Abstract
Gene transfer vectors based on lentiviruses can transduce terminally differentiated cells in the brain; however, their ability to reverse established behavioral deficits in animal models of neurodegeneration has not previously been tested. When recombinant feline immunodeficiency virus (FIV)-based vectors expressing β-glucuronidase were unilaterally injected into the striatum of adult β-glucuronidase deficient [mucopolysaccharidosis type VII (MPS VII)] mice, an animal model of lysosomal storage disease, there was bihemispheric correction of the characteristic cellular pathology. Moreover, after the injection of FIV-based vectors expressing β-glucuronidase into brains of β-glucuronidase-deficient mice with established impairments in spatial learning and memory, there was dramatic recovery of behavioral function. Cognitive improvement resulting from expression of β-glucuronidase was associated with alteration in expression of genes associated with neuronal plasticity. These data suggest that enzyme replacement to the MPS VII central nervous system goes beyond restoration of β-glucuronidase activity in the lysosome, and imparts improvements in plasticity and spatial learning.
Mucopolysaccharidosis type VII (MPS VII), or Sly syndrome, is a lysosomal storage disease (LSD) resulting from mutations in β-glucuronidase (1). Similar to many other LSDs, there are both systemic and central nervous system (CNS) components, and patients show progressive disease involvement. In β-glucuronidase deficiency, lysosomes become progressively laden with undegraded glycosaminoglycans, leading to mental retardation and loss of hearing and vision. Patients also suffer from dysostosis multiplex, joint abnormalities, and hepatosplenomegaly.
An animal model for MPS VII, the β-glucuronidase-deficient gusmps mouse (2, 3), has been invaluable in testing enzyme, cell, and gene therapies (4–9). MPS VII mice exhibit progressive lysosomal accumulation in multiple organs, including but not limited to bone, spleen, liver, lung, kidney, retina, and brain (2, 10). Early work by Birkenmeier and colleagues (11, 12) demonstrated that enzyme replacement therapy or bone marrow transplant was sufficient for correction of the visceral manifestations of the disorder. However, there remained significant storage within the brain. Until recently, the functional affect of CNS storage was unknown. Recent work by Sands and coworkers (13) demonstrated that MPS VII mice have progressive learning impairment as measured by a Morris water maze, as well as gradual loss of vision. Protection against the onset of storage pathology and the functional deficits of learning impairment could be accomplished by enzyme therapy or adeno-associated viral (AAV)-mediated therapy, initiated immediately after birth (13–15). Similarly, in an animal model of metachromatic leukodystrophy, HIV-based vectors could protect against disease incipience (16).
The collective incidence of LSDs is ≈1 in 7,000 live births, with 65% affecting the CNS (17). In most instances, disease diagnosis occurs well after the onset of pathology. As such, recovery of function, rather than protection from disease onset, will be an important benchmark for efficacy of gene therapy for the LSDs. Our prior studies with recombinant adenoviruses, and others' using AAV- or HIV-based vectors, established that focal expression of β-glucuronidase within diseased rodent striata could reduce storage pathology in both hemispheres (4, 5, 8, 18). The enzyme is secreted from transduced cells and taken up by nontransduced cells, leading to a zone of correction that extends beyond the site of transduction. In this study we set out to test the hypothesis that recombinant viral vectors based on feline immunodeficiency virus (FIV) could revert not only the pathologic phenotype, but more importantly, the established behavioral dysfunction.
Materials and Methods
In Vivo Delivery and Transgene Assays.
Animal studies were approved by the Animal Care and Use Committee of the University of Iowa. β-Glucuronidase-deficient mice were from The Jackson Laboratories and our own breeding colony. C57BL/6 mice were from Harlan Sprague (Indianapolis, IN). For virus injections, mice were anesthetized with ketamine/xylazine (100–125 mg/kg ketamine/10–12.5 mg/kg xylazine). The bregma was exposed by incision and used as a zero coordinate for stereotactic injections into the striatum or ventricle as described (4, 19).
For histological studies, the mice were injected unilaterally with 5 μl [1 × 106 transducing units (TU)] of vector. Animals were killed 3, 6, 9, 15, and 18 weeks later, and their brains were analyzed for enzyme activity, volume, in situ RNA hybridization, and storage vacuoles as described (4).
Vectors.
FIV packaging constructs were generated from the full-length FIV molecular clone, FIV-34TF10 (National Institutes of Health AIDS Research and Reference Reagent Program), as described (20). The FIV construct, pVETLCβgal (pVETLCβ; ref. 20), was generated by inserting cytomegalovirus (CMV)-β-galactosidase into the pVETL FIV backbone. To construct pVETLRβgluc, a Rous sarcoma virus (RSV) promoter was isolated from pUC19RSV (J. K. Yee, personal communication), and the resulting fragment was inserted into pVETL to generate pVETLRSV. The β-glucuronidase cDNA was isolated from pAdRSV4 (21) and ligated into pVETLRSV to make pVETLRβgluc(+polyA). The poly(A) tail was removed to generate pVETLRβgluc. For FIVMCS, PVETLRSV was modified to contain a multiple cloning site (MCS) downstream from the RSV promoter. The vesicular stomatitis virus (VSV)-G envelope plasmid, pCMV-G, has been described (22). Pseudotyped FIV vectors expressing β-glucuronidase (FIVβgluc) and β-galactosidase (FIVβgal) vector particles were done by transient transfection of plasmids into 293T cells plated one day earlier at 2.8 × 106 cells per 10-cm dish. Cotransfections were made with a 1:2:1 molar ratio of FIV packaging, FIV vector, and pCMV-G constructs. Harvested supernatants (32 and 48 h later) were passed through 0.45-μm Nalgene filters and concentrated by centrifugation (23). Vector titers determined by serial dilution on HT1080 cells and by quantitative PCR of transduced target cells ranged from 1 × 108 to 2 × 108 TU/ml (20).
Repeated Acquisition and Performance Chamber (RAPC) Analyses.
The RAPC used to assess spatial learning and memory was done essentially as described (24–26). Mice (n = 8 each; MPS VII and control mice) were habituated to a reward solution before introduction to the RAPC. The habituation protocol required water deprivation for 12–16 h followed by exposure to a 0.2% saccharin solution for 30 min twice a day for 2 days, after which regular drinking water was provided ad libitum. Mice were introduced to the RAPC during four habituation sessions followed by four baseline experimental sessions (see Fig. 3, baseline sessions 1–4). A 12-h water deprivation period preceded behavioral sessions, with ad libitum water on nontest days. A session was 3 presentations each of the repeated acquisition (RA) component and the performance (P) component with 3 trials per presentation. In the RA component, the specific door sequence changed unpredictably with each successive session. During all P component sessions, the sequence of doors leading to the saccharin reward was identical. A discriminative stimulus (static audio signal) was played to signal that the P component was in effect. Thus, there were a total of 18 trials per session (3 trials per presentation × 3 presentations per component × 2 components per session). Latency was measured as the time required for the mouse to leave the start box, navigate through the four compartments, and consume the saccharin solution in the goal box. Mice were placed manually in the goal box if they failed to reach it within 10 min on any trial. Errors were defined as attempts to go through a locked door. Five weeks after the pretreatment sessions, MPS VII and control mice were split into groups (n = 4 per each treatment) and injected with FIVβgluc or FIVβgal (1 × 106 TU) into the striatum. Four weeks later, mice were reassessed according to the above protocol. Sessions 4, 5, and 6 were separated by 3 days each. A timeline for behavioral testing is detailed in Fig. 3A.
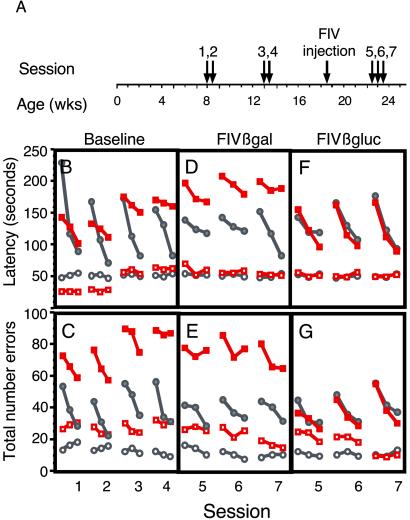
Figure 3.
Behavioral analyses in untreated and FIV-transduced mice. RAPC analysis was used to investigate baseline and posttreatment differences between MPS VII mice and age-matched heterozygous controls. (A) Behavioral sessions before and after gene transfer were performed according to the time line (Materials and Methods). MPS VII mice demonstrated significant baseline impairment in learning (solid red squares) relative to control mice (solid gray circles) as seen by both measures (latency in B and errors in C). There were no significant differences in the performance component of the assay between MPS VII (open red squares) and control (open gray circles) mice. After baseline testing, both groups were segregated randomly before bilateral striatal injection with FIVβgal or FIVβgluc. Behavioral tests on FIVβgal-treated (D and E) and FIVβgluc-treated (F and G) mice resumed 4 weeks after injection. FIVβgal-injected MPS VII mice continued to demonstrate a severe learning impairment in both latency and error (D and E). MPS VII mice injected with FIVβgluc exhibited no significant differences in learning compared with control mice in error or latency measures (F and G; P ≥ 0.05), and reflect a recovery of the learning impairment seen in the baseline measurements (B and C). Solid symbols, learning component. Open symbols, performance component.
Statistics for Behavioral Analyses.
Baseline differences (pretreatment) in errors and latencies were evaluated by using repeated-measures ANOVA (RMANOVA) with component (RA and P) and session (1–4) as within group factors and β-glucuronidase status (control vs. MPS VII) as a between-group factor. These were followed, where appropriate, by one-factor ANOVAs (β-glucuronidase status) for individual session data. Statistical assessment of changes in these measures after treatment were carried out separately for each treatment (FIVβgal and FIVβgluc) and for each component (RA and P) in RMANOVAs with β-glucuronidase status (control vs. MPS VII) as a between-group factor and session as within group factors. Subsequent one-factor ANOVAs were used, where appropriate, for determining differences between control vs. MPS VII groups for each session.
Microarray Study.
Both striata of 16-week-old mice were injected with either 5 μl of FIVβgluc or FIVMCS (5 × 105 to 1 × 106 TU total; n = 8 per treatment). Eight weeks later the mice were killed, the brain was removed, meninges were dissected away, and brains were cut at the level of the dorsal hippocampus midsagitally. Hippocampi were removed, and before total RNA isolation, two groups of four hippocampi each were pooled, homogenized in Trizol (GIBCO/BRL) by using a Pro-200 homogenizer (PRO Scientific, Oxford, CT) and frozen on liquid nitrogen. RNA was prepped by using the standard Trizol protocol and assessed by gel electrophoresis and spectrophotometry. Target preparation was performed as directed (Affymetrix, Santa Clara, CA) with all components generated throughout the procedure (cDNA, full-length cRNA, and fragmented cRNA) analyzed by gel electrophoresis to assess size distribution.
Gene expression analysis was done by the Affymetrix Mu11K high-density oligonucleotide array at the University of Rochester Microarray Core Facility. Hybridization, staining, washing, and scanning were performed per the manufacturer's protocol. All arrays were assessed for “array performance” by statistical analysis of control transcripts spiked into the hybridization mixture. In addition, several genes were identified on each array to assess the overall quality of array signal intensity. The results demonstrated that the arrays were within a 0.31-fold difference of each other at baseline, allowing for data normalization by the global scaling approach.
The Affymetrix Microarray Analysis Suite was used to generate the comparative analysis. The fold change represented for any transcript between the FIVMCS and FIVβgluc was calculated after global scaling to a target intensity of 2,500 (to normalize any differences in overall signal intensity among arrays). Super scoring was applied to all probe sets of 8 probe pairs or more. Data reported reflect the average fold changes in gene expression from two biological replicates (4 hippocampi each) for each condition. Data in Fig. 4D show the fold-change data from pair-wise comparisons of selected genes, of which all changed in the same direction. The false discovery rate of genes identified by our microarray analyses was determined by using significance analysis of microarrays (SAM) (27). A “two-class, unpaired” analysis within SAM was used to measure the effect of treatment as a function of genetically similar subjects (inbred strain, sex matched, littermates), and data were normalized by global scaling with Δ = 0.5, based on the false positive distribution. By SAM, a total of 517 genes (4.7%) were called significant, and an additional 253 genes were called false positive. All genes discussed in Fig. 4 met SAM criteria for statistical significance.
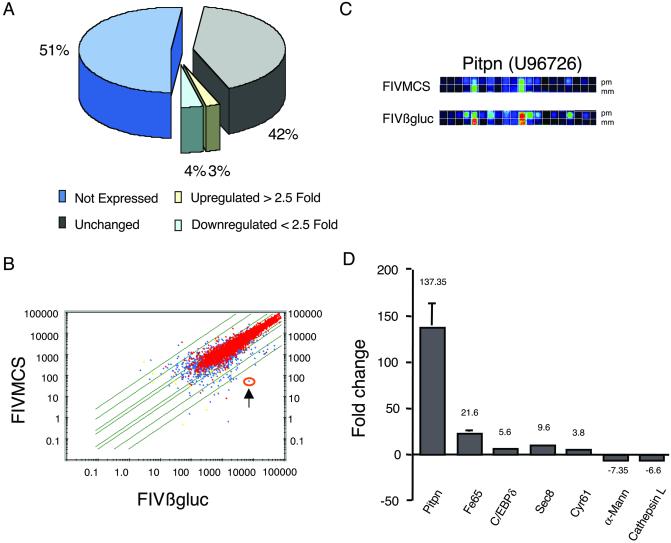
Figure 4.
Microarray analysis of murine hippocampus after treatment with FIV vectors. Hippocampi from FIVMCS- and FIVβgluc-treated animals were extracted 8 weeks after injection, and gene expression was analyzed on Affymetrix high-density oligonucleotide arrays. (A) A representation of the effects of FIVβgluc gene transfer on gene expression in the hippocampus. (B) A scatter plot depicts the average difference distribution of all genes examined, comparing FIVMCS with FIVβgluc samples. Red points depict genes that are called present, whereas blue points represent genes changing from absent to present or vice versa. Green lines indicate the magnitude of change with intervals of 2, 10, and 30-fold relative to baseline. One gene of interest, Pitpn (U96726), is circled in orange (arrow) and exhibits a significant change in gene expression as depicted by differences in the raw data images (C). (C) The increase in selective binding to the perfect match (pm) vs. the mismatch (mm) probes. (D) The increase or decrease in fold expression of RNA specific to selected genes from FIVβgluc relative to FIVMCS-treated mice. Data normalization and analyses were completed by using algorithms in the Affymetrix Microrarray Analysis suite and Data Mining Tools and significance analysis of microarrays (27).
Microarray Validation: Quantitative Real-Time PCR (QPCR) and Data Analysis.
Levels of Pitpn (U96726), Fe65 (P46933), Fisp-12 (M70642), C/EBP (X61800), and Egr2 (Krox20, X06746) expression were examined in cDNAs archived from the microarray experiment by using TaqMan chemistry with specific probes and primers designed with PRIMER EXPRESS V.1.0. The following dye combinations were used: FAM (5-carboxyfluorescein; reporter), TAMRA (N,N,N′,N′-tetramethyl-6-carboxyrhodamine; quencher), and ROX (6-carboxy-X-rhodamine; reference). A validation experiment was done with a probe designed to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (M32599) to determine the relative probe efficiency. This probe was used as a reference gene for comparative analyses. The absolute value of the slope of log input amount vs. ΔCT (CT = threshold cycle) was less than 0.1 for all comparisons, allowing for ΔΔCT determinations of relative quantitation of gene expression in FIVβgluc-treated mice vs. FIVMCS-treated mice (28). After optimization, cDNAs were diluted 1:100 with 1 μl used for each 25 μl PCR mixture containing 12.5 μl of ABI 2× Universal Master Mix, 1.25 μl of forward and reverse primers (final range 200–900, nM depending on primer set), and 1 μl of probe (final range 50–200 nM, depending on probe/primer set). Reactions were performed in triplicate and replicated three times. Thus, data in Table 1 reflect nine reactions per sample being tested; t test's for each sample achieved P < 0.001 or better. All reactions were run in an ABI 7700, and data were collected at all temperature phases during cycles. Raw data were analyzed by using the sequence detection software (Applied Biosystems) and relative quantitation using the comparative CT method was performed in Microsoft excel.
Table 1.
Validation of microarray data by QPCR
| Gene of interest (GOI) | Treatment | GOI average CT | GAPDH average CT | ΔCT GOI − GAPDH | ΔΔCT ΔCT − ΔCTMCS | Fold change relative to MCS |
|---|---|---|---|---|---|---|
| Pitpn (U96726) | FIVMCS | 25.02 ± 0.80 | 19.18 ± 0.18 | 5.84 ± 0.82 | 0.00 ± 0.82 | 1.0 |
| FIVβgluc | 20.07 ± 0.75 | 21.69 ± 0.46 | −0.99 ± 0.89 | −6.83 ± 0.88 | 114.04 | |
| Fe65 (P46933) | FIVMCS | 28.62 ± 0.52 | 19.18 ± 0.18 | 9.44 ± 0.55 | 0.00 ± 0.55 | 1.0 |
| FIVβgluc | 26.99 ± 0.77 | 21.69 ± 0.46 | 5.30 ± 0.90 | −4.14 ± 0.90 | 17.67 | |
| C/EBPδ (X61800) | FIVMCS | 31.71 ± 0.32 | 19.18 ± 0.18 | 12.53 ± 0.37 | 0.00 ± 0.37 | 1.0 |
| FIVβgluc | 29.88 ± 0.87 | 21.69 ± 0.46 | 8.19 ± 0.99 | −4.35 ± 0.99 | 20.30 | |
| Egr2 (X06746) | FIVMCS | 22.35 ± 0.35 | 19.18 ± 0.18 | 3.17 ± 0.39 | 0.00 ± 0.39 | 1.0 |
| FIVβgluc | 21.15 ± 0.51 | 21.69 ± 0.46 | −0.54 ± 0.69 | −3.71 ± 0.69 | 13.12 |
TaqMan chemistry was used to measure gene expression by means of real-time quantitative PCR (QPCR; Materials and Methods). A comparative CT method allowed calculation of relative changes in gene expression of mice treated with FIVβgluc vs. FIVMCS. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a reference for all comparative analyses because microarray data revealed statistical consistency throughout experimental conditions and replicates. t tests on the replicates of each sample achieved P < 0.001 or better for every sample.
Results and Discussion
Evaluation of FIV Accessory Proteins for Gene Delivery to the CNS.
FIV is distantly related to HIV. This nonprimate lentivirus does not replicate in human cells, and there has been no evidence of seroconversion among individuals exposed to FIV through repeated exposure by infected cats (29). FIV has two accessory proteins, Vif and Orf2. FIV Vif is the functional equivalent of HIV Vif, and is required for a productive infection in certain feline cells, for example, peripheral blood mononuclear cells (30, 31). FIV Orf2 is a weak transactivator of the FIV long terminal repeat (LTR), and therefore functionally similar to HIV Tat (32). We first determined whether Vif and Orf2 were required for gene transfer to mouse brain; vectors devoid of accessory proteins would be preferable if they are as functional as the parental vector in vivo (20). Because the FIV-based system uses a hybrid CMV/FIV LTR, we hypothesized that Orf2 would be dispensable, similar to that found in vitro (20). Recombinant FIV vectors expressing the reporter gene encoding Escherichia coli β-galactosidase were generated by using the packaging constructs shown in Fig. 1A (20) and were injected into the striatum of mice. Full coronal sections were evaluated for β-galactosidase activity by histochemistry from 3 (n = 6; not shown) to 18 weeks (n = 8; Fig. 1B). In all cases, intrastriatal injections resulted in β-galactosidase activity in the ipsilateral striatum, corpus callosum, and the neocortex. Results with FIVβgalΔvifΔorf2 were not significantly different from FIVβgalwt (P > 0.05; Fig. 1C) as regards volume of transgene-positive brain (8.8 ± 2.4% and 9.7 ± 2.6% positive for FIVβgalΔvifΔorf2 and FIVβgalwt, respectively). Also, there was no decline in the β-galactosidase expression over time (8.8 ± 2.4% vs. 7.8 ± 1.7% positive at 3 and 18 weeks, respectively). Consistent with experiments with HIV-based vectors in rats (23), we found that the FIVβgal pseudotyped with the vesicular stomatitis virus (VSV)-G envelope readily transduced neuronal cells (Fig. 1D), with occasional glial fibrillary acidic protein, β-galactosidase double-positive (glial) cells also noted (Fig. 1E). Neuronal tropism was also found by using the amphotropic envelope from Moloney murine leukemia virus (data not shown).
Figure 1.
FIV accessory proteins are not required for transduction of rodent CNS. FIV vectors encoding β-galactosidase containing both or neither of the Vif and Orf2 accessory proteins were generated as described (Materials and Methods) and injected into mice striata. (A) FIV packaging, vector, and envelope constructs. (B) Photomicrograph of a representative section stained for β-galactosidase activity. Mice were injected with FIVβgalΔvifΔorf2 18 weeks earlier. (C) The volume of β-galactosidase expression in FIV-injected hemispheres. (D) A representative confocal photomicrograph of the injected striatum after immunohistochemical staining for β-galactosidase (green) and NeuN (red) antigens. Cell soma colabeled for β-galactosidase and NeuN appear yellow in this merged image. (E) Occasional transduced glia could be identified in sections stained for glial fibrillary acid protein (GFAP, red) and β-galactosidase (green; arrowhead).
FIV-Mediated Gene Transfer of β-Glucuronidase to MPS VII Mice.
There are several important criteria for efficacy of FIV-mediated gene therapy to brain for treatment of the LSDs. First, does FIV-mediated gene transfer lead to adequate levels of β-glucuronidase expression for secretion and uptake into nearby nontransduced cells, and does the expressed enzyme lead ultimately to evidence of enzyme activity at sites distant from the injection? Second, is expression sufficient for reduction of the notable lysosomal pathology in affected brain in transduced and nontransduced cells? Finally, do FIV vectors expressing β-glucuronidase have a favorable impact on the disease course when introduced into animals with evident disease pathology and functional behavioral deficits? We first tested whether unilateral injection to the brains of 8-week-old MPS VII mice with the Vif- and Orf2-deleted FIVβgluc (Fig. 1A; n = 6) resulted in transduction of cells near the injection site. Although in situ hybridization for vector-encoded β-glucuronidase showed focal transduction (Fig. 2A), histological assay for β-glucuronidase (4) showed that levels of activity extended well beyond the area of transduced cells (Fig. 2B), with 18.1% ± 2.5% of the hemisphere positive for enzyme activity 18 weeks after vector introduction. In tissues from mice killed 3 weeks after gene transfer, histological correction of storage pathology was observed in the ipsilateral striatum and ipsilateral cortex, and modest reductions in storage product were seen in the contralateral cortex (data not shown). Six weeks after injection of FIVβgluc, there was notable correction of cellular morphology in regions of the injected (not shown) and contralateral hemispheres of the brain (Fig. 2 D, F, and H), as compared with the lysosome-laden cortical (Fig. 2C), striatal (Fig. 2E), or hippocampal (Fig. 2G) tissues from control MPS VII mice. The absence of lysosomal inclusions was maintained through the course of the study (18 weeks), supporting the hypothesis that persistent expression of β-glucuronidase from FIV-transduced cells, ≈2–5% of which may be secreted (13), resulted in correction of cells at increasing distances over time.
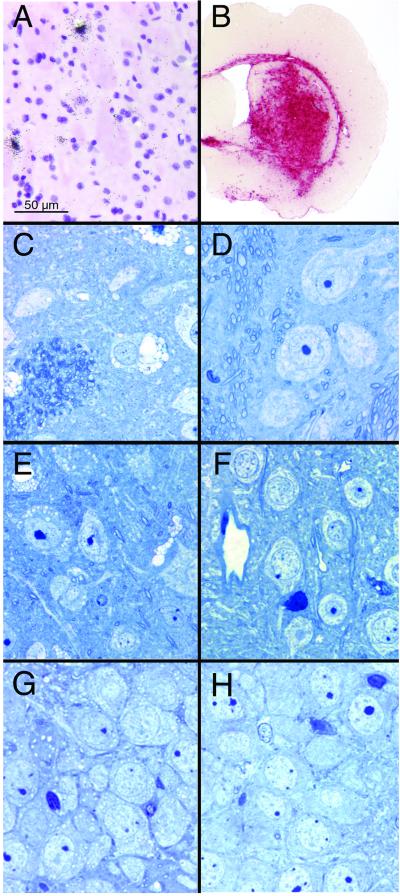
Figure 2.
β-Glucuronidase expression after FIV-mediated gene transfer. (A) Transgene-positive cells near the region of the injection as revealed by in situ RNA analyses. (B) β-Glucuronidase activity in the brain of a MPS VII mouse injected with FIVβgluc and stained for β-glucuronidase activity (dark red reaction product). (C, E, and G) Representative examples of the lysosomal storage in the striatum (C), cortex (E) and hippocampus (G) of 8- to 12-week-old MPS VII mice. (D, F, and H) Correction of the storage defect in the contralateral striatum (D), cortex (F) and hippocampus (H) 6 weeks after injection of FIVβgluc into an 8-week-old MPS VII mouse.
β-Glucuronidase-deficient mice have storage pathology in the eye and brain by several weeks after birth, leading to progressive decline in retinal and neuronal function as measured by electroretinograms or the Morris water maze, respectively (13, 33). By 8 weeks of age, there is sufficient motor impairment precluding further use of the Morris water maze. Thus, to test for functional recovery resulting from introduction of FIVβgluc into diseased mouse brain, the RAPC was used (24–26). The RAPC is a hippocampus-dependent paradigm that is capable of differentiating between spatial learning and other effects such as motor, motivational, and sensory disturbances that can confound analyses of spatial learning. Importantly, this testing paradigm allows for same-animal comparisons of pretreatment vs. posttreatment behavior in an environment devoid of strenuous physical activity.
Baseline testing.
Behavioral testing using the RAPC was initiated on β-glucuronidase-deficient and age-matched heterozygous control littermates at 8 weeks of age, a time at which brain pathology is evident, to define baseline learning and performance abilities (Fig. 3A; Materials and Methods). Both latency (the time to reach the goal) and total errors (the number of mistakes made) were measured. In sessions 1 and 2, performed at 8 and 8.5 weeks of age, respectively, both MPS VII and control mice (n = 8 per group) showed reductions in the time to reach the goal box from the first to the last trial of each session (Fig. 3B). Similar results were seen for numbers of errors (Fig. 3C). However, the MPS VII mice showed significant impairments in learning relative to control mice (Fig. 3 B and C; P = 0.0001, P = 0.029 for latency and errors, respectively). At 13 and 13.5 weeks of age, β-glucuronidase-deficient mice showed further impairments in behavior, with increased latency and numbers of errors (sessions 3 and 4; Fig. 3 B and C). There was no evidence of impaired behavior of the control mice. Additionally, the relative difference in the performance component as compared with the learning component for either group remained unchanged (P > 0.05).
Behavioral assays after FIV-mediated gene transfer.
MPS VII and control mice were injected bilaterally at 18.5 weeks of age (n = 4 per group) with FIVβgluc or FIVβgal after baseline testing, with behavioral reassessment initiated 4 weeks later. β-Glucuronidase-deficient mice transduced with FIVβgluc showed dramatic improvements in latencies and errors to levels indistinguishable from controls (Fig. 3 F and G; P > 0.05). By contrast, FIVβgal-injected MPS VII mice continued to demonstrate severe learning impairments in error and latency measures relative to control mice (Fig. 3 D and E; P = 0.0003 and P = 0.0019 for error and latency, respectively). The data in Fig. 3 F and G, in concert with those shown in Fig. 2, suggest that therapies leading to reduction of storage pathology can significantly improve established learning deficits.
Molecular Correlates for Improved Pathology and Behavior.
We next asked whether alterations in gene expression consistent with improvements in learning and memory or improvements in lysosomal function occurred as a result of FIVβgluc gene transfer. MPS VII mice (16 weeks of age) received intrastriatal injections of FIVβgluc or FIVMCS, a vector that expresses no transgene (n = 8 per group). FIVMCS, rather than FIVβgal, was used to avoid potential confounding effects caused by β-galactosidase expression. Eight weeks later, mice were killed, and RNA was isolated from dissected hippocampi. mRNAs were analyzed by using Affymetrix high-density oligonucleotide arrays.
After FIVβgluc injection into striatum, 93% of the genes and expressed sequence tags represented on the array did not change significantly relative to empty vector, or were not expressed, whereas 3% and 4% were up- or down-regulated greater than 2.5-fold, respectively (Fig. 4A). A scatter plot of average difference values between treatment groups illustrates the relative distribution of gene expression for the data set (Fig. 4B). Raw image data for pitpn, which encodes the α isoform of phosphatidylinositol transfer protein (PITPα), are shown in Fig. 4C. Reductions in pitpn expression occur in the mouse degeneration mutant vibrator (34), suggesting a requirement for PITPα in maintenance of neuronal function. In our studies, pitpn expression was increased approximately 137-fold relative to control tissues (Fig. 4D). QPCR validation studies confirmed elevated levels of mRNA specific to pitpn in isolated hippocampi (Table 1; refs. 28 and 35).
Although animals were killed 8 weeks after gene transfer, we found elevated expression of several genes implicated in learning and memory relative to FIVMCS-injected controls (Fig. 4D, Table 1). C/EBPδ has been shown to increase as a result of stimulation of cAMP signaling in hippocampal neurons (36, 37). In FIVβgluc-transduced mice, the expression of C/EPBδ was elevated 5.6- and 20-fold by microarray and QPCR analysis, respectively. Egr2 (Krox 20), another immediate early gene implicated in learning and memory (38), was elevated approximately 10-fold by QPCR. Egr2 is also important for peripheral nerve myelination (39). Expression of Cyr61, an extracellular matrix protein found in the human CNS, was shown to be induced on stimulation of m1 and m3 muscarinic acetylcholine receptors (40). We found a 3.8-fold increase in hippocampal Cyr61 mRNA.
Fe65 interacts with the γ-secretase-liberated tail of amyloid precursor protein (APP), and with the histone acetyltransferase Tip60 (41). Recent studies by Greengard and colleagues (42) showed that Fe65 also interacts with APP at the plasma membrane to foster axonal migration. In our studies, we noted significant elevations in Fe65 (Fig. 4D and Table 1). The role of Fe65 after FIVβgluc-mediated gene transfer is intriguing, and may imply enhanced neurite outgrowth and improved synaptic function. In support of this hypothesis, Sec8 expression was increased 9.6-fold (Fig. 4D). Sec8 is localized to regions of neurite outgrowth and is required for synaptogenesis (43).
Secondary elevations in lysosomal enzyme activity occurs in LSDs, and gene or enzyme therapy for the deficiency often normalizes these levels. For β-glucuronidase deficiency, enzyme replacement can reduce elevated α-galactosidase, β-galactosidase, and β-hexosaminidase levels (8, 11). It is currently unknown whether these secondary changes occur as a result of increased transcription or decreased protein degradation. We found that although reductions in RNA specific to β-hexosaminidase and α-galactosidase did not change greater than 2.5-fold, cathepsin L and α-mannosidase were significantly reduced (Fig. 4D). Whether this observation is reflective of the entire injected hemisphere, or only the harvested hippocampus, was not tested.
The combined results show that β-glucuronidase replacement reversed the severe neurological deficit in mice with established brain lysosomal storage disease. The data show that neuronal impairment has not occurred in aged MPS VII mice to a degree that function cannot be recovered. Before these studies, efficacy after gene, cell, or enzyme therapy to adult animal models with existing disease was assessed by clearance of the characteristic lysosomal distention observed within multiple cell types. We demonstrate that a functional assay of learning and memory is a more appropriate endpoint as we progress in the evaluation of vectors to treat human CNS disorders. Finally, our gene expression analyses using high-density oligonucleotide arrays and QPCR implied that β-glucuronidase treatment improved CNS function in manners beyond simple reconstitution of enzyme levels.
Acknowledgments
We thank Inês Martins, Qinwen Mao, Phil Sheridan, Kim Wahoski, and Christine McLennan for assistance, and Michael J. Welsh for critical discussions. This work was supported by National Institutes of Health Grants NS34568, DK54759, HD33531 (to B.L.D.), and MH57047 (to H.J.F.), and the Roy J. Carver Foundation (to B.L.D.).
Abbreviations
- MPS VII
Mucopolysaccharidoses type VII
- FIV
feline immunodeficiency virus
- LSD
lysosomal storage disease
- CNS
central nervous system
- TU
transducing units
- CMV
cytomegalovirus
- RAPC
repeated acquisition and performance chamber
- RA
repeated acquisition
- P
performance
- QPCR
quantitative real time PCR
- MCS
multiple cloning site
- RSV
Rous sarcoma virus
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 5760.
References
- 1.Sly W S, Quinton B A, McAlister W H, Rimoin D L. J Pediatr. 1973;82:249–257. doi: 10.1016/s0022-3476(73)80162-3. [DOI] [PubMed] [Google Scholar]
- 2.Birkenmeier E H, Davisson M T, Beamer W G, Ganschow R E, Vogler C A, Gwynn B, Lyford K A, Maltais L M, Wawrzyniak C J. J Clin Invest. 1989;83:1258–1266. doi: 10.1172/JCI114010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sands M S, Birkenmeier E H. Proc Natl Acad Sci USA. 1993;90:6567–6571. doi: 10.1073/pnas.90.14.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghodsi A, Stein C, Derksen T, Yang G, Anderson R D, Davidson B L. Hum Gene Ther. 1998;9:2331–2340. doi: 10.1089/hum.1998.9.16-2331. [DOI] [PubMed] [Google Scholar]
- 5.Stein C S, Ghodsi A, Derksen T, Davidson B L. J Virol. 1999;73:3424–3429. doi: 10.1128/jvi.73.4.3424-3429.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghodsi A, Stein C, Derksen T, Martins I, Anderson R D, Davidson B L. Exp Neurol. 1999;160:109–116. doi: 10.1006/exnr.1999.7205. [DOI] [PubMed] [Google Scholar]
- 7.Daly T M, Vogler C, Levy B, Haskins M E, Sands M S. Proc Natl Acad Sci USA. 1999;96:2296–2300. doi: 10.1073/pnas.96.5.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosch A, Perret E, Desmaris N, Trono D, Heard J M. Hum Gene Ther. 2000;11:1139–1150. doi: 10.1089/10430340050015194. [DOI] [PubMed] [Google Scholar]
- 9.Snyder E Y, Taylor R M, Wolfe J H. Nature (London) 1995;374:367–370. doi: 10.1038/374367a0. [DOI] [PubMed] [Google Scholar]
- 10.Lazarus H S, Sly W S, Kyle J W, Hageman G S. Exp Eye Res. 1993;56:531–541. doi: 10.1006/exer.1993.1067. [DOI] [PubMed] [Google Scholar]
- 11.Vogler C, Sands M, Higgins A, Levy B, Grubb J, Birkenmeier E H, Sly W S. Pediatr Res. 1993;34:837–840. doi: 10.1203/00006450-199312000-00028. [DOI] [PubMed] [Google Scholar]
- 12.Birkenmeier E H, Barker J E, Vogler C A, Kyle J W, Sly W S, Gwynn B, Levy B, Pegors C. Blood. 1991;78:3081–3092. [PubMed] [Google Scholar]
- 13.O'Connor L H, Erway L C, Vogler C A, Sly W S, Nicholes A, Grubb J, Holmberg S W, Levy B, Sands M S. J Clin Invest. 1998;101:1394–1400. doi: 10.1172/JCI1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sands M S, Vogler C, Torrey A, Levy B, Gwynn B, Grubb J, Sly W S. J Clin Invest. 1997;99:1596–1605. doi: 10.1172/JCI119322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frisella W A, O'Connor L H, Vogler C A, Roberts M, Walkley S, Levy B, Daly T M, Sands M S. Mol Ther. 2001;3:351–358. doi: 10.1006/mthe.2001.0274. [DOI] [PubMed] [Google Scholar]
- 16.Consiglio A, Quattrini A, Martino S, Bensadoun J C, Dolcetta D, Trojani A, Benaglio G, Marchesini S, Cestari V, Oliverio A, et al. Nat Med. 2001;7:310–316. doi: 10.1038/85454. [DOI] [PubMed] [Google Scholar]
- 17.Meikle P J, Hopwood J J, Clague A E, Carey W F. J Am Med Assoc. 1999;281:249–254. doi: 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- 18.Skorupa A F, Fisher K J, Wilson J M, Parente M K, Wolfe J H. Exp Neurol. 1999;160:17–27. doi: 10.1006/exnr.1999.7176. [DOI] [PubMed] [Google Scholar]
- 19.Franklin K B J, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic; 1997. pp. 1–190. [Google Scholar]
- 20.Johnston J C, Gasmi M, Lim L E, Elder J H, Yee J-K, Jolly D J, Campbell K P, Davidson B L, Sauter S L. J Virol. 1999;73:4991–5000. doi: 10.1128/jvi.73.6.4991-5000.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davidson B L, Doran S E, Shewach D S, Latta J M, Hartman J W, Roessler B J. Exp Neurol. 1994;125:258–267. doi: 10.1006/exnr.1994.1028. [DOI] [PubMed] [Google Scholar]
- 22.Yee J K, Miyanohara A, LaPorte P, Bouic K, Burns J C, Friedman T. Proc Natl Acad Sci USA. 1994;91:9564–9568. doi: 10.1073/pnas.91.20.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blömer U, Naldini L, Kafri T, Trono D, Verma I M, Gage F H. J Virol. 1997;71:6641–6649. doi: 10.1128/jvi.71.9.6641-6649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brooks A I, Cory-Slechta D A, Murg S M, Federoff H J. Neurobiol Learn Mem. 2000;74:241–258. doi: 10.1006/nlme.1999.3951. [DOI] [PubMed] [Google Scholar]
- 25.Brooks A I, Cory-Slechta D A, Bowers W J, Murg S L, Federoff H J. Hum Gene Ther. 2000;11:2341–2352. doi: 10.1089/104303400750038453. [DOI] [PubMed] [Google Scholar]
- 26.Brooks A I, Cory-Slecta D A, Federoff H J. Proc Natl Acad Sci USA. 2000;97:13378–13383. doi: 10.1073/pnas.230169397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tusher V G, Tibshirani R, Chu G. Proc Natl Acad Sci USA. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.PE Applied Biosystems. User Bulletin #2. ABI Prism 7700 Sequence Detection System. 1997. , Technical Notes (Perkin–Elmer, St. Pete Beach, FL), pp. 1–35. [Google Scholar]
- 29.Yamamoto J K, Hansen H, Ho E W, Morishita T Y, Okuda T, Sawa T R, Nakamura R M, Pedersen N C. J Am Vet Med Assoc. 1989;194:213–220. [PubMed] [Google Scholar]
- 30.Shacklett B L, Luciw P A. Virology. 1994;204:860–867. doi: 10.1006/viro.1994.1609. [DOI] [PubMed] [Google Scholar]
- 31.Tomonaga K, Norimine J, Shin Y S, Fukasawa M, Miyazawa T, Adachi A, Toyosaki T, Kawaguchi Y, Kai C, Mikami T. J Virol. 1992;66:6181–6185. doi: 10.1128/jvi.66.10.6181-6185.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Parseval A, Elder J H. J Virol. 1999;73:608–617. doi: 10.1128/jvi.73.1.608-617.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bastedo L, Sands M S, Lambert D T, Pisa M A, Birkenmeier E, Chang P L. J Clin Invest. 1944;94:1180–1186. doi: 10.1172/JCI117434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamilton B A, Smith D J, Mueller K L, Kerrebrock A W, Bronson R T, van Berkel V, Daly M J, Kruglyak L, Reeve M P, Nembauser J L, et al. Neuron. 1997;18:711–722. doi: 10.1016/s0896-6273(00)80312-8. [DOI] [PubMed] [Google Scholar]
- 35.Bustin S A. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 36.Taubenfeld S M, Wiig K A, Monti B, Dolan B, Pollonini G, Alberini C M. J Neurosci. 2001;21:84–91. doi: 10.1523/JNEUROSCI.21-01-00084.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yukawa K, Tanaka T, Tsuji S, Akira S. J Biol Chem. 1998;273:31345–31351. doi: 10.1074/jbc.273.47.31345. [DOI] [PubMed] [Google Scholar]
- 38.Pearse D, Mirza A, Leah J. Brain Res. 2001;894:193–208. doi: 10.1016/s0006-8993(01)01993-x. [DOI] [PubMed] [Google Scholar]
- 39.Nagarajan R, Svaren J, Le N, Araki T, Watson M. Neuron. 2001;30:355–368. doi: 10.1016/s0896-6273(01)00282-3. [DOI] [PubMed] [Google Scholar]
- 40.Albrecht C, von der Kammer H, Mayhaus M, Klaudiny J, Schweizer M, Nitsch R M. J Biol Chem. 2000;275:28929–28936. doi: 10.1074/jbc.M003053200. [DOI] [PubMed] [Google Scholar]
- 41.Cao X, Südhof T C. Science. 2001;293:115–120. doi: 10.1126/science.1058783. [DOI] [PubMed] [Google Scholar]
- 42.Sabo S L, Ikin A F, Buxbaum J D, Greengard P. J Cell Biol. 2001;153:1403–1414. doi: 10.1083/jcb.153.7.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hazuka C D, Foletti D L, Hsu S C, Kee Y, Hopf F W, Scheller R H. J Neurosci. 1999;19:1324–1334. doi: 10.1523/JNEUROSCI.19-04-01324.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]