Regeneration is a process that occurs in many tissues, but in the nervous system it has the special meaning of axon growth, not cell replacement. Axon regeneration is a motile process, and growth cones at the elongating axon tip express receptors that mediate responses to environmental signals. In the peripheral nervous system, regeneration occurs spontaneously after nerve injury. In the central nervous system (CNS), however, damaged nerves do not regenerate, which is why brain and spinal cord injuries are so devastating. It is now well established that the lack of regeneration in the CNS is explained in large part by an unfavorable growth environment, and by the presence of growth inhibitory molecules. Schwab and his colleagues (1) were the first to discover a potent growth inhibitory activity in the CNS, and to show that much of this inhibitory activity is associated with myelin. Subsequently, two important myelin-derived growth inhibitory proteins have been identified, myelin-associated glycoprotein (MAG) and Nogo (2–6). To understand growth inhibition in the CNS, the neuronal receptors to growth inhibitory molecules need to be characterized. The neuronal receptor for Nogo was discovered last year, and it is a protein with no obvious intracellular signaling domain (7). By contrast, the MAG receptor has remained elusive. The report by Vyas et al. in this issue of PNAS (8) provides evidence that gangliosides GD1a and GT1b are neuronal receptors that mediate inhibition by MAG.
To understand growth inhibition, the neuronal receptors to growth inhibitory molecules need to be characterized.
MAG was the first myelin-derived growth inhibitory molecule to be reported, and the discovery of the inhibitory activity of MAG was made independently by two groups (2, 3). MAG had been cloned long before its growth inhibitory activity was revealed (9), and the new role for MAG was unexpected. The more recent discovery of Nogo created tremendous interest, especially because three different groups published their findings simultaneously (4–6). The Schwab group (10) accomplished the biochemical purification of Nogo, and provided the peptide data used for cloning. The comparison of MAG and Nogo show similar growth inhibitory activity (5). Thus, MAG, like Nogo, is a very potent growth inhibitory protein, but MAG has not received as much attention. This is, in part, because the discovery of MAG as a growth inhibitory protein was at first controversial, which led to confusion in the scientific community. MAG had been previously described as a cell adhesion molecule that favored axon growth (11), and was also known to be present in peripheral nerve myelin. These facts seemed at odds with the new growth inhibitory activity. It is now clear that embryonic and postnatal neurons respond differently to MAG, and that there is a developmental switch in the neuronal response to MAG (12). Although early experiments were performed with embryonic neurons that are not inhibited by MAG, this was not realized at the time. Other important studies have now clarified this issue and shown that adult neurons are inhibited by MAG. Filbin and collaborators (13) discovered that high cAMP levels in developing neurons override the growth inhibitory response to MAG. Growth inhibition by MAG can be blocked with drugs that increase cAMP signaling (14, 15). Also, the presence of MAG in peripheral nerve myelin was confusing. In retrospect, the lack of inhibitory activity previously reported for peripheral nerve myelin resulted from copurification of laminin from the basal lamina. Laminin can override the growth inhibitory activity of MAG (16). Moreover, despite its presence in peripheral nerve myelin, MAG does not block regeneration. Peripheral nerve myelin is cleared before axon regeneration by macrophages. In mutant mice that cannot clear myelin, there is a lack of axon regeneration. When MAG-null mutant mice were bred with the mice that fail to clear myelin, peripheral nerve regeneration was restored (17). These experiments highlight the importance of MAG in blocking axon regeneration in vivo.
