Abstract
The topoisomerase (topo) III enzymes are found in organisms ranging from bacteria to humans, yet the precise cellular function of these enzymes remains to be determined. We previously found that Drosophila topo IIIβ can relax plasmid DNA only if the DNA is first hypernegatively supercoiled. To investigate the possibility that topo IIIβ requires a single-stranded region for its relaxation activity, we formed R-loops and D-loops in plasmids. In addition to containing a single-stranded region, these R-loops and D-loops have the advantage of being covalently closed and supercoiled, thus allowing us to assay for supercoil relaxation. We found that topo IIIβ preferentially cleaves, rather than relaxes, these substrates. The cleavage of the R-loops and D-loops, which is primarily in the form of nicking, occurs to a greater extent at a temperature that is lower than the optimal temperature for relaxation of hypernegatively supercoiled plasmid. In addition, the cleavage can be readily reversed by high salt or high temperature, and the products fail to enter the gel in the absence of proteinase K treatment and are not observed with an active-site Y332F mutant of topo IIIβ, indicating that the cleavage is mediated by a topoisomerase. We mapped the cleavage to the unpaired strand within the loop region and found that the cleavage occurs along the length of the unpaired strand. These studies suggest that the topo III enzyme behaves as a structure-specific endonuclease in vivo, providing a reversible DNA cleavage activity that is specific for unpaired regions in the DNA.
Cellular processes such as replication, transcription, and recombination rely on the action of topoisomerases to relieve the accompanying flexural and torsional stress these processes impart upon the DNA (reviewed in refs. 1 and 2). The topoisomerases act through a simple, yet elegant, mechanism whereby the active-site tyrosine covalently attaches to the DNA phosphodiester backbone, thereby introducing a transient break into the DNA. Passage of an additional strand (or strands) of DNA through this break, followed by religation of the break, produces a change in the overall topology of the DNA. While much has been discovered about the in vitro and in vivo activities of the topoisomerase (topo) I and topo II enzymes, much less is known about the activities of topo III.
The topo III enzymes are ubiquitous in nature, having been found in organisms ranging from bacteria to humans. Interestingly, it has been suggested that these enzymes may play a role in some aspect of nucleic acid metabolism other than the regulation of DNA supercoiling. This suggestion is based both upon the weak in vitro relaxation activity of topo III (3–5) and upon genetic information gained by mutations made in bacteria and yeast (6–9). Relative to its ability to relax DNA, bacterial topo III has a much greater activity in decatenation (3), but it can also knot and unknot single-stranded circles of RNA as well (10).
Insight into the biological function(s) of topo III comes through the identification of genetic, physical, and functional interactions between topo III and members of the RecQ family of helicases (11–15). These helicases, which show a 3′→5′ directionality of unwinding relative to the strand to which they bind, are thought to be associated with a variety of cellular processes, including replication, repair, and recombination (reviewed in refs. 16–18). Mutations in three of the five known human RecQ homologs, RecQ4, BLM, and WRN, lead to the diseases Rothmund–Thomson syndrome, Bloom's syndrome, and Werner's syndrome, respectively. These diseases are all characterized by reduced genome stability and a tendency toward certain neoplasias (19). Much biochemistry has been done to identify the preferred in vitro substrates for these helicases (20, 21). The enzymes display a strong preference for binding and unwinding branched substrates that contain a 3′ single-stranded DNA overhang. A 3′ overhang is not a structural requirement, however, as a blunt-ended duplex containing a centrally located bubble, and three-way and four-way synthetic Holliday junctions are all efficiently unwound. Interestingly, the Bloom's syndrome helicase has also been shown to bind and melt synthetic D-loops (22).
D-loops and R-loops are bubble-like structures that form when one strand of a double helix is displaced by the annealing of a complementary strand of either DNA (D-loop) or RNA (R-loop). These structures are of great interest because they are associated with a variety of important biological processes (reviewed in ref. 23). For example, D-loop formation is an essential step in the recombination-mediated repair of double-strand breaks in DNA. In addition, D-loops have the potential to form during replication. R-loops are also thought to prime replication in bacterial or viral genomic DNA, as well as mammalian mitochondrial DNA (24–26). In addition, R-loop formation has been associated with recombination events that occur at mammalian immunoglobulin class switch sequences (27, 28). Interestingly, it has been proposed that one of the primary roles of Escherichia coli topo I is to suppress R-loop formation during transcriptional elongation that could otherwise impair cell growth (29, 30). In the absence of topo I, such as a topA mutant, extensive R-loop formation occurs, especially when the cells are grown at low temperature (31). top3 has been identified as a multicopy suppressor of the topA mutation, and thus topo III is thought to have a role in suppressing R-loop formation as well (32).
We found that Drosophila topo IIIβ, like human topo IIIα, shows a marked preference for relaxing hypernegatively supercoiled DNA (4, 33, 34). This unique specificity is likely due to the presence of single-stranded regions in the hypernegatively supercoiled DNA, which has a much greater superhelical density than that of natively isolated plasmid. To test this hypothesis and further investigate the substrate preferences of Drosophila topo IIIβ, we formed D-loops and R-loops in plasmids. Unlike the D-loops and R-loops formed with oligonucleotides, these plasmid-based hybrids have the added advantage of being covalently closed and supercoiled. Surprisingly, we observed a cleavage rather than relaxation activity with these D-loop and R-loop substrates. The possible biological significance of this finding is discussed here.
Experimental Procedures
R-Loop and D-Loop Preparation.
R-loops were prepared according to the method of Lee and Clayton (35) with modifications made by Dudas and Kreuzer (36). Briefly, radiolabeled RNA was made by transcription of an appropriate PCR fragment with T7 RNA polymerase (United States Biochemical) in the presence of [α-32P]CTP (Perkin–Elmer/NEN). The RNA was purified, treated with DNase I, then annealed to plasmid. R-loop formation was monitored both by the incorporation of labeled oligonucleotide and by the slight shift in gel electrophoretic mobility (see Fig. 1A for an example).
Figure 1.
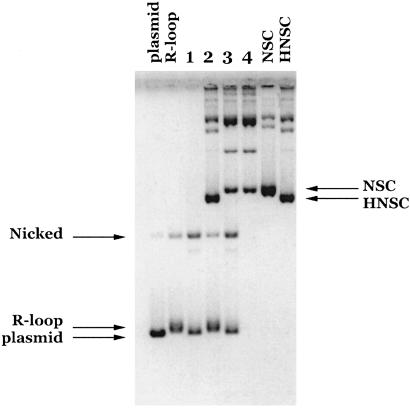
Cleavage of R-loops and D-loops by Drosophila topo IIIβ. (A) Drosophila topo IIIβ was incubated with synthetic pBR322 (1) or pKK405 (2) R-loops under standard buffer conditions for 1 hr at either 37°C or 23°C. In the two right-most lanes, topo IIIβ was incubated with both R-loop and plasmid under standard conditions for 1 hr at 23°C. After electrophoresis, the gel was stained with ethidium bromide. R-loops and their parental plasmid markers are as indicated. (B) Drosophila topo IIIβ was incubated with synthetic pBR322 (1) or pKK405 (2) D-loops under standard buffer conditions for 1 hr at either 37°C or 23°C. After electrophoresis, the gel was dried and subjected to PhosphorImager analysis. (C) Drosophila topo I was incubated with pBR322 R-loop under standard conditions for 1 hr at 30°C. After electrophoresis, the gel was stained with ethidium bromide. Fully relaxed plasmid and R-loop markers are as indicated.
D-loops were prepared according to the method of Mazin and Kowalczykowski (37). Briefly, oligonucleotides (GATCCTTTTAAATTAAAAATGAAGTTTTAAATCAATCTAAAGTATATATGAGTAAACTTGGTCTGACAGTTACCAATGCTTAATCAGTGAGGCACCTATCTCAG, which anneals at residues 3224–3327 in pBR322, and GAATCTAAGTCCATCCATTAACAACCAATAACAATTGAATAGAGAACAATATGAGATTAGAAGATCTTCAAGAAGAATTGAAGAAAGATGTGTTTATAGATTCG, which anneals at residues 5204–5307 in pKK405) were 5′-end-labeled with T4 polynucleotide kinase (United States Biochemical) and [γ-32P]ATP (Perkin–Elmer/NEN), then purified over a Bio-Spin 6 column (Bio-Rad). Labeled oligonucleotide was then coated with RecA protein (New England Biolabs). Single-stranded-DNA-binding protein (SSB) (United States Biochemical) was added to the reaction to displace oligonucleotide from the secondary binding site of RecA before plasmid DNA was added to stimulate strand exchange. Reactions were stopped with EDTA and SDS, then the mixtures were treated with proteinase K.
The resulting R-loops and D-loops were purified over Sephacryl S-400 columns (Pharmacia Biotech). Column fractions were analyzed by dot blot and PhosphorImager analysis (Molecular Dynamics).
Protein Purification and Enzyme Assays.
We previously published the purification protocol for Drosophila topo IIIβ (33). Standard topo IIIβ reactions were incubated for 1 hr in 40 mM Hepes–KOH (pH 7.5)/1 mM MgCl2, at a temperature specified in the text. Purification of Drosophila topo I-ND423 was also reported previously (38). Standard topo I reactions were for 1 hr at 30°C in 10 mM Tris⋅HCl (pH 7.9)/50 mM KCl/10 mM MgCl2/100 mM NaCl/0.1 mM EDTA. All reactions were stopped by the addition of 10 mM EDTA, 0.2% SDS, and 0.15 mg/ml proteinase K. Samples were heated at 37°C for 15 min before analysis by 1.2% agarose gel electrophoresis.
An active-site mutant of topo IIIβ was made by using Stratagene's QuikChange XL site-directed mutagenesis kit, with mutagenic primers (5′-GCAGGGCTACATCAGCTTTCCGCGAACAGAGACC and 5′-GGTCTCTGTTCGCGGAAAGCTGATGTAGCCCTGC) to convert the original codon from tyrosine-332 to phenylalanine (underlined). The mutation was confirmed by DNA sequencing, and the protein was expressed and purified as reported above for the wild-type protein.
Samples for Southern blotting and DNA sequencing were purified after topo IIIβ reaction by extraction with phenol and precipitation with ethanol. The DNA was resuspended and dialyzed against appropriate buffer, then digested with restriction enzyme (New England Biolabs). The digested material was purified by extraction with phenol and precipitation with ethanol, then resuspended in TE buffer (10 mM Tris/1 mM EDTA, pH 7.9).
Southern Blots, Linear PCR, and DNA Sequencing.
For Southern blots, DNA samples were separated by alkaline 1.2% agarose gel electrophoresis, then transferred to a nitrocellulose filter. Oligonucleotide sequences used for Southern probes are as follows: MAP1 (5′-ATCGTGGCCGGCATCAC-3′), MAP3 (5′-GTCGCCATGATCGCGTA-3′), MAP4 (5′-ATAAATCTGGAGCCGGT-3′), and MAP5 (5′-CTGATTTCTCGTAACGAT-3′). The probes were prepared by 5′-end-labeling and purifying oligonucleotides, as described above.
Sequencing ladders were made by using the United States Biochemical Sequenase Version 2.0 kit, with 32P 5′-end-labeled oligonucleotides as primers. For analysis of DNA cleavage products, 1 ng of DNA template was mixed with a 10,000-fold molar excess of a single 32P-labeled primer and subjected to 10 cycles of linear PCR using cloned Pfu polymerase (Stratagene). The PCR cycle was 1 min denaturation at 94°C, 1 min annealing at 50°C, 1 min extension at 72°C, with temperature ramped from 50°C to 72°C. After the reactions were terminated, DNA samples were heated at 75°C for 2 min and analyzed on a 6% polyacrylamide/8 M urea gel.
Results
Cleavage of Plasmid-Based R-Loops and D-Loops by Drosophila topo IIIβ.
The topo III enzymes show a preference for relaxing plasmid DNA that contains a single-stranded region. For example, E. coli topo III shows a greater relaxation activity at 52°C than at 30°C, presumably because the DNA is partially melted at the higher temperature (3). Saccharomyces cerevisiae topo III also shows a greater relaxation activity when a single-stranded loop of 29 nucleotides is introduced into the plasmid (5). To investigate the possibility that Drosophila topo IIIβ requires a single-stranded region for its relaxation activity, we prepared two synthetic R-loops and their corresponding D-loops in plasmids. To follow the formation of these structures, the annealed RNA (for R-loop) and DNA (for D-loop) were radiolabeled.
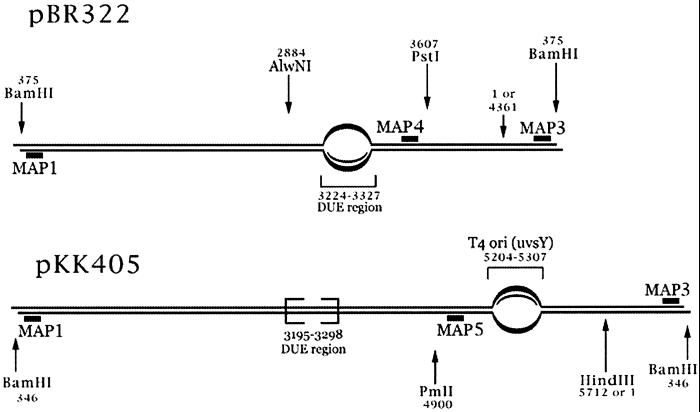
For both R-loop substrates, homologous RNA was annealed to a region of plasmid where DNA base composition is about 70% A+T. For pBR322, the RNA was annealed within a DNA-unwinding element (or DUE region) located on the plasmid between the ampicillin-resistance gene and the origin of DNA replication. The pKK405 plasmid is a derivative of pBR322, with a 1.35-kb insert containing the T4 phage ori(uvsY) origin of replication. Although pKK405 does contain the DUE region from pBR322, its RNA was annealed within the T4 ori(uvsY) insert (see Fig. 4). The efficiency of RNA uptake for both plasmids was on the order of 90–95%, as quantitated by PhosphorImager analysis (data not shown). R-loop formation was also revealed by a slightly reduced gel electrophoretic mobility (Fig. 1A). This high efficiency allowed the R-loop reaction products to be followed by ethidium bromide staining after gel electrophoresis.
Figure 4.
Strategy for mapping the R-loop and D-loop cleavage sites. BamHI-linearized structures of pBR322 R-loop/D-loop (Upper) and pKK405 R-loop/D-loop (Lower) substrates are shown. The area of the loop is indicated by the bubble and its position within the plasmid is numbered. Restriction sites are shown by arrows and their positions are also numbered. The short black bars represent the oligonucleotides that were 5′-end-labeled and used as probes for Southern blot analysis or as DNA sequencing primers.
The R-loops were incubated with Drosophila topo IIIβ for 1 hr at 37°C, a temperature optimal for the relaxation of hypernegatively supercoiled DNA. A significant fraction of both R-loop substrates was nicked, but the cleavage was considerably enhanced at a lower temperature of 23°C (Fig. 1A). It is interesting to note that a portion of the nicked material retained the labeled RNA, as detected by PhosphorImager analysis (data not shown).
When R-loop was mixed with plasmid, only R-loop appeared to be cleaved, indicating specificity of the enzyme for substrate containing a single-stranded region (Fig. 1A). Rather than being fully relaxed, the R-loop was nicked by topo IIIβ. The nicked DNA can be distinguished from fully relaxed DNA both by the absence of a ladder of topoisomers (which is usually observed in fully relaxed DNA under the conditions used in these experiments; see Fig. 1C), and by analysis of the DNA products by gel electrophoresis in the presence of ethidium bromide (data not shown). A similar experiment was performed with Drosophila topo I (Fig. 1C). In this case, the type I topoisomerase was able to fully relax the R-loop.
Corresponding D-loops were also prepared in the same A+T-rich regions of pBR322 and pKK405 by using radiolabeled oligonucleotides (Experimental Procedures). The efficiency of D-loop formation was much less than that of R-loop (on average 10–20%), making detection of the D-loop reaction products by ethidium bromide staining of the gel difficult. Cleavage of the radiolabeled D-loops by Drosophila topo IIIβ was readily detected by PhosphorImager analysis, however, because some of the nicked material retained the labeled DNA (Fig. 1B). Similar to the result with the R-loop substrates, the cleavage for both D-loops was enhanced at a lower temperature of 23°C, in comparison with the reaction at 37°C. Because of the low efficiency of D-loop formation, the majority of the DNA in the reaction was plasmid rather than D-loop. Ethidium bromide staining of the gel revealed that the majority of the DNA was untouched by topo IIIβ (data not shown). This observation again suggests a unique specificity for cleavage of substrate containing a single-stranded region. When similar experiments were performed with Drosophila topo I, the D-loops, like the R-loops, were efficiently relaxed (data not shown). Taken together, these experiments demonstrate that Drosophila topo IIIβ preferentially cleaves both synthetic R-loops and D-loops, without exhibiting any significant relaxation activity toward these substrates.
Cleavage of R-Loop Under Conditions Where Hypernegatively Supercoiled Plasmid Is Relaxed.
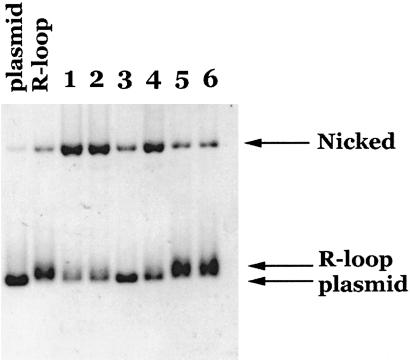
Our earlier results showed that Drosophila topo IIIβ can specifically relax hypernegatively supercoiled DNA (33). The final DNA products of the complete topo IIIβ reaction have a superhelical density similar to that of plasmid DNA. To further examine the substrate specificity of topo IIIβ, we performed a mixing experiment in which R-loop and hypernegatively supercoiled DNA were treated with topo IIIβ in the same reaction (Fig. 2). Under conditions where the hypernegatively supercoiled DNA was relaxed to the level of plasmid DNA supercoiling (as indicated by an upward shift in mobility), the R-loop was cleaved (Fig. 2, lane 3).
Figure 2.
Cleavage of synthetic R-loop under conditions where hypernegatively supercoiled (HNSC) plasmid is relaxed. topo IIIβ was incubated with pBR322 R-loop (lane 1), HNSC plasmid (lane 4), or both R-loop and HNSC plasmid (lane 3) under standard conditions for 1 hr at 37°C. Lane 2 is a no-enzyme control, containing both R-loop and HNSC plasmid. Parental pBR322 plasmid, pBR322 R-loop, and negatively supercoiled (NSC) and hypernegatively supercoiled (HNSC) plasmid markers are as indicated.
In addition to the relaxation, a significant portion of the hypernegatively supercoiled DNA was also cleaved in the mixing reaction. Because efficient R-loop cleavage requires a stoichiometric amount of topo IIIβ, we carried out experiments in which topo IIIβ was incubated at increasing concentrations (enzyme-to-DNA molar ratios from 1 to 50) with either the hypernegatively supercoiled DNA or the R-loop to determine what effect enzyme concentration had on the reaction. While we found that the ratio of relaxed vs. cleaved hypernegatively supercoiled DNA depends on the amount of topo IIIβ enzyme, we observed only cleavage of R-loop, not relaxation, in the range of enzyme concentration tested (data not shown). Titration of topo IIIβ in the R-loop reactions thus confirms that the preferred activity for topo IIIβ on R-loops is cleavage rather than relaxation.
R-Loop and D-Loop Cleavage Is Mediated by a Topoisomerase.
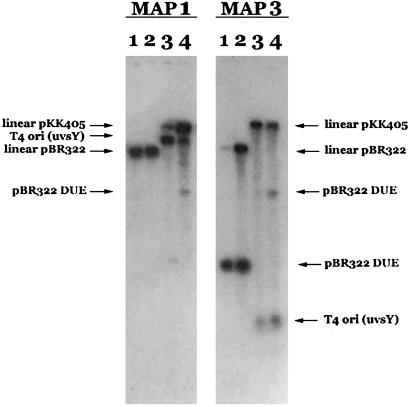
A hallmark of the topoisomerase cleavage reaction is that religation can be affected by a shift in either the temperature or buffer conditions. This reversibility is in contrast to the irreversibility of cleavage reactions carried out by nucleases. To further characterize the R-loop and D-loop cleavage reactions, we investigated whether the cleavage was reversible. R-loop was incubated with topo IIIβ under standard buffer conditions at 23°C. After 1 hr, aliquots were removed and stopped (Fig. 3, lane 1), or subjected to an increase in temperature to 70°C for 10 min (Fig. 3, lane 3), or subjected to an increase in salt concentration for an additional 1 hr at 23°C (to 10 mM MgCl2, 0.5 M NaCl, or 1 M NaCl; Fig. 3, lanes 4, 5, and 6, respectively) before the reaction was stopped. As a control, an aliquot of enzyme was continued at 23°C for an additional hour (Fig. 3, lane 2). All conditions tested resulted in the disappearance of nicked material and a concomitant appearance of R-loop.
Figure 3.
R-loop cleavage is reversible. topo IIIβ was incubated with R-loop under standard conditions at 23°C. Aliquots were removