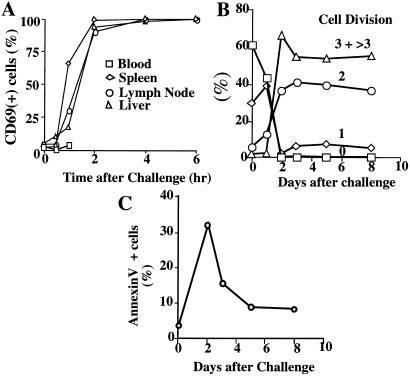
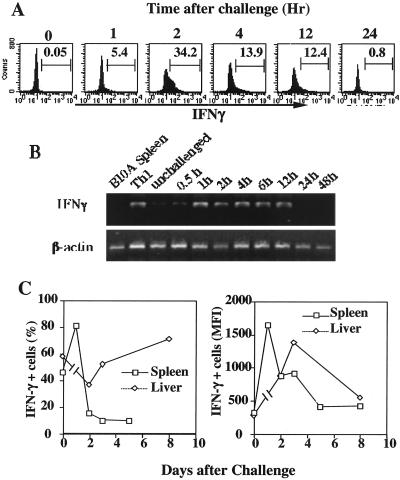
Within 1–2 h of challenge, memory cells in the spleen, lymph node, and liver showed a striking induction of CD69 (Fig. 2A). IFNγ was rapidly produced by spleen cells; 5% of the cells were IFNγ+ at 1 h after in vivo challenge, and 34% of the cells at 2 h. Thereafter, the frequency of IFNγ+ cells fell and had returned to undetectable levels at 24 h after challenge (Fig. 3A). IFNγ mRNA was detectable within 1 h of challenge and persisted for 12 h (Fig. 3B).
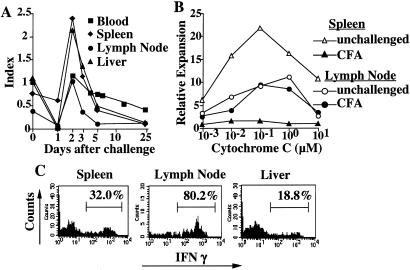
Figure 2.
Challenged memory cells are transiently activated and undergo cell division but rapidly express Annexin V. (A) Cells were obtained at various times after cytochrome C challenge and stained with anti-Vα11, anti-Vβ3, and anti-CD69. The percent of CD69+ cells among transferred cells (Vα11 and Vβ3 positive) was determined. (B) Spleen cells were obtained from mice that had received CFSE-labeled TH1 cells 30 days earlier. CFSE fluorescence in transferred Vα11, Vβ3 cells at various times after challenge was determined as a measure of cell division. The method was unable to distinguish 3 cell divisions from >3 cell divisions. (C) Spleen cells isolated in B were also stained with PE-Annexin V and PI. Percent of PI-negative, Annexin V-positive cells among transferred cells was measured.
Figure 3.
Memory cells respond to challenge with rapid IFNγ production. (A) Spleen cells were isolated from recipients at various times after i.v. challenge with 100 μg of cytochrome C. Cells were fixed immediately and stained with anti-IFNγ antibodies. Transferred memory cells were identified by CFSE fluorescence, and their IFNγ content was measured by flow cytometry. (B) mRNA was obtained from these spleen cells and expression of IFNγ mRNA was determined by reverse transcription–PCR. (C) Spleen and liver cells were isolated from unchallenged mice or at various times after challenge. Cells were stimulated with immobilized anti-CD3 and anti-CD28 for 6 h. Monensin was added for the final 2 h. The cells were stained anti-Vα11, anti-Vβ3, and anti-IFNγ and analyzed by flow cytometry.
Splenic memory cells showed enhanced responsiveness to in vitro stimulation with immobilized anti-CD3 and anti-CD28. One day after antigen challenge, 80% of the cells became IFNγ+, with a mean fluorescence intensity of ≈1600, compared with 45% of the cells from nonchallenged mice becoming IFNγ+ on stimulation with a mean fluorescence intensity of ≈400 (Fig. 3C). Thereafter, the splenic transgenic cells showed diminished responsiveness (<20% of the cells became IFNγ+ in response to stimulation at 2 days after challenge and the mean fluorescence intensity fell). Transgenic cells in the liver retained a level of response similar to that before challenge.