Like the determination of the function of MAG, the identification of the MAG receptor has had an ignominious fate because it has proven difficult to characterize. It was expected that the MAG receptor would be a typical transmembrane protein, but a definitive protein receptor has eluded discovery, even though many have tried. MAG binds sialic acid on sialyated glycans and sialoglycoproteins (18, 19). Neuraminidase treatment of neurons to remove sialic acid blocks the inhibitory effect of MAG on neurite growth (12, 19, 20). Mutational studies of MAG further demonstrated the importance of MAG-binding to sialic acid for biological activity (19). The major brain gangliosides that include GD1a and GT1b were shown to interact with MAG (21), but gangliosides are glycolipids, not proteins. Notably, Vinson et al. (22) showed that neurite growth is reduced by addition of soluble GD1a and GT1b to neurons plated on MAG Chinese hamster ovary cells, and that anti-GD1a antibodies bock growth inhibition by MAG. These experiments clearly indicated that gangliosides bind to MAG, and suggest GT1b as a candidate receptor. The question remained whether gangliosides on neuronal cell surfaces can be considered true MAG receptors. In their article in this issue of PNAS, Vyas et al. (8) show that GD1a and GT1b can account for neurite growth inhibition on inhibitory substrates. To be MAG receptors, gangliosides should: (i) bind MAG and be expressed by neurons that are inhibited by MAG; (ii) trigger growth inhibition on permissive substrates when activated; and (iii) be known to signal to intracellular intermediates that mediate growth inhibitory signaling. Clear evidence is presented by Vyas et al. (8) that gangliosides GD1a and GT1b meet these criteria.
The key experiment by Vyas et al. (8) reveals that ganglioside clustering is sufficient for inhibition of neurite growth. In cells grown on permissive substrates, multivalent clustering of gangliosides totally blocked neurite growth in the absence of inhibitory molecules. In addition, Vyas et al. (8) confirm the effects of neuraminidase, and demonstrate with specific antibodies that both GD1a and GT1b can mediate growth inhibition. They also showed that blockade of neuronal ganglioside synthesis, either enzymatically or by studying neurons from transgenic mice engineered to lack gangliosides, prevented growth inhibition by MAG. Evidence that ganglioside clustering by antibodies is sufficient to signal growth inhibition has also been provided by Vinson et al. (22). A critical aspect of this latter study was to show that inhibition of neurite growth by anti-GT1b antibodies is reversed by an inhibitor to Rho kinase (22). Vyas et al. (8) agree that Rho is likely to be the key signaling intermediate.
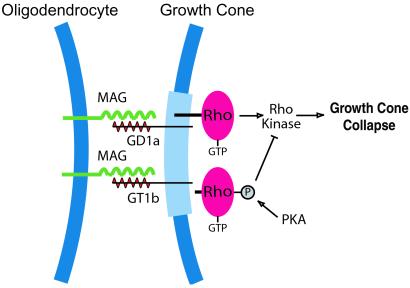
How can gangliosides signal growth cone collapse in the absence of an intracellular signaling domain? Likely, through ganglioside clustering in membrane rafts that include known signaling proteins on the cytoplasmic side (Fig. 1). In membrane rafts, glycosyl domains such as those that form the complex structure of gangliosides have such importance in signaling that the microdomain signaling complexes have been called “glycosynapse” (23). Clustering of gangliosides in a glycosynapse would activate proteins in the raft specialized for signaling. The best candidate is Rho, a small GTPase known to partition in membrane rafts (23, 24) and that mediates growth inhibition by MAG (25). Rho activates, among many substrates, a serine/threonine kinase called Rho kinase (Fig. 1). Microdomain signaling complexes that function as a unit for MAG signaling are consistent with the known effects of cAMP in blocking MAG inhibition. Elevated cAMP activates protein kinase A (PKA). Phosphorylation of Rho by PKA causes Rho to dissociate from the membrane (26), which also would dissociate Rho from the ganglioside raft. Thus, elevated cAMP can indirectly block the Rho signaling pathway by disrupting the receptor signaling complex. The idea of glycosynapses in growth cones is supported by the observation that growth cones have several different types of membrane rafts (27).
Figure 1.
Model showing MAG activation of Rho by binding gangliosides in membrane rafts. MAG expressed on the surface of oligodendrocytes interacts with gangliosides GD1a or GT1b present in growth cone membrane microdomains (light blue). Rho associates with ganglioside rafts on the cytoplasmic side. Signaling from activated Rho through Rho kinase provokes growth cone collapse and prevents axon elongation. Phosphorylation of Rho by protein kinase A causes Rho to dissociate from the membrane (26), and blocks growth cone collapse.
