Abstract
BACKGROUND & AIMS:
Hepatocellular carcinoma (HCC) is a leading cause of cancer death. HCC is preventable with about 70% of HCC attributable to modifiable risk factors. Glucagon-like peptide-1 receptor agonists (GLP-1RAs), Food and Drug Administration–approved medications for treating type 2 diabetes mellitus (T2DM), have pleiotropic effects on counteracting risk factors for HCC. Here we evaluate the association of GLP-1RAs with incident HCC risk in a real-world population.
METHODS:
This retrospective cohort included 1,890,020 patients with a diagnosis of T2DM who were prescribed GLP-1RAs or other non-GLP-1RA anti-diabetes medications and had no prior diagnosis of HCC. Incident (first-time) diagnosis of HCC and hepatic decompensating events during a 5-year follow-up was compared between cohorts of patients prescribed GLP-1 RAs vs other anti-diabetes medications. Time-to-first-event analysis was performed using Kaplan-Meier survival analysis with hazard ratio and 95% confidence interval calculated.
RESULTS:
GLP-1RAs were associated with a lower risk of incident HCC with hazard ratio of 0.20 [0.14–0.31], 0.39 [0.21–0.69], 0.63 [0.26–1.50] compared with insulin, sulfonylureas, and metformin, respectively. GLP-1RAs were associated with a significantly lower risk of hepatic decompensation compared with 6 other anti-diabetes medications. Reduced risks were observed in patients without and with different stages of fatty liver diseases, with more profound effects in patients without liver diseases. Similar findings were observed in patients with and without obesity and alcohol or tobacco use disorders. GLP-1RA combination therapies were associated with decreased risk for HCC and hepatic decompensations compared with monotherapies.
CONCLUSIONS:
GLP-1RAs were associated with a reduced risk of incident HCC and hepatic decompensation compared with other anti-diabetes medications in patients with T2DM. These findings provide supporting evidence for future studies to investigate the underlying mechanisms and their clinical use.
Keywords: Hepatocellular Carcinoma, Glucagon-Like Peptide-1 Receptor Agonists, Type 2 Diabetes Mellitus, Cancer Prevention, Real-World Evidence
Liver cancer, among which 72% is hepatocellular carcinoma (HCC), is the sixth most common cancer and the third leading cause of cancer deaths worldwide.1 In the United States, the incidence of liver cancer has tripled over the past 4 decades.2 Each year in the United States, about 25,000 men and 11,000 women get liver cancer, and about 19,000 men and 9000 women die from the disease.3 About 70% of HCC in the United States is attributable to modifiable risk factors including type 2 diabetes (T2DM), obesity, alcohol or tobacco use disorders, metabolic dysfunction-associated steatotic liver disease (MASLD), and metabolic dysfunction-associated steatohepatitis (MASH).4–6 Glucagon-like peptide-1 receptor agonists (GLP-1RAs) are medications approved by the Food and Drug Administration for treating T2DM. GLP-1RAs have pleiotropic effects on lowering plasma glucose, inducing weight loss, reducing desire for alcohol drinking and tobacco smoking, and modulating immune functions.7–9 GLP-1 receptors are present in human hepatocytes and have direct roles in decreasing nonalcoholic fatty liver disease.10–13 However, there are no clinical studies to investigate the potential benefits of GLP-1RAs in reducing HCC risk. Because GLPRAs reduce major modifiable risk factors for HCC, we hypothesized that GLP-1RAs are associated with reduced HCC incidence and hepatic decompensation. Here we used an electronic health record (EHR) analytic platform to conduct a nationwide multicenter retrospective cohort study in patients with T2DM who were prescribed GLP-1RAs vs non-GLP-1 RA anti-diabetes medications to determine whether GLP-1RAs were associated with changes in HCC incidence and hepatic decompensation. Separate analyses were performed in patients with and without obesity, with different stages of liver diseases (MASLD, MASH, or liver fibrosis/cirrhosis), and in patients with and without alcohol or tobacco use disorders. We further examined whether combination therapies with GLP-1RAs provided additional benefits compared with monotherapies.
Methods
Database
We used the TriNetX platform to access de-identified EHRs of 111.7 million patients from 63 health care organizations across 50 states, covering diverse age, race/ethnic, income, and insurance groups and clinical settings.14 TriNetX built-in analytic functions allow patient-level analyses, while only reporting population-level data. The MetroHealth System institutional review board determined that the research as described in this study was not human subject research and institutional review board approval was not required. TriNetX platform has been used for retrospective cohort studies15–26 including cancer cohort studies.19,26 Similar to this study, we have examined the association of GLP-1RAs with colorectal cancer incidence in patients with T2DM27 and the associations of GLP-1RA (semaglutide) with suicidal ideations28 and with cannabis use in patients with obesity and patients with T2DM.29
Study Population
The study population comprised 1,890,020 patients with a diagnosis of T2DM who were prescribed GLP-1RAs or other non-GLP-1RA anti-diabetes medications (insulin, metformin, dipeptidyl-peptidase-4 (DPP-4) inhibitors, sodium-glucose cotransporter-2 (SGLT2) inhibitors, sulfonylureas, thiazolidinediones) from January 2013 to February 2019 and had no HCC diagnosis before the medication prescription. The study population was divided into exposure and comparison cohorts for each comparison. For comparing GLP-1RAs with insulins, the study population was divided into the following: GLP-1RA(+)Insulin(−) cohort: 47,578 patients prescribed GLP-1RA but not insulins from January 2010 to February 2019, and Insulin(+)GLP-1RA(–) cohort: 1,148,166 patients prescribed insulin from January 2010 to February 2019 and never prescribed GLP-1RAs. Similar designs were used for comparing GLP-1RAs with metformin, DPP-4 inhibitors, SGLT2 inhibitors, sulfonylureas, and thiazolidinediones. Other non-GLP-1RA anti-diabetes medication classes were not examined because of limited sample sizes.
Cohorts were propensity score matched (1:1 using nearest neighbor greedy matching) for risk factors for HCC1,5,6 including demographics (age, gender, race, ethnicity), adverse socioeconomic determinants of health (housing and economic circumstances, upbringing, education, physical environment, social environment), lifestyle factors (exercise, diet, smoking, alcohol drinking), family history of cancers, genetic susceptibility to cancer, obesity (3 International Classification of Diseases, 10th Revision [ICD-10] diagnosis codes and 15 body mass index [BMI] categories ranging from BMI 30 to BMI 70 or greater), T2DM complications (10 different categories), hypertension, alcohol use disorders, benign neoplasm of liver, chronic hepatitis, MASLD, MASH, liver fibrosis, and cirrhosis; and procedures including cancer screening, bariatric surgery, atherosclerotic cardiovascular diseases including disorders of lipoprotein metabolism and other lipidemias, heart diseases, cerebrovascular diseases, and peripheral artery disease; and prior prescription of anti-diabetes medications, aspirin, statins, and nonsteroidal anti-inflammatory analgesics (details in Table 1 and Supplementary Table 1). Two outcomes were examined: first-time diagnosis of HCC (ICD-10 code C22.0 “liver cell carcinoma”)30 and hepatic decompensation events (ascites, spontaneous bacterial peritonitis, hepatic encephalopathy, esophageal varices).31 Patients were followed for 5 years after the index event or time zero (the prescription of GLP-1RA for the exposure cohort vs the prescription of other non-GLP-1RA anti-diabetes medications for the comparison cohorts that occurred in January 2010 to February 2019). The outcomes were compared between the propensity score matched GLP-1RAs and non-GLP-1RA anti-obesity medications cohorts. Kaplan-Meier analysis was used to estimate the probability of outcome at daily time intervals with censoring applied. When the last fact (the outcome of interests, death, or other medical encounters) in the patient’s record is in the time window for analysis, the patient was censored on the day after the last fact in the record. Hazard ratios (HRs) and 95% confidence intervals (95% Cis) were used to describe the relative hazard of the outcomes based on a comparison of time to event rates.
Table 1.
Characteristics of the GLP-1RA(+)Insulin(−) and Insulin(+)GLP-1RA(−) Cohorts Before and After Propensity Score Matching
| Before propensity score matching |
After propensity score matching |
|||||
|---|---|---|---|---|---|---|
| GLP-1RA(+) insulin(−) | Insulin(+) GLP-1RA(−) | SMD | GLP-1RA(+) Insulin(−) | Insulin(+) GLP-1RA(−) | SMD | |
| Total number | 47,578 | 1,148,166 | 46,470 | 46,470 | ||
|
| ||||||
| Age at index event, y, mean ± SD | 56.0 ± 11.9 | 62.1 ± 15.8 | 0.44a | 56.1 ± 11.9 | 56.3 ± 14.7 | 0.01 |
|
| ||||||
| Sex (%) | ||||||
| Female | 54.7 | 46.5 | 0.16a | 54.6 | 56.2 | 0.03 |
| Male | 41.5 | 50.7 | 0.19a | 41.8 | 40.3 | 0.03 |
| Unknown | 3.8 | 2.8 | 0.06 | 3.7 | 3.5 | 0.008 |
|
| ||||||
| Ethnicity, % | ||||||
| Hispanic/Latinx | 9.1 | 8.9 | 0.008 | 9.0 | 9.0 | 0.001 |
| Not Hispanic/Latinx | 66.0 | 64.6 | 0.03 | 66.1 | 66.4 | 0.007 |
|
| ||||||
| Unknown | 24.8 | 26.5 | 0.04 | 24.9 | 24.6 | 0.006 |
|
| ||||||
| Race, % | ||||||
| American Indian or Alaska Native | 0.5 | 0.5 | 0.004 | 0.5 | 0.5 | 0.004 |
| Asian | 2.5 | 3.7 | 0.07 | 2.5 | 2.4 | 0.006 |
| Black | 13.4 | 17.4 | 0.11a | 13.5 | 12.7 | 0.02 |
|
| ||||||
| Native Hawaiian or other Pacific Islander | 0.4 | 0.9 | 0.06 | 0.4 | 0.4 | 0.01 |
| White | 66.4 | 61.8 | 0.09 | 66.3 | 67.4 | 0.02 |
| Unknown | 13.9 | 12.6 | 0.04 | 13.9 | 13.8 | 0.001 |
|
| ||||||
| Adverse socioeconomic determinants of health, % | 1.7 | 1.3 | 0.03 | 1.7 | 1.6 | 0.005 |
|
| ||||||
| Problems related to lifestyle, % | 3.4 | 2.1 | 0.08 | 3.2 | 3.2 | 0.001 |
|
| ||||||
| Family history of cancer | 5.1 | 2.6 | 0.13a | 4.9 | 4.8 | 0.005 |
|
| ||||||
| Genetic susceptibility to cancer | 0.1 | 0.1 | 0.02 | 0.1 | 0.1 | 0.001 |
|
| ||||||
| Obesity categories, % | ||||||
| Morbid (severe) obesity due to excess calories | 18.3 | 7.1 | 0.34a | 17.7 | 17.4 | 0.008 |
| Obesity, unspecified | 30.3 | 12.7 | 0.44a | 29.5 | 29.1 | 0.007 |
| Other obesity due to excess calories | 2.9 | 0.5 | 0.19a | 2.5 | 2.4 | 0.01 |
| BMI 30.0–30.9 | 1.4 | 0.7 | 0.06 | 1.3 | 1.1 | 0.02 |
| BMI 31.0–31.9 | 1.5 | 0.7 | 0.07 | 1.3 | 1.2 | 0.009 |
| BMI 32.0–32.9 | 1.6 | 0.7 | 0.08 | 1.5 | 1.3 | 0.01 |
| BMI 33.0–33.9 | 1.6 | 0.7 | 0.08 | 1.5 | 1.5 | 0.002 |
| BMI 34.0–34.9 | 1.8 | 0.7 | 0.09 | 1.7 | 1.6 | 0.007 |
| BMI 35.0–35.9 | 2.1 | 0.7 | 0.12a | 1.9 | 1.8 | 0.007 |
| BMI 36.0–36.9 | 1.9 | 0.7 | 0.11a | 1.8 | 1.7 | 0.005 |
| BMI 37.0–37.9 | 1.8 | 0.6 | 0.11a | 1.7 | 1.6 | 0.003 |
| BMI 38.0–38.9 | 1.8 | 0.6 | 0.11a | 1.6 | 1.6 | 0.004 |
| BMI 39.0–39.9 | 1.3 | 0.5 | 0.09 | 1.2 | 1.2 | 0.009 |
| BMI 40.0–44.9 | 5.3 | 1.8 | 0.19a | 4.9 | 4.5 | 0.02 |
| BMI 45.0–49.9 | 3.1 | 1.0 | 0.15a | 2.9 | 2.8 | 0.005 |
| BMI 50.0–59.9 | 2.2 | 0.8 | 0.12a | 2.0 | 1.9 | 0.008 |
| BMI 60.0–69.9 | 0.6 | 0.2 | 0.06 | 0.6 | 0.6 | 0.002 |
| BMI ≥70 | 0.2 | 0.1 | 0.03 | 0.2 | 0.2 | 0.01 |
|
| ||||||
| T2DM categories, % | ||||||
| Type 2 diabetes mellitus without complications | 75.1 | 58.1 | 0.37a | 74.6 | 74.4 | 0.005 |
| Type 2 diabetes mellitus with hyperglycemia | 29.8 | 17.9 | 0.28a | 29.0 | 28.6 | 0.01 |
| Type 2 diabetes mellitus with hyperosmolarity | 0.9 | 0.5 | 0.04 | 0.9 | 0.8 | 0.01 |
| Type 2 diabetes mellitus with ketoacidosis | 0.2 | 0.5 | 0.05 | 0.2 | 0.1 | 0.02 |
| Type 2 diabetes mellitus with kidney complications | 6.6 | 8.7 | 0.08 | 6.6 | 6.0 | 0.02 |
| Type 2 diabetes mellitus with ophthalmic complications | 4.9 | 5.6 | 0.03 | 4.9 | 4.6 | 0.02 |
| Type 2 diabetes mellitus with neurological complications | 10.4 | 9.6 | 0.03 | 10.3 | 9.8 | 0.02 |
| Type 2 diabetes mellitus with circulatory complications | 2.9 | 2.9 | 0.001 | 2.9 | 2.7 | 0.01 |
| Type 2 diabetes mellitus with other specified complications | 32.4 | 20.4 | 0.28a | 31.7 | 30.9 | 0.02 |
| Type 2 diabetes mellitus with unspecified complications | 7.3 | 4.0 | 0.14a | 7.0 | 6.8 | 0.008 |
|
| ||||||
| Pre-existing medical conditions, procedures, medications, % | ||||||
| Alcohol-related disorders | 1.4 | 2.9 | 0.11a | 1.4 | 1.3 | 0.007 |
| Tobacco use disorder | 8.2 | 10.1 | 0.07 | 8.2 | 7.8 | 0.02 |
| Benign neoplasm of liver | 0.1 | 0.1 | 0.008 | 0.1 | 0.1 | 0.002 |
| Chronic viral hepatitis | 0.6 | 1.3 | 0.07 | 0.6 | 0.5 | 0.02 |
| Unspecified viral hepatitis | 0.7 | 1.5 | 0.08 | 0.7 | 0.6 | 0.009 |
| Chronic hepatitis, not elsewhere classified | 0.1 | 0.2 | 0.02 | 0.1 | 0.1 | 0.005 |
| Alcoholic liver disease | 0.2 | 0.7 | 0.09 | 0.2 | 0.2 | 0.002 |
| Fatty (change of) liver, not elsewhere classified (MASLD) | 7.0 | 2.5 | 0.21a | 6.7 | 6.4 | 0.009 |
| Metabolic dysfunction-associated steatohepatitis (MASH) | 1.1 | 0.4 | 0.09 | 1.0 | 1.0 | 0.002 |
| Fibrosis and cirrhosis of liver | 1.1 | 2.0 | 0.07 | 1.0 | 1.0 | 0.006 |
| Hepatic fibrosis | 0.7 | 1.0 | 0.04 | 0.6 | 0.6 | 0.002 |
| Liver cirrhosis | 0.9 | 1.9 | 0.09 | 0.9 | 0.8 | 0.003 |
| Hepatic encephalopathy | 0.0 | 0.1 | 0.04 | 0.0 | 0.0 | 0.006 |
| Esophageal varices | 0.2 | 0.6 | 0.06 | 0.2 | 0.2 | <.001 |
| Spontaneous bacterial peritonitis | 0.0 | 0.1 | 0.03 | 0.0 | 0.0 | <.001 |
| Ascites | 0.4 | 1.6 | 0.12a | 0.4 | 0.3 | 0.01 |
| Hypertension | 61.5 | 54.7 | 0.14a | 61.5 | 61.5 | 0.02 |
| Disorders of lipoprotein metabolism and other lipidemias | 59.0 | 40.6 | 0.37a | 58.2 | 58.1 | 0.003 |
| Ischemic heart diseases | 13.1 | 22.4 | 0.25a | 13.3 | 12.1 | 0.03 |
| Other forms of heart disease | 16.5 | 28.0 | 0.28a | 16.7 | 15.2 | 0.04 |
| Cerebrovascular diseases | 4.8 | 10.9 | 0.23a | 4.9 | 4.4 | 0.03 |
| Diseases of arteries, arterioles and capillaries | 6.7 | 11.2 | 0.16a | 6.7 | 6.2 | 0.02 |
| Bariatric surgery | 1.7 | 0.6 | 0.11a | 1.6 | 1.6 | 0.005 |
| Encounter for screening for malignant neoplasms | 26.2 | 11.9 | 0.37a | 25.6 | 25.4 | 0.004 |
| Insulins | 19.0 | 6.7 | 0.38a | 18.2 | 17.0 | 0.03 |
| Metformin | 58.4 | 22.0 | 0.80a | 57.5 | 57.6 | 0.001 |
| DPP-4 inhibitors | 20.0 | 4.9 | 0.47a | 19.0 | 18.9 | 0.003 |
| SGLT2 inhibitors | 9.8 | 0.7 | 0.42a | 8.3 | 7.7 | 0.02 |
| Sulfonylureas | 29.6 | 13.6 | 0.40a | 29.1 | 29.1 | 0.001 |
| Thiazolidinediones | 8.7 | 3.9 | 0.20a | 8.6 | 8.6 | <.001 |
| Aspirin | 24.9 | 22.4 | 0.06 | 24.9 | 24.0 | 0.02 |
| Nonsteroidal anti-inflammatory analgesics | 15.9 | 9.2 | 0.20a | 15.6 | 15.0 | 0.02 |
| Atorvastatin | 27.6 | 15.0 | 0.31a | 27.0 | 26.3 | 0.02 |
| Lovastatin | 2.1 | 1.6 | 0.04 | 2.1 | 2.0 | 0.004 |
| Pravastatin | 7.7 | 4.5 | 0.13a | 7.5 | 7.5 | 0.004 |
| Rosuvastatin | 8.4 | 3.6 | 0.20a | 8.1 | 8.2 | 0.003 |
| Simvastatin | 16.0 | 11.5 | 0.13a | 16.0 | 15.7 | 0.008 |
NOTE. Shown are cohorts before and after propensity score matching for the listed variables. The status of variables was based on the presence of related clinical codes anytime to 1 day before the index event (prescription of GLP-1RAs or insulins in 1/2010–2/2019).
SD, standard deviation; SMD, standardized mean differences.
SMD greater than 0.1, a threshold indicating cohort imbalance. Adverse socioeconomic determinants of health include housing and economic circumstances, upbringing, education, physical environment, and social environment. Problems with lifestyle included tobacco use, lack of physical exercise, inappropriate diet and eating habits and others.
Insulin is known to be associated with increased risk for HCC.32–34 Because patients with T2DM were often prescribed both insulins and GLP-1RAs or other anti-diabetes medications, insulins could mask the potential beneficial effects of GLP-1RAs. We further compared GLP-1RAs with the other 5 anti-diabetes medications in patients with patients who were not prescribed any insulins after the index event (prescriptions of GLP-1RAs or other anti-diabetes medications).
GLP-1RAs were compared with each of the 6 non-GLP-1RA anti-diabetes medications in separate analyses in patients with T2DM with and without a prior history of obesity. The status of obesity was based on the presence or absence of ICD-10 codes for obesity and related BMI codes (details in Supplementary Table 1) before the index event.
Separate analyses were performed in 3 T2DM patient populations without and with different stages of fatty liver diseases: (1) patients without MASLD, MASH, or liver fibrosis and cirrhosis; (2) patients with MASLD or MASH; and (3) patients with liver fibrosis and cirrhosis. The status of these liver diseases was based on the presence or absence of related ICD-10 codes (details in Supplementary Table 1) before the index event. Because of sample limitations, we did not further examine the outcomes in patients with MASLD and with MASH separately.
Separate analyses were performed in patients with T2DM with and without a prior history of alcohol or tobacco use disorders. The status of alcohol or tobacco use disorders was based on the presence or absence of related ICD-10 codes (details in the Supplementary Table 1) before the index event. Because of sample limitations, we did not further examine the outcomes in patients with alcohol use disorder and tobacco use disorder separately.
We compared the risk of HCC incidence and hepatic decompensation between combination therapies with GLP-1RAs with monotherapies without GLP-1RAs for all 6 medication classes. In addition, combination therapies with GLP-1RAs were also compared with monotherapy of GLP-1RAs.
The data were collected and analyzed on March 4, 2024, within the TriNetX Analytics Platform. Details of clinical codes for defining study populations, outcomes, and confounding variables are in Supplementary Table 1.
Results
Risks of HCC Incidence and Hepatic Decompensating Events in Patients With T2DM: GLP-1 RAs vs Non-GLP-1RA Anti-diabetes Medications
GLP-1RAs were separately compared with each of the 6 non-GLP-1RA anti-diabetes medications (insulins, metformin, DPP-4 inhibitors, SGLT2 inhibitors, sulfonylureas, thiazolidinediones). For comparing GLP-1RAs with insulins, the study population included 1,195,744 patients with T2DM who were prescribed GLP-1RAs or insulins but not from January 2010 to February 2019. The GLP-1RA(+) Insulin(−) cohort compared with the Insulin(+)GLP-1RA(−) cohort was younger, included more women, more White and fewer Black people, had a higher prevalence of obesity, diabetes with hyperglycemia, MAFLD, and prior prescriptions of non-GLP-1RA, statins, and nonsteroidal anti-inflammatory analgesics and anti-diabetes medications. After propensity score matching, the 2 cohorts (46,470 in each, mean age 56.2 years, 55.4% women, 13.1% Black, 66.9% White, 9.0% Hispanic) were balanced (Table 1). The characteristics for comparing GLP-1RA to the other 5 non-GLP-1RA anti-diabetes medication classes were in Supplementary Tables 2–6.
GLP-1RAs were associated with a significantly lower risk for HCC incidence compared with insulin (HR, 0.20; 95% CI, 0.14–0.31) and sulfonylureas (HR, 0.78; 95% CI, 0.65–0.93). No significant difference was observed when comparing GLP-1RAs with metformin, DPP-4 inhibitors, SGLT2 inhibitors, or thiazolidinediones (Figure 1A). However, among patients who were not prescribed insulin after the prescriptions of GLP-1RA or other anti-diabetes medications, GLP-1RAs were associated with a more profound lower risk for HCC compared with sulfonylureas (HR, 0.39; 95% CI, 0.21–0.69). GLP-1RAs were associated with a lower risk of HCC compared with metformin or DPP-4 inhibitors (Figure 1A). However, the difference was not statistically significant, which may be due to the small sample sizes because most patients were prescribed insulins.
Figure 1.

Risk of incident (first-time diagnosis) HCC and hepatic decompensating events in patients with T2DM. (A) Comparison of the risk of incident HCC between propensity score–matched cohorts. (B) Comparison of the risk of hepatic decompensating events between propensity score–matched cohorts. Outcomes were followed for 5 years after the index event (prescription of GLP-1RA vs specific non-GLP-1RA anti-diabetes medication classes [but not both] in 1/2010–2/2019). Hazard rates were calculated using Kaplan-Meier analysis to estimate the probability of outcome at daily time intervals with censoring applied. Overall risk = number of patients with outcomes during the follow-up time window/number of patients in the cohort at the beginning of the time window. A plus sign (+) indicates that a patient was prescribed GLP-1RA or non-GLP-1RA anti-diabetes medication, while a minus sign (−) indicates that they were not. SU, sulfonylureas, TZD, thiazolidinediones.
GLP-1RAs were associated with a significantly lower risk for hepatic decompensating events compared with all 6 non-GLP-1RA anti-diabetes medication classes (Figure 1B). A similar lower risk was observed in patients without insulin prescriptions, although some of the differences were not statistically significant due to limited sample sizes.
In summary, GLP-1RAs were associated with a decreased risk of HCC compared with insulin and sulfonylureas. In patients without insulin prescriptions, GLP-1RAs were associated with a decreased risk of HCC compared with metformin, DPP-4 inhibitors, and sulfonylureas, with more profound effects than in the overall population. GLP-1RAs were associated with a decreased risk of hepatic decompensating events compared with all 6 other anti-diabetes medication classes, with more profound effects in patients who were not prescribed insulins.
Risks of HCC Incidence and Hepatic Decompensating Events in Patients With T2DM, With and Without Obesity: GLP-1 RAs vs Non-GLP-1RA Anti-diabetes Medications
Obesity has been independently associated with increased risk for HCC.35 Obesity and T2DM often co-occur in patients. As shown in Table 1, the prevalence of obesity including severe obesity in the GLP-1RA(+)Insulin(−) and Insulin(+)GLP-1RA(−) cohorts ranged from 20% to 50%. Here we further examined the associations of GLP-1RAs with HCC and hepatic decompensation compared with other anti-obesity medications in patients with and without obesity.
Among patients with T2DM and obesity, GLP-1RAs were associated with a significantly lower risk for HCC incidence compared with insulin (HR, 0.20; 95% CI, 0.14–0.31), lower but not significant compared with sulfonylureas and metformin. A similar trend was found for patients without obesity (Figure 2A). GLP-1RAs were associated with a lower risk for hepatic decompensating events compared with all 6 non-GLP-1RA anti-diabetes medication classes, similar in patients with and without obesity (Figure 2B).
Figure 2.

Risk of incident (first-time diagnosis) HCC and hepatic decompensating events in patients with T2DM, with and without obesity. (A) Comparison of the risk of incident HCC between propensity score–matched cohorts. (B) Comparison of the risk of hepatic decompensating events between propensity score–matched cohorts. Outcomes were followed for 5 years after the index event.
In summary, the associations of GLP-1RAs with HCC and hepatic decompensating event were similar in patients with and without obesity, suggesting its effects are independent of weight loss.
Risks of HCC Incidence and Hepatic Decompensating Events in Patients With T2DM, Without and With Different Stages of Fatty Liver Diseases: GLP-1 RAs vs Non-GLP-1RA Anti-diabetes Medications
We then examined the associations of GLP-RAs with HCC and hepatic decompensating events in patients with T2DM, without and with different stages of fatty liver diseases. Among patients with no MASLD, MASH, or liver fibrosis and cirrhosis, GLP-1RAs were associated with a significantly lower risk for HCC incidence compared with insulin (HR, 0.18; 95% CI, 0.11–0.31), lower but not significant risk compared with metformin, DPP-4 inhibitors, SGLT2 inhibitors, and sulfonylureas. Among patients with a prior diagnosis of MASLD or MASH, GLP-1RAs were associated with a significantly lower risk for HCC incidence compared with insulin (HR, 0.34; 95% CI, 0.17–0.70) but not with other anti-diabetes medications. Among patients with a prior diagnosis of liver fibrosis and cirrhosis, GLP-1RAs were associated with a significantly lower risk for HCC incidence compared with insulin (HR, 0.27; 95% CI, 0.12–0.63) but not with other anti-diabetes medications (Figure 3A).
Figure 3.
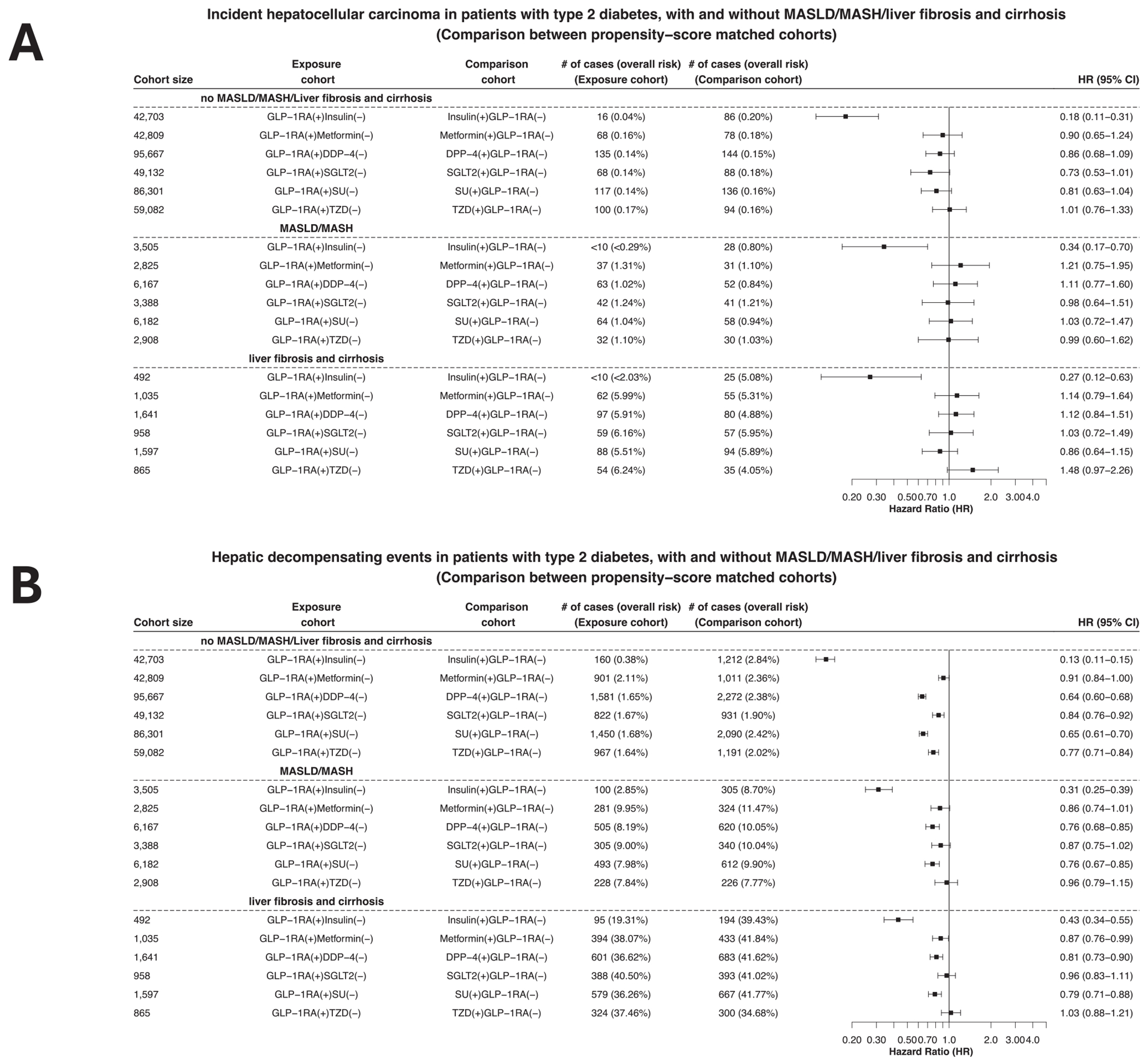
Risk of the incident (first-time diagnosis) HCC and hepatic decompensating events in patients with T2DM, with and without MASLD, MASH, and liver fibrosis and cirrhosis. (A) Comparison of the risk of incident HCC between propensity score–matched cohorts. (B) Comparison of the risk of hepatic decompensating events between propensity score–matched cohorts. Outcomes were followed for 5 years after the index event.
For the outcome of hepatic decompensating events, GLP-1RAs were associated with decreased risk in patients without MASLD, MASH, or liver fibrosis and cirrhosis compared with all 6 non-GLP-1RA anti-diabetes medications. Among patients with a prior diagnosis of MASLD or MASH, GLP-1RAs were associated with a significantly lower risk for hepatic decompensating events compared with other anti-diabetes medications except for thiazolidinediones. Among patients with a prior diagnosis of liver fibrosis and cirrhosis, GLP-1RAs were associated with a significantly lower risk for HCC incidence compared with other anti-diabetes medications except for SGLT2 inhibitors and thiazolidinediones (Figure 3B).
In summary, GLP-1RAs were associated with reduced risks of HCC and hepatic decompensating events in patients without and with fatty liver diseases. The effects were more profound effects in those without than those with fatty liver diseases.
Risk of HCC Incidence and Hepatic Decompensating Events in Patients With T2DM, With and Without Alcohol or Tobacco Use Disorders: GLP-1 RAs vs Non-GLP-1RA Anti-diabetes Medications
We then examined the associations of GLP-RAs with HCC and hepatic decompensating events in patients with and without alcohol or tobacco use disorders. Among patients with no prior diagnosis of alcohol or tobacco use disorders, GLP-1RAs were associated with a significantly lower risk for HCC incidence compared with insulin (HR, 0.22; 95% CI, 0.14–0.35), lower but not significant risk compared with metformin, DPP-4 inhibitors, and sulfonylureas. Among patients with a prior diagnosis of alcohol or tobacco use disorders, GLP-1RAs were associated with a lower, but not significant, risk for HCC incidence compared with insulin due to small sample sizes (Figure 4A).
Figure 4.

Risk of the incident (first-time diagnosis) HCC and hepatic decompensating events in patients with T2DM, with and without alcohol or tobacco use disorders. (A) Comparison of the risk of incident HCC between propensity score–matched cohorts. (B) Comparison of the risk of hepatic decompensating events between propensity score–matched cohorts. Outcomes were followed for 5 years after the index event.
For the outcome of hepatic decompensating events, GLP-1RAs were associated with a decreased risk in patients without alcohol or tobacco use disorders compared with all 6 non-GLP-1RA anti-diabetes medications. Among patients with alcohol or tobacco use disorders, GLP-1RAs were associated with a significantly lower risk for hepatic decompensating events compared with other anti-diabetes medications except for metformin and SGLT2 inhibitors (Figure 4B).
Risk of HCC Incidence and Hepatic Decompensating Events in Patients With T2DM: GLP-1RA Combinations vs Monotherapies
GLP-1RA combination therapies were associated with decreased risk for HCC compared with monotherapies without GLP-1RAs for all 6 medication classes. The reduction was similar across 6 medication classes, significant for insulin and metformin, but not for other medications, which might be because of their smaller sample sizes. These consistent reductions compared with all 6 monotherapies suggest that GLP-1RAs had protective effects on HCC, which was independent of the types of combining medications. In addition, GLP-1RA combinations have additional beneficial effects compared with monotherapies of non-GLP-1RA anti-diabetes medications such as metformin (Figure 5A, top panel).
Figure 5.

Comparing GLP-1RA combination therapies with monotherapies for the risk of the incident (first-time diagnosis) HCC and hepatic decompensating events in patients with T2DM. (A) Comparison of the risk of incident HCC between propensity score–matched cohorts. (B) Comparison of the risk of hepatic decompensating events between propensity score–matched cohorts. Outcomes were followed for 5 years after the index event (prescription of GLP-1RA for the GLP-RA combination therapies vs specific anti-diabetes medication classes for monotherapies from January 2010 to February 2019).
Compared with GLP-1RA monotherapy, GLP-1RA combination therapies with insulin were associated with a higher risk for HCC, suggesting the harmful effects of insulin on HCC could not be reversed by GLP-1RAs. GLP-1RAs combined with SGLT2 inhibitors or thiazolidinediones compared with GLP-1RA monotherapy were associated with decreased risk for HCC, suggesting that potential preventive effects of SGLT2 inhibitors or thiazolidinediones on HCC prevention and that GLP-1RA combinations with these medications were better in preventing HCC than GLP-1RAs alone (Figure 5A, bottom panel).
Similar findings were observed for hepatic decompensating events. GLP-1RA combination therapies were consistently associated with decreased risk for hepatic decompensating events compared with all 6 different monotherapies of non-GLP-1RA anti-diabetes medications, suggesting GLP-1RA’s beneficial effects on hepatic decompensating events (Figure 5B, top panel). The results comparing GLP-1RA combination therapies with GLP-1RA monotherapy indicate that insulins are harmful and GLP-1RAs could not reverse the harmful effects. These results also revealed that metformin, SGLT2 inhibitors, sulfonylureas, and thiazolidinediones are beneficial for hepatic decompensating events and GLP-1RA combinations with these medications were better at improving liver functions than GLP-1RA monotherapy (Figure 5B, bottom panel).
In summary, the data comparing GLP-1RA combination therapies with monotherapies suggest that GLP-1RAs are beneficial in preventing HCC and reducing hepatic decompensation and GLP-1RA combinational therapies conferred additional benefits compared with monotherapies.
Discussion
Here we document a potential benefit of GLP-1RAs, alone and in combination with other anti-diabetes medications, in preventing HCC and reducing hepatic decompensation in a real-world population of 1.89 million patients with T2DM. Our results show that GLP-1RAs were effective in patients with different stages of fatty liver diseases (MASLD, MASH), or liver fibrosis and cirrhosis, but most effective in patients without prior liver diseases. Our results also show that GLP-1RAs were associated with reduced risk for HCC and hepatic decompensation events in patients with and without obesity and in patients with and without alcohol or tobacco use disorders. These results extend preclinical and clinical studies that GLP-1RAs reduced obesity, T2DM, MASLD, MASH, and alcohol or tobacco use disorders,8,9,13,36,37 all of which are major risk factors for HCC5 However, to the best of our knowledge, no studies have examined GLP-1RAs in HCC prevention. Thus, the potential beneficial effects of GLP-1RA on HCC prevention deserve attention in that HCC remains one of the leading causes of cancer death.1
GLP-1RAs were associated with a significantly decreased risk for HCC compared with insulins and sulfonylureas. Previous studies suggest that treatment of T2DM with insulins may increase the incidence of certain malignancies, including HCC.32–34 Sulfonylureas lower blood glucose by stimulating insulin secretion and increasing insulin blood level.38 Studies have shown that sulfonylureas were associated with an increased risk of HCC incidence.34,39,40 Epidemiological, clinical, and preclinical studies have shown that insulin and insulin-like growth factor signaling promote cancer risk and prognosis.32 Therefore it is important to separate the potential beneficial effects of GLP-1RAs from the harmful effects of insulins and sulfonylureas. In comparing GLP-1RA combination therapies with monotherapies, we showed that GLP-1RA combination therapies were associated with decreased risk for HCC and hepatic decompensation events compared with corresponding monotherapy of non-GLP-1RA anti-diabetes medications, consistent across all 6 medications including insulins and sulfonylureas. These results strongly support beneficial effects of GLP-1RAs independent of the comparison medications.
A meta-analysis of 10 observational studies of 334,307 patients with T2DM showed a 50% and 46% reduction in HCC incidence with metformin and thiazolidinediones, respectively.34 DPP-4 is a membrane-associated peptidase and plays crucial roles in the development of various chronic liver diseases including nonalcoholic fatty liver disease, and HCC through its peptidase activity, immune modulation, degradation of extracellular matrix, and lipid accumulation.41 SGLT2 inhibitors have multiple pleiotropic effects including glucose lowering, weight loss, blood pressure reduction, improvement in liver enzymes, and steatosis.42,43 These previous studies supported the potential beneficial effects of metformin, DPP-4 inhibitors, SGLT2 inhibitors, and thiazolidinediones on HCC. By comparing GLP-1RAs with these medications, we showed no significant differences in HCC incidence though significant reductions in hepatic decompensation events, suggesting that GLP-1RAs have similar beneficial effects in HCC prevention. However, in patients without insulins, GLP-1RAs were associated with decreased risk for HCC compared with metformin and DPP-4 inhibitors, although the difference was not statistically significant because of small sample sizes. These results indicate that GLP-1RAs may have stronger protective effects on HCC than metformin and DPP-4 inhibitors.
In comparing GLP-1RA combination therapies with monotherapies, we showed that GLP-1RA combination therapies were associated with decreased risk for HCC and hepatic decompensation events compared with corresponding monotherapy of metformin, DPP-4 inhibitors, SGLT2 inhibitors, or thiazolidinediones. These results strongly support the beneficial effects of GLP-1RAs independent of these medications and that GLP-1RA combination therapies offer additional benefits to these beneficial comparison medications.
Obesity is a risk factor for multiple cancers, including the colon, rectum, gastric cardia, liver, gallbladder, pancreas, and kidney and esophagus cancer, with a relative risk of 1.8 for HCC.44 GLP-1RAs promote weight loss by reducing the appetite and feelings of hunger, slowing the release of food from the stomach, and increasing feelings of fullness after eating.45 We compared GLP-1RAs with other anti-diabetes medications in T2DM patients with and without obesity. We showed that GLP-1RAs were associated with a decreased risk for HCC incidence and hepatic decompensation events compared with other medications (insulins, metformin, DPP-4 inhibitors, SGLT2 inhibitors, and sulfonylureas) in patients without obesity. These results indicate the potential effects of GLP-1RAs on HCC and liver functions in addition to those associated with weight loss.
Excess alcohol consumption and tobacco use are established risk factors for HCC.46–48 Preclinical and clinical studies have shown that GLP-1RAs reduced drinking and relapse8,49,50 and nicotine intake and smoking cessation.51,52 We examined the associations of GLP-RAs with HCC and hepatic decompensating events in patients with and without alcohol or tobacco use disorders. Among patients with no prior diagnosis of alcohol or tobacco use disorders, GLP-1RAs were associated with a decreased risk for HCC compared with insulin, metformin, DPP-4 inhibitors, and sulfonylureas and reduced risk for hepatic decompensation compared with all 6 non-GLP-1RA anti-diabetes medications. These results suggest the potential effects of GLP-1RAs on preventing HCC and improving liver functions in addition to their functions in curbing the desire for alcohol drinking and tobacco smoking.
MASLD is characterized by hepatic steatosis in the absence of heavy alcohol consumption and may progress to MASH and hepatic fibrosis and cirrhosis.53 MASLD, particularly MASH and MASH-associated hepatic fibrosis and cirrhosis, contributes to the development of HCC.54,55 MASLD is the fastest-growing cause of HCC in the United States.56 GLP-1RAs were shown to have beneficial effects on MASH resolution but not MASH-related liver fibrosis.13,57 We show that in patients without MASLD, MASH, or liver fibrosis and cirrhosis, GLP-1RAs were associated with a decreased risk for HCC incidence compared with insulin, metformin, DPP-4 inhibitors, SGLT2 inhibitors, and sulfonylureas. Among patients with MASLD, MASH, or liver fibrosis and cirrhosis, GLP-1RAs were associated with a lower risk for HCC incidence compared with insulin but not with other anti-diabetes medications. In addition, GLP-1RAs were associated with lower risks for hepatic decompensations compared with all 6 non-GLP-1RA anti-diabetic medications in patients without and with these fatty liver diseases and the effects were more profound in patients without these diseases.
MASLD is the most common cause of liver diseases, with an estimated prevalence of 20% to 30% in general population,58 40% to 70% among patients with T2DM, and 50% to 90% among patients with obesity.56,58–60 However, liver diseases including MASLD are often underdiagnosed and underrecorded in patient EHRs. In a large primary-care record study of 17,669,973 adults, 176,114 or 1% had a recorded diagnosis of MASLD.61 In our study, the prevalence of MASLD was 2.5% to 7.0% in patients with T2DM, which is significantly lower than the actual 40% to 70%. This underdiagnosis suggests that the study population without documented fatty liver diseases might include patients with MASLD, MASH, or liver fibrosis, which could have underestimated the beneficial effects of GLP-1RAs in preventing HCC and improving liver functions in this study population. In summary, these observations suggest the need for clinical trials to test the early use of GLP-1RAs as an intervention to prevent the development of irreversible progression to HCC in patients with T2DM.
We showed that combination therapies with GLP-1RAs were associated with consistently reduced risk for HCC and hepatic decompensation compared with all 6 corresponding monotherapies. These consistent reductions indicate that GLP-1RAs had protective effects on HCC independent of the specific types of combining medications and that combinations with GLP-1RA have additional beneficial effects compared with monotherapies. Although GLP-1RA plus insulin was better than insulin monotherapy in preventing HCC, GLP-1RAs did not reverse the harmful effects of insulins on HCC risk because patients prescribed GLP-1RAs plus insulin still had an increased risk for HCC or hepatic decompensation compared with those prescribed GLP-1RAs alone.
HCC was mainly driven by chronic liver inflammation.62 Deregulation of the liver’s central role in immunoregulation is a hallmark of MASLD, MASH, liver fibrosis, and HCC. Inflammatory pathways, including cytokine signaling, innate immune signaling, and adaptive immunity contribute to inflammation-mediated HCC development.62 GLP-1RAs have been shown to significantly reduce chronic and systematic inflammation and reduce serum levels of C-reactive protein, interleukin-6 and tumor necrosis factor-α.62–64 Studies show that GLP-1RAs also modulate innate immune response in innate immune cells,65 and can reverse the negative effects of MASLD by modulating M2 Kupffer cells.66 Although our study is observational and could not elucidate mechanisms underlying the observed beneficial effects of GLP-1RA on HCC and liver functions, the consistent findings from various populations stratified by their insulin use, the status of obesity, status of fatty liver diseases, and alcohol and tobacco use suggest that GLP-1RA may reduce HCC in addition to their effects on weight loss, improving on liver diseases or curbing alcohol use or smoking. These additional beneficial effects of GLP-1RAs on HCC may be mediated through immune modulation on liver function, which may represent an effective immunomodulating approach for HCC and liver diseases. However, further studies in elucidating these mechanisms are needed.
Our study has its limitations. First, as a retrospective observational study, no causal inferences can be drawn. In addition, we could not directly examine the underlying mechanism of how GLP-1RAs may have effects on HCC, largely because of the limitations of data and analytics tools. To probe potential mechanisms, we designed several sub-analyses in patients stratified by the status of obesity, alcohol or tobacco use disorders, and fatty liver diseases. Second, patients in our study were drawn from the TriNetX platform. Results need to be validated in other populations and platforms. Third, retrospective observational studies have inherent limitations, including unmeasured or uncontrolled confounders and biases. Although we controlled for an extensive list of more than 80 variables, these could not be fully eliminated. Fourth our follow-up time was 5 years. HCC often takes years to develop and its age of onset in the United States is 60 to 70.5 For our study, the mean age was 56.2 years, and the study population was high-risk because all patients had T2DM. Although our study observed a statistically lower risk for GLP-1RAs compared with non-GLP-1RA anti-diabetes medications, future longer-term studies are necessary. Fifth, the study period was 2010 to 2019 to allow for a 5-year follow-up for all patients at the time of data collection and analysis in March 2024. Semaglutide was approved for treating T2DM in December 2017 and gained popularity after its approval in 2021 for weight loss. Preclinical and clinical studies showed that it reduces alcohol intake, tobacco smoking, MASLD, and MASH.8,13,36,37 Ongoing clinical trials are evaluating the beneficial effects of semaglutide on heavy drinking, tobacco cessation, MASLD, and MASH. Although the GLP-1RAs in our study included semaglutide, the sample size was limited for separate analyses for semaglutide. Therefore, future work is warranted to examine long-term associations of semaglutide with HCC incidence in patients with T2DM and with obesity. Sixth, patient EHRs had limited information about medication duration and adherence, therefore these variables could not be controlled in our study. Although we controlled demographics, adverse socioeconomic status, and medical encounters for cancer screening, and all the patients in the study population had medical encounters for their T2DM diagnosis and were subsequently prescribed anti-diabetes medications, we could not explicitly control for resource access and health care utilization.
In summary, findings from this study show that GLP-1RA was associated with a lower risk for HCC incidence and hepatic decompensation compared with non-GLP-1 RA anti-diabetes medications in a real-world population of patients with T2DM, a high-risk population for HCC. However, the effect of GLP-1 RAs on HCC risk compared with other anti-diabetes medications should be interpreted with caution owing to the inherent and heterogeneous cancer-modifying effect of the comparator group and different underlying molecular mechanisms. Given the alarming increase in HCC and its poor prognosis, as well as a significantly increased risk for HCC in patients with T2DM, further preclinical and clinical studies are warranted to investigate the underlying mechanisms and to support its use clinically for HCC prevention.
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2024.04.029.
WHAT YOU NEED TO KNOW.
BACKGROUND AND CONTEXT
About 70% of hepatocellular carcinoma is attributable to modifiable risk factors. Medications targeting these modifiable risk factors are beneficial for hepatocellular carcinoma prevention.
NEW FINDINGS
Based on a cohort study of 1,890,020 patients with type 2 diabetes, glucagon-like peptide-1 receptor agonists were associated with a lower risk of hepatocellular carcinoma compared with other anti-diabetes medications.
LIMITATIONS
This is a retrospective observational study with inherent limitations including unmeasured or uncontrolled confounders and biases.
CLINICAL RESEARCH RELEVANCE
These findings provide preliminary evidence of the potential benefit of glucagon-like peptide-1 receptor agonists for hepatocellular carcinoma prevention and hepatic function improvement in a real-world high-risk population of patients with type 2 diabetes, supporting future clinical studies to investigate its clinical use for hepatocellular carcinoma prevention and liver function improvement.
BASIC RESEARCH RELEVANCE
Findings from this study call for preclinical studies of the underlying mechanism.
Funding
We acknowledge support from National Cancer Institute Case Comprehensive Cancer Center (CA221718, CA043703), American Cancer Society (RSG-16–049–01 – MPC), The Landon Foundation-AACR (15–20–27-XU), NIH Director’s New Innovator Award Program (DP2HD084068), National Institute on Aging (AG057557, AG061388, AG062272, AG07664), National Institute on Alcohol Abuse and Alcoholism (AA029831). The funding source had no role in this study including design, execution, analyses, interpretation of the data, or decision to submit results.
Abbreviations used in this paper:
- BMI
body mass index
- CI
confidence interval
- DPP-4 inhibitors
dipeptidyl-peptidase-4 inhibitors
- EHRs
electronic health records
- GLP-1RAs
Glucagon-like peptide-1 receptor agonists
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- ICD-10
International Classification of Diseases, 10th Revision
- MASH
metabolic dysfunction-associated steatohepatitis
- MASLD
metabolic dysfunction-associated steatotic liver disease
- SGLT2 inhibitors
sodium-glucose cotransporter-2 inhibitors
- T2DM
type 2 diabetes mellitus
Footnotes
CRediT Authorship Contributions
Lindsey Wang (Formal analysis: Lead; Investigation: Lead; Methodology: Lead; Validation: Lead; Visualization: Lead; Writing – original draft: Supporting; Writing review & editing: Supporting)
Nathan A. Berger, MD (Conceptualization: Equal; Funding acquisition: Lead; Investigation: Equal; Methodology: Equal; Supervision: Lead; Validation: Equal; Writing– original draft: Equal; Writing – review & editing: Equal)
David C. Kaelber, MD, PhD, MPH (Investigation: Supporting; Methodology: Supporting; Project administration: Supporting; Resources: Supporting; Software: Supporting)
Rong Xu, PhD (Conceptualization: Equal; Formal analysis: Supporting; Funding acquisition: Supporting; Investigation: Equal; Methodology: Supporting; Project administration: Equal; Resources: Equal; Supervision: Equal; Validation: Supporting; Visualization: Supporting; Writing – original draft: Equal; Writing – review & editing: Equal)
Conflicts of interest
The authors disclose no conflicts.
Data Availability
This study used population-level aggregate and de-identified data collected by the TriNetX platform and are available from TriNetX, LLC (https://trinetx.com/) but third-party restrictions apply to the availability of these data. The data were used under license for this study with restrictions that do not allow for the data to be redistributed or made publicly available. To gain access to the data, a request can be made to TriNetX (join@trinetx.com), but costs might be incurred, and a data-sharing agreement would be necessary. Data specific to this study including diagnosis codes and cohort characteristics in aggregated format are included in the manuscript as tables, figures, and supplementary files. All the statistical analyses in this study including propensity score matching and Kaplan-Meier Survival analyses were conducted within the TriNetX platform by using its built-in functions. Data and code to reproduce the analyses can be accessed at https://github.com/liwang0904/GLP-HCC
References
- 1.National Cancer Institute. Liver Cancer Causes, Risk Factors, and Prevention. Available at: https://www.cancer.gov/types/liver/what-is-liver-cancer/causes-risk-factors. Updated May 15, 2024.
- 2.American Cancer Society. Cancer Facts & Figures 2023. Available at: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2023-cancer-facts-figures.html. Accessed November 6, 2023.
- 3.Centers for Disease Control and Prevention. Liver Cancer Basics 2022. Available at: https://www.cdc.gov/cancer/liver/index.htm. Accessed November 2, 2023.
- 4.Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin 2018;68:31–54. [DOI] [PubMed] [Google Scholar]
- 5.McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology 2021;73(Suppl 1):4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz JM, Carithers RL Jr. Epidemiology and risk factors for hepatocellular carcinoma. UpToDate, January 26, 2024. Available at: https://medilib.ir/uptodate/show/3599. Accessed March 1, 2024. [Google Scholar]
- 7.Bendotti G, Montefusco L, Lunati ME, et al. The anti-inflammatory and immunological properties of GLP-1 receptor agonists. Pharmacol Res 2022;182:106320. [DOI] [PubMed] [Google Scholar]
- 8.Chuong V, Farokhnia M, Khom S, et al. The glucagon-like peptide-1 (GLP-1) analogue semaglutide reduces alcohol drinking and modulates central GABA neurotransmission. JCI Insight 2023;8:e170671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lengsfeld S, Burkard T, Meienberg A, et al. Effect of dulaglutide in promoting abstinence during smoking cessation: a single-centre, randomized, double-blind, placebo-controlled, parallel group trial. EClinicalMedicine 2023;57:101865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta NA, Mells J, Dunham RM, et al. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology 2010;51:1584–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mantovani A, Byrne CD, Targher G. Efficacy of peroxisome proliferator-activated receptor agonists, glucagon-like peptide-1 receptor agonists, or sodium-glucose cotransporter-2 inhibitors for treatment of non-alcoholic fatty liver disease: a systematic review. Lancet Gastroenterol Hepatol 2022;7:367–378. [DOI] [PubMed] [Google Scholar]
- 12.Targher G, Mantovani A, Byrne CD. Mechanisms and possible hepatoprotective effects of glucagon-like peptide-1 receptor agonists and other incretin receptor agonists in non-alcoholic fatty liver disease. Lancet Gastroenterol Hepatol 2023;8:179–191. [DOI] [PubMed] [Google Scholar]
- 13.Newsome PN, Buchholtz K, Cusi K, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med 2021;384:1113–1124. [DOI] [PubMed] [Google Scholar]
- 14.TriNetX - The World’s Largest, Living Ecosystem of Real-World Data and Evidence. TriNetX 2021. Available at: https://trinetx.com/. Accessed May 6, 2023.
- 15.Wang L, Wang Q, Davis PB, et al. Increased risk for COVID-19 breakthrough infection in fully vaccinated patients with substance use disorders in the United States between December 2020 and August 2021. World Psychiatry 2022;21:124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Berger NA, Kaelber DC, et al. Incidence rates and clinical outcomes of SARS-CoV-2 infection with the omicron and delta variants in children younger than 5 years in the US. JAMA Pediatr 2022;178:811–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Davis PB, Kaelber DC, et al. Comparison of mRNA-1273 and BNT162b2 vaccines on breakthrough SARS-CoV-2 infections, hospitalizations, and death during the delta-predominant period. JAMA 2022;327:678–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L, Davis PB, Kaelber DC, et al. COVID-19 breakthrough infections and hospitalizations among vaccinated patients with dementia in the United States between December 2020 and August 2021. Alzheimers Dement 2023;19:421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang W, Kaelber DC, Xu R, et al. Breakthrough SARS-CoV-2 infections, hospitalizations, and mortality in vaccinated patients with cancer in the US between December 2020 and November 2021. JAMA Oncol 2022;8:1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Kaelber DC, Xu R, et al. COVID-19 breakthrough infections, hospitalizations and mortality in fully vaccinated patients with hematologic malignancies: a clarion call for maintaining mitigation and ramping-up research. Blood Rev 2022;54:100931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Berger NA, Xu R. Risks of SARS-CoV-2 breakthrough infection and hospitalization in fully vaccinated patients with multiple myeloma. JAMA Netw Open 2021;4:e2137575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Volkow ND, Berger NA, et al. Association of COVID-19 with endocarditis in patients with cocaine or opioid use disorders in the US. Mol Psychiatry 2023;28:543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao Z, Winhusen TJ, Gorenflo M, et al. Repurposing ketamine to treat cocaine use disorder: integration of artificial intelligence-based prediction, expert evaluation, clinical corroboration and mechanism of action analyses. Addiction 2023;118:1307–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olaker VR, Kendall EK, Wang CX, et al. Association of recent SARS-CoV-2 infection with new-onset alcohol use disorder, January 2020 through January 2022. JAMA Netw Open 2023;6:e2255496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, Volkow ND, Berger NA, et al. Cardiac and mortality outcome differences between methadone, buprenorphine and naltrexone prescriptions in patients with an opioid use disorder. J Clin Psychol 2023;79:2869–2883. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Xu R, Kaelber DC, et al. Time trend and association of early-onset colorectal cancer with diverticular disease in the United States: 2010–2021. Cancers 2022;14:4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Wang W, Kaelber DC, et al. GLP-1 receptor agonists and colorectal cancer risk in drug-naive patients with type 2 diabetes, with and without overweight/obesity. JAMA Oncol 2024;10:256–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang W, Volkow ND, Berger NA, et al. Association of semaglutide with risk of suicidal ideation in a real-world cohort. Nat Med 2024;30:168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang W, Volkow ND, Berger NA, et al. Association of semaglutide with reduced incidence and relapse of cannabis use disorder in real-world populations: a retrospective cohort study. Mol Psychiatry 2024. Mar 14; 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ICD10data.com. 2024. ICD-10-CM Diagnosis Code C22.0. Available at: https://www.icd10data.com/ICD10CM/Codes/C00-D49/C15-C26/C22-/C22.0. Accessed November 7, 2023.
- 31.Lo Re V 3rd, Lim JK, Goetz MB, et al. Validity of diagnostic codes and liver-related laboratory abnormalities to identify hepatic decompensation events in the Veterans Aging Cohort Study. Pharmacoepidemiol Drug Saf 2011;20:689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pollak M The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer 2012;12:159–169. [DOI] [PubMed] [Google Scholar]
- 33.Talamantes S, Lisjak M, Gilglioni EH, et al. Non-alcoholic fatty liver disease and diabetes mellitus as growing aetiologies of hepatocellular carcinoma. JHEP Rep 2023;5:100811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh S, Singh PP, Singh AG, et al. Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. Am J Gastroenterol 2013;108:881–891; quiz 892. [DOI] [PubMed] [Google Scholar]
- 35.Ohki T, Tateishi R, Sato T, et al. Obesity is an independent risk factor for hepatocellular carcinoma development in chronic hepatitis C patients. Clin Gastroenterol Hepatol 2008;6:459–464. [DOI] [PubMed] [Google Scholar]
- 36.Reardon S Could new weight-loss drugs like ozempic treat addiction? Scientific American 2023. Available at: https://www.scientificamerican.com/article/could-new-weight-loss-drugs-like-ozempic-treat-addiction/. Accessed July 12, 2023.
- 37.Bandyopadhyay S, Das S, Samajdar SS, et al. Role of semaglutide in the treatment of nonalcoholic fatty liver disease or non-alcoholic steatohepatitis: a systematic review and meta-analysis. Diabetes Metab Syndr 2023;17:102849. [DOI] [PubMed] [Google Scholar]
- 38.Hemmingsen B, Sonne DP, Metzendorf M-I, et al. Insulin secretagogues for prevention or delay of type 2 diabetes mellitus and its associated complications in persons at increased risk for the development of type 2 diabetes mellitus. Cochrane Database Syst Rev 2016;10:CD012151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee J-Y, Jang S-Y, Nam CM, et al. Incident hepatocellular carcinoma risk in patients treated with a sulfonylurea: a nationwide, nested, case-control study. Sci Rep 2019;9:8532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang C-H, Lin J-W, Wu L-C, et al. Oral insulin secretagogues, insulin, and cancer risk in type 2 diabetes mellitus. J Clin Endocrinol Metab 2012;97:E1170–E1175. [DOI] [PubMed] [Google Scholar]
- 41.Itou M, Kawaguchi T, Taniguchi E, et al. Dipeptidyl peptidase-4: a key player in chronic liver disease. World J Gastroenterol 2013;19:2298–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patoulias D, Michailidis T. SGLT-2 inhibitor and GLP-1 receptor agonist treatment for patients with nonalcoholic fatty liver disease and type 2 diabetes mellitus: Is their combination the optimal treatment option? J Clin Transl Hepatol 2022;10:574–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar J, Memon RS, Shahid I, et al. Antidiabetic drugs and non-alcoholic fatty liver disease: A systematic review, meta-analysis and evidence map. Dig Liver Dis 2021;53:44–51. [DOI] [PubMed] [Google Scholar]
- 44.Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body fatness and cancer—viewpoint of the IARC Working Group. N Engl J Med 2016;375:794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ard J, Fitch A, Fruh S, et al. Weight loss and maintenance related to the mechanism of action of glucagon-like peptide 1 receptor agonists. Adv Ther 2021;38:2821–2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Donato F, Tagger A, Gelatti U, et al. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol 2002;155:323–331. [DOI] [PubMed] [Google Scholar]
- 47.Hassan MM, Hwang L-Y, Hatten CJ, et al. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology 2002;36:1206–1213. [DOI] [PubMed] [Google Scholar]
- 48.Trichopoulos D, Bamia C, Lagiou P, et al. Hepatocellular carcinoma risk factors and disease burden in a European cohort: a nested case-control study. J Natl Cancer Inst 2011;103:1686–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aranäs C, Edvardsson CE, Shevchouk OT, et al. Semaglutide reduces alcohol intake and relapse-like drinking in male and female rats. EBioMedicine 2023;93:104642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klausen MK, Jensen ME, Møller M, et al. Exenatide once weekly for alcohol use disorder investigated in a randomized, placebo-controlled clinical trial. JCI Insight 2022;7:e159863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yammine L, Verrico CD, Versace F, et al. Exenatide as an adjunct to nicotine patch for smoking cessation and prevention of postcessation weight gain among treatment-seeking smokers with pre-diabetes and/or overweight: study protocol for a randomised, placebo-controlled clinical trial. BMJ Open 2023;13:e072707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klausen MK, Thomsen M, Wortwein G, et al. The role of glucagon-like peptide 1 (GLP-1) in addictive disorders. Br J Pharmacol 2022;179:625–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chan W-K, Chuah K-H, Rajaram RB, et al. Metabolic dysfunction-associated steatotic liver disease (MASLD): a state-of-the-art review. J Obes Metab Syndr 2023;32:197–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ascha MS, Hanouneh IA, Lopez R, et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010;51:1972–1978. [DOI] [PubMed] [Google Scholar]
- 55.Kanwal F, Kramer JR, Mapakshi S, et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology 2018;155:1828–1837.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2021;18:223–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Loomba R, Abdelmalek MF, Armstrong MJ, et al. Semaglutide 2·4 mg once weekly in patients with non-alcoholic steatohepatitis-related cirrhosis: a randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol Hepatol 2023;8:511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rinella ME. Nonalcoholic fatty liver disease. JAMA 2015;313:2263. [DOI] [PubMed] [Google Scholar]
- 59.Williamson RM, Price JF, Glancy S, et al. Prevalence of and risk factors for hepatic steatosis and nonalcoholic fatty liver disease in people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes Care 2011;34:1139–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Divella R, Mazzocca A, Daniele A, et al. Obesity, nonalcoholic fatty liver disease and adipocytokines network in promotion of cancer. Int J Biol Sci 2019;15:610–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alexander M, Loomis AK, Fairburn-Beech J, et al. Real-world data reveal a diagnostic gap in non-alcoholic fatty liver disease. BMC Med 2018;16:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ringelhan M, Pfister D, O’Connor T, et al. The immunology of hepatocellular carcinoma. Nat Immunol 2018;19:222–232. [DOI] [PubMed] [Google Scholar]
- 63.Mehdi SF, Pusapati S, Anwar MS, et al. Glucagon-like peptide-1: a multi-faceted anti-inflammatory agent. Front Immunol 2023;14:1148209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Newsome P, Francque S, Harrison S, et al. Effect of semaglutide on liver enzymes and markers of inflammation in subjects with type 2 diabetes and/or obesity. Aliment Pharmacol Ther 2019;50:193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen J, Mei A, Wei Y, et al. GLP-1 receptor agonist as a modulator of innate immunity. Front Immunol 2022;13:997578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Z, Feng P-P, Zhao Z-B, et al. Liraglutide protects against inflammatory stress in non-alcoholic fatty liver by modulating Kupffer cells M2 polarization via cAMP-PKA-STAT3 signaling pathway. Biochem Biophys Res Commun 2019;510:20–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study used population-level aggregate and de-identified data collected by the TriNetX platform and are available from TriNetX, LLC (https://trinetx.com/) but third-party restrictions apply to the availability of these data. The data were used under license for this study with restrictions that do not allow for the data to be redistributed or made publicly available. To gain access to the data, a request can be made to TriNetX (join@trinetx.com), but costs might be incurred, and a data-sharing agreement would be necessary. Data specific to this study including diagnosis codes and cohort characteristics in aggregated format are included in the manuscript as tables, figures, and supplementary files. All the statistical analyses in this study including propensity score matching and Kaplan-Meier Survival analyses were conducted within the TriNetX platform by using its built-in functions. Data and code to reproduce the analyses can be accessed at https://github.com/liwang0904/GLP-HCC