Most transferred cells underwent three or more cell divisions within 2 days of antigenic challenge (Fig. 2B). The frequency of Annexin V-positive cells also peaked on day 2 (Fig. 2C), suggesting that stimulation was associated with rapid activation followed by rapid apoptosis (13). Because the frequency of transgenic cells was markedly diminished in the blood, in the lymphoid organs, and in a major tissue site, we conclude that most of the cells were eliminated as a result of antigen challenge.
Memory Cells That Survive Antigen Challenge Are Anergic.
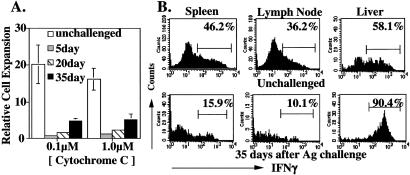
We assessed the responsiveness of the cells surviving after antigen challenge by testing antigen-driven expansion and cytokine production. We first established that the degree of expansion of memory TH1 cells from unchallenged donors was independent of the frequency of the transgenic cells. We then compared the relative expansion of transgenic cells from antigen-challenged recipients to that of cells from unchallenged recipients. Transgenic cells remaining in the spleen (Fig. 4A), lymph node and liver (data not shown) 5 days after challenge showed profound unresponsiveness, which was only slightly less pronounced at 20 days. Even at 30–35 days, surviving splenic and liver (data not shown) transgenic T cells displayed substantially diminished expansion in response to antigenic challenge. At 35 days after challenge, anergy in the spleen and lymph node transgenic cell populations was also observed at the level of IFNγ production in response to challenge with immobilized anti-CD3 and anti-CD28 (Fig. 4B). By contrast, surviving liver cells show a more vigorous IFNγ response than did nonchallenged liver cells, indicating that cells in this tissue site had escaped from anergy, at least at the level of cytokine production.
Figure 4.
Properties of memory cells surviving Ag challenge. (A) Spleen cells from unchallenged recipient mice, and from recipient mice that had been challenged 5, 20, or 35 days earlier were isolated; 2 × 106 of these cells were cultured with 0.1 or 1.0 μM cytochrome C peptide in individual wells of a 24-well plate. The number of transgenic cells was assessed 4 days later and relative cell expansion calculated as described in Materials and Methods. (B) Thirty-five days after Ag challenge of recipient mice, a suspension of spleen and lymph node cells and of liver lymphocytes was prepared. Cells were stimulated with immobilized anti-CD3/anti CD28 and the frequency of IFN-γ producing cells determined by staining of fixed/permeabilized cells as in Fig. 3C.
In Vivo Primed Memory Cells Are also Eliminated by Antigen Challenge.
To test the possibility that only in vitro primed memory cells would be eliminated by antigen challenge, 30 × 106 CD4 T cells from 5C.C7 Rag2−/− donors were transferred to normal B10.A mice. Miniosmotic pumps containing 1 mg of cytochrome C were implanted in the recipients and removed 7 days later. The frequency of transgenic cells among blood CD4 T cells was 6.7% before implantation of the pump. On day 3, it had risen to 25.5%, and was 20.1% on day 7, 19.1% on day 14, 10.8% on day 24, and 4.4% on day 64. On day 64, three of the mice were challenged with 100 μg of cytochrome C i.v. and 2 were challenged with saline. All of the cytochrome C-challenged mice showed rapid depletion of transgenic cells from the blood (Fig. 5A). By day 3 after challenge, the frequency of transgenic cells had returned toward normal (mean index = 0.68), but by day 7 their frequency had declined. On day 9, the indices in the three challenged mice were 0.43, 0.34, and 0.11 compared with indices of 0.95 and 1.02 in the two unchallenged animals. The five mice were killed 35 days after challenge. Essentially no transgenic cells were present in the spleen, liver, or lung of two of the three cytochrome C challenged mice (Fig. 5B), whereas these cells were retained in the saline-challenged mice. Thus, TCR transgenic T cells primed in vivo showed a vigorous expansion but, on challenge 64 days later, were depleted in frequency in a manner similar to that observed with T cells from the same donors that had been primed in vitro.
Figure 5.
Memory cells primed in vivo are eliminated by antigen challenge. 5C.C7 TCR transgenic cells were transferred into B10.A mice. These animals were immunized by implantation of a miniosmotic pump containing cytochrome C. Sixty-four days later, three mice were challenged i.v. with cytochrome C (100 μg). (A) The frequency of transgenic cells in the blood was measured before challenge and on days 1, 2, 3, 5, 7, and 9. (B) The mice were killed on day 35 after challenge and the frequency of transgenic cells in the blood, spleen, liver, and lung was measured.
Challenge with Cytochrome C in CFA Induces a Complex Response Pattern.
It might well be anticipated that the responses we have observed reflect the state of the antigen used for challenge and thus of the APCs that present cytochrome C peptide to the resting memory cells. To test the importance of the form of the antigen, B10.A mice that had received in vitro primed TH1 cells from 5C.C7 Rag2−/− donors were challenged with cytochrome C (100 μg) emulsified in CFA. Cells rapidly disappeared from the blood, lymph node (including the lymph nodes draining the challenge site), and liver, but not from the spleen. They could be detected in these organs at day 2, and their frequency in liver and spleen was increased, but thereafter their frequency declined in all of the organs, although the actual cell number was more sustained in the lymph nodes because of their increased size (Fig. 6A). Although the transgenic cells present in the draining lymph nodes at day 23–25 were not anergic, either in terms of their proliferative response to antigen (Fig. 6B) or their cytokine production to in vitro stimulation (Fig. 6C), transgenic cells remaining in the spleen showed striking proliferative anergy and liver cells had a marked impairment in IFNγ production in response to in vitro challenge. Thus, even challenge in CFA did not result in overall expansion of memory cells; their total numbers diminished and, although anergy was not observed in the draining lymph nodes, memory cells in other sites in the challenged mouse were anergic.
Figure 6.
Challenge in presence of CFA leads to slower elimination of memory cells and to “split” anergy. Recipient mice were challenged s.c., in the back, with 100 μg of cytochrome C emulsified in CFA (CFA) or not (unchallenged). (A) Frequency of memory cells in blood, spleen, lymph node, and liver at various times after challenge with cytochrome C in CFA. (B) Spleen and lymph node cells from recipient mice challenged with cytochrome C in CFA 23–25 days earlier or from unchallenged mice were cultured with 0.001–10 μM cytochrome C peptide in vitro. Relative expansion was determined 4 days later. (C) Spleen, lymph node, and liver lymphoid cells from recipient mice challenged with cytochrome C in CFA 32 days earlier were stimulated in vitro with immobilized anti-CD3/anti-CD28. Intracellular IFNγ content was measured 6 h later.
Discussion
Here we observed that resting 5C.C7 TH1 memory cells are rapidly eliminated when challenged intravenously with cytochrome C. The elimination of memory cells was preceded by a wave of activation; all of the specific memory cells in the blood and the great majority of those in lymph nodes and in the liver left these organs within a few hours.
Within the spleen, cells rapidly became activated and produced IFNγ. Both IFNγ-producing cells and IFNγ mRNA were detectable within 1 h of challenge and peaked in frequency and in amount in 2 h. This wave of activation was also associated with cell division, which began on day 2. Most of the cells underwent three or more divisions by the end of day 2. However, at this time Annexin V was expressed on a substantial fraction of the memory cells. Thereafter, the memory cells diminished in frequency in all sites we examined, reaching levels only slightly above the frequency of Vα11, Vβ3 cells in normal B10.A mice by 8 days. The small proportion of surviving cells was anergic both in terms of their limited in vitro expansion in response to antigenic challenge and of the low frequency of the cells that produced IFNγ in response to challenge with immobilized anti-CD3 and anti-CD28.
When mice possessing resting memory T cells were challenged with cytochrome C in CFA, a generally similar pattern of early response was observed. In particular, the cells rapidly left lymph nodes, including the draining lymph nodes, but not the spleen. Important differences existed, however. The frequency of cells in the blood fell from prechallenge levels but was sustained above background. In the spleen and the liver, the frequency of transferred memory cells was only slightly above background on day 25. Even in the draining lymph node, the fall in the frequency of transgenic cells was striking. When examined on day 5 after challenge, the remaining cells in the draining lymph nodes and in other sites displayed anergy as shown by a marked limitation in antigen-driven expansion in vitro (data not shown). However, by day 25, the draining lymph node cells showed normal responses to challenge with cytochrome C and APC in terms of lymphocyte expansion; 80% of them produced IFNγ upon challenge with immobilized anti-CD3 and anti-CD28. By contrast, transgenic memory cells in the spleen and liver continued to be anergic on day 25, revealing an unanticipated “split” in the responsiveness of memory cells from mice challenged with what should have been a highly efficient immunization protocol.
The anergy of cells remaining after challenge is consistent with recent findings that a state of anergy is induced in LCMV-specific CD8 T cells that had responded to infection; this anergy persisted for ≈30 days after viral infection (S. Kaech and R. Ahmed, personal communication).
What is the physiological significance of the burst of activation followed by elimination of resting memory TH1 cells challenged with antigen? First, we need to point to some caveats in considering these results. The cells examined all expressed a single TCR, so the possibility exists that the observed elimination and induction of anergy may be limited to a set of T cells that express receptors in a given range of affinity for the ligand. However, we observed a generally similar loss of cells when BALB/c mice bearing memory DO.11.10 TH1 cells were challenged with their cognate antigen, ovalbumin, although the kinetics of cell loss was somewhat slower. Second, the memory cells we used in most experiments were primed in vitro and were highly polarized as a result of two rounds of TH1-inducing stimuli. Do the properties of in vitro-primed cells with a particular TCR adequately reflect the behavior of normally primed cells that express a range of TCRs? Although we have not yet examined “conventional” T cells primed in vivo, priming 5C.C7 cells in vivo by using antigen in a miniosmotic pump gave rise to memory cells that showed a generally similar behavior to in vitro primed cells on challenge. What is particularly striking is the expansion of naive cells as a result of primary stimulation contrasted to their failure to expand on secondary stimulation, followed by a rapid diminution in their frequency. In unpublished work, it has been observed that 5C.C7 cells from primed transgenic mice are also highly susceptible to death when challenged after cell transfer (S.Z.B.-S., unpublished observations).
It could be argued that highly polarized cells are particularly prone to antigen-driven cell death. Indeed, anergy can be induced in vitro in cytochrome C-specific T cell clones with high concentrations of antigen in the absence of costimulation (12). However, the in vivo priming experiment we performed resulted in striking elimination of transgenic cells upon challenge. It seems likely that priming with a miniosmotic pump without any other adjuvant would be a less vigorous procedure that the in vitro priming we have used, suggesting rapid activation followed by elimination may be quite general among CD4 memory T cells. Nonetheless, under conditions of physiologic priming, one may generate a spectrum of memory T cells that vary in their susceptibility to elimination, with the most “vigorously” primed being eliminated and the less vigorously primed cells actually expanding upon challenge. This spectrum would allow both the burst of activation and cytokine production that we have observed and, at the same time, expansion of memory cells, equipping the animal for yet a second challenge. Such possibilities await testing.
These experiments suggest that it might be possible to eliminate resting memory T cells in vivo. Indeed, Critchfield et al. successfully deleted recently primed TCR transgenic T cells specific for myelin basic protein by a series of challenges with myelin basic protein, providing a potential approach for treatment of autoimmune diseases (13). Their model differs from ours in that the transferred cells were recently primed and thus may still have had an activated memory phenotype; indeed, the authors argued that the death was due to effects of IL-2 (“propriocidal” cell death). By contrast, in unpublished work, we observed that IL-2 is actually protective in the model of elimination of resting memory T cells by antigenic challenge.
Elimination of resting memory T cell might be potentially valuable in episodic autoimmune diseases; it might be possible to eliminate resting memory cells by antigenic challenge during quiescent periods. However, if that elimination were to be attempted, it would have to be done under cover of agents, such as anti-cytokine antibodies, that could block the potentially dangerous effects of the transient burst of cytokine production that occurs. Indeed, Gefter and his colleagues had attempted to deplete cells mediating atopic responses by treating animals and humans with peptides containing epitopes of key allergens (14, 15). Although they concluded that the mechanism responsible for diminution of responses by challenge with specific peptides was anergy rather than elimination, they actually performed their key experiments with naive rather than memory T cells.
The unexpected sensitivity of highly polarized memory TH1 T cells to elimination by antigen may provide approaches for manipulating immune responses; however, as we argue above, any interventions based on these concepts must proceed with the recognition that elimination is part of a program in which vigorous activation and cytokine production occurs initially.
Acknowledgments
We thank Ms. Shirley Starnes for excellent editorial assistance.
Abbreviations
- cRPMI
complete RPMI
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- PI
proridium iodide
- CFA
complete Freund's adjuvant
- TCR
T cell antigen receptor
References
- 1.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J, Zajac A J, Miller J D, Slansky J, Ahmed R. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 2.Doherty P C, Christensen J P. Annu Rev Immunol. 2000;18:561–592. doi: 10.1146/annurev.immunol.18.1.561. [DOI] [PubMed] [Google Scholar]
- 3.Jenkins M K, Khoruts A, Ingulli E, Mueller D L, McSorley S J, Reinhardt R L, Itano A, Pape K A. Annu Rev Immunol. 2001;19:23–45. doi: 10.1146/annurev.immunol.19.1.23. [DOI] [PubMed] [Google Scholar]
- 4.Lenardo M, Chan K M, Hornung F, McFarland H, Siegel R, Wang J, Zheng L. Annu Rev Immunol. 1999;17:221–253. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 5.Hu-Li J, Huang H, Ryan J, Paul W E. Proc Natl Acad Sci USA. 1997;94:3189–3194. doi: 10.1073/pnas.94.7.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Sasson S Z, Makedonski K, Hu-Li J, Paul W E. Eur J Immunol. 2000;30:1308–1317. doi: 10.1002/(SICI)1521-4141(200005)30:5<1308::AID-IMMU1308>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 7.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Nature (London) 1999;14:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 8.Ben-Sasson S Z, Gerstel R, Hu-Li J, Paul W E. J Immunol. 2001;166:112–120. doi: 10.4049/jimmunol.166.1.112. [DOI] [PubMed] [Google Scholar]
- 9.Seder R A, Paul W E, Davis M M, Fazekas de St. Groth B. J Exp Med. 1992;176:1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.London C A, Lodge M P, Abbas A K. J Immunol. 2000;164:265–272. doi: 10.4049/jimmunol.164.1.265. [DOI] [PubMed] [Google Scholar]
- 11.London C A, Perez V L, Abbas A K. J Immunol. 1999;162:766–773. [PubMed] [Google Scholar]
- 12.Schwartz R H. Science. 1990;248:1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- 13.Critchfield J M, Racke M K, Zuniga-Pflucker J C, Cannella B, Raine C S, Goverman J, Lenardo M J. Science. 1994;263:1139–1143. doi: 10.1126/science.7509084. [DOI] [PubMed] [Google Scholar]
- 14.Falb D, Briner T J, Sunshine G H, Bourque C R, Lugman M, Gefter M L, Kamradt T. Eur J Immunol. 1996;26:130–135. doi: 10.1002/eji.1830260120. [DOI] [PubMed] [Google Scholar]
- 15.Wallner B P, Gefter M L. Clin Immunol Immunopathol. 1996;80:105–109. doi: 10.1006/clin.1996.0102. [DOI] [PubMed] [Google Scholar]