Much experimental evidence supports MAG signaling to Rho. Dominant negative mutation of Rho blocks the ability of neurons to respond to the growth inhibitory property of MAG, and inactivation of Rho allows long neurite growth on MAG substrates (25). Inactivation of Rho kinase reverses inhibitory ganglioside signaling (22). More recently, Rho–GTP pull down assays have provided direct evidence of MAG signaling to Rho (M. Winton, C. Dubreuil, N. Leclerc, D. Lasko, and L.M., unpublished data). Inactivation of Rho or inactivation of Rho kinase not only prevents growth inhibition, but promotes axon regeneration and functional recovery in vivo after spinal cord injury (28). Taken together, these data provide strong evidence that gangliosides are true MAG receptors that block axon growth by activating Rho. This finding is important because of the widespread expression of GD1a and GT1b in the nervous system.
Inhibitory signaling of MAG through Rho is also of significance in terms of signaling by other growth inhibitory proteins that are likely to use the same pathway. In their experiments, Vyas et al. (8) also tested substrates of extracted myelin proteins containing other inhibitory proteins (2). Surprisingly, their treatments to block ganglioside interaction with MAG completely blocked growth inhibition by the more complex inhibitory substrates. These results suggest that ganglioside signaling to Rho is a dominant signaling pathway and Rho may be a convergent point for signaling by different inhibitory receptors, such as the Nogo receptor. If Rho is essential for inhibitory signaling, then it could become an important therapeutic target to overcome growth inhibition in the CNS. Thus, further study of ganglioside membrane microdomains and Rho signaling will be of critical importance to understanding mechanisms of growth cone collapse and growth inhibition. The identification of the MAG receptor is another step forward for studies of spinal cord injury and axon regeneration in the nervous system.
Acknowledgments
I thank Matthew Winton for help preparing Fig. 1.
Note Added in Proof.
A new report suggests that MAG binding to GT1b signals to Rho through p75 neurotrophin receptor (29).
Footnotes
See companion article on page 8412.
References
- 1.Schwab M E, Kapfhammer J P, Bandtlow C E. Annu Rev Neurosci. 1993;16:565–595. doi: 10.1146/annurev.ne.16.030193.003025. [DOI] [PubMed] [Google Scholar]
- 2.McKerracher L, David S, Jackson J L, Kottis V, Dunn R, Braun P E. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- 3.Mukhopadhyay G, Doherty P, Walsh F S, Crocker P R, Filbin M T. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 4.Chen M S, Huber A B, van der Haar M, Frank M, Schnell L, Spillmann A A, Christ F, Schwab M E. Nature (London) 2000;403:434–438. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- 5.Prinjha R, Moore S E, Vinson M, Blake S, Morrow R, Christie G, Michalovich D, Simmons D L, Walsh F S. Nature (London) 2000;403:383–384. doi: 10.1038/35000287. [DOI] [PubMed] [Google Scholar]
- 6.GrandPré T, Nakamura F, Vartanian T, Strittmatter S M. Nature (London) 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- 7.Fournier A E, GrandPre T, Strittmatter S M. Nature (London) 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- 8.Vyas A A, Patel H V, Fromholt S E, Heffer-Lauc M, Vyas K A, Dang J, Schachner M, Schnaar R L. Proc Natl Acad Sci USA. 2002;99:8412–8417. doi: 10.1073/pnas.072211699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arquint M, Roder J, Chia L-S, Down J, Wilkinson D, Bayley H, Braun P, Dunn R. Proc Natl Acad Sci USA. 1987;84:600–604. doi: 10.1073/pnas.84.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spillmann A A, Bandtlow C E, Lottspeich F, Keller F, Schwab M E. J Biol Chem. 1999;273:19283–19293. doi: 10.1074/jbc.273.30.19283. [DOI] [PubMed] [Google Scholar]
- 11.Johnson P W, Abramow-Newerly W, Seilheimer B, Sadoul R, Tropak M B, Arquint M, Dunn R J, Schachner M, Roder J C. Neuron. 1989;3:377–385. doi: 10.1016/0896-6273(89)90262-6. [DOI] [PubMed] [Google Scholar]
- 12.DeBellard M E, Tang S, Mukhopadhyay G, Shen Y J, Filbin M T. Mol Cell Neurosci. 1996;7:89–101. doi: 10.1006/mcne.1996.0007. [DOI] [PubMed] [Google Scholar]
- 13.Cai D, Qiu J, Cao Z, McAtee M, Bregman B S, Filbin M T. J Neurosci. 2001;21:4731–4739. doi: 10.1523/JNEUROSCI.21-13-04731.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai D, Shen Y, De Bellard M, Tang S, Filbin M T. Neuron. 1999;22:89–101. doi: 10.1016/s0896-6273(00)80681-9. [DOI] [PubMed] [Google Scholar]
- 15.Song H-j, Ming G-I, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, Poo M-m. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- 16.David S, Braun P E, Jackson J L, Kottis V, McKerracher L. J Neurosci Res. 1995;42:594–602. doi: 10.1002/jnr.490420417. [DOI] [PubMed] [Google Scholar]
- 17.Schafer M, Fruttiger M, Montag D, Schachner M, Martini R. Neuron. 1996;16:1107–1113. doi: 10.1016/s0896-6273(00)80137-3. [DOI] [PubMed] [Google Scholar]
- 18.Kelm S, Pelz A, Schauer R, Filbin M T, Tang S, DeBellard M E, Schnaar R L, Mahoney J A, Hartnell A, Bradfield P, Crocker P R. Curr Biol. 1994;4:965–972. doi: 10.1016/s0960-9822(00)00220-7. [DOI] [PubMed] [Google Scholar]
- 19.Tang S, Shen Y J, DeBellard M E, Mukhopadhyay G, Salzer J L, Crocker P R, Filbin M T. J Cell Biol. 1997;138:1355–1366. doi: 10.1083/jcb.138.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tropak M B, Roder J C. J Neurochem. 1997;68:1753–1763. doi: 10.1046/j.1471-4159.1997.68041753.x. [DOI] [PubMed] [Google Scholar]
- 21.Yang L J, Zeller C B, Shaper N L, Kiso M, Hasegawa A, Shapiro R E, Schnaar R L. Proc Natl Acad Sci USA. 1996;93:814–818. doi: 10.1073/pnas.93.2.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vinson M, Strijbos P J, Rowles A, Facci L, Moore S E, Simmons D L, Walsh F S. J Biol Chem. 2001;276:20280–20285. doi: 10.1074/jbc.M100345200. [DOI] [PubMed] [Google Scholar]
- 23.Hakomori S. Proc Natl Acad Sci USA. 2002;99:225–232. doi: 10.1073/pnas.012540899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michaely P A, Mineo C, Ying Y S, Anderson R G. J Biol Chem. 1999;274:21430–21436. doi: 10.1074/jbc.274.30.21430. [DOI] [PubMed] [Google Scholar]
- 25.Lehmann M, Fournier A E, Selles-Navarro I, Dergham P, Leclerc N, Tigyi G, McKerracher L. J Neurosci. 1999;19:7537–7547. doi: 10.1523/JNEUROSCI.19-17-07537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lang P, Gesbert F, Delespine-Carmagnat M, Stancou R, Pouchelet M, Bertoglio J. EMBO J. 1996;15:510–519. [PMC free article] [PubMed] [Google Scholar]
- 27.He Q, Meiri K F. Mol Cell Neurosci. 2002;19:18–31. doi: 10.1006/mcne.2001.1060. [DOI] [PubMed] [Google Scholar]
- 28. Dergham, P., Ellezam, B. Essagian, C., Avedissian, H., Lubell, W. D. & McKerracher, L. (2002) J. Neurosci., in press. [DOI] [PMC free article] [PubMed]
- 29.Yamashita T H, Higuchi H, Tohyama M. J Cell Biol. 2002;157:565–570. doi: 10.1083/jcb.200202010. [DOI] [PMC free article] [PubMed] [Google Scholar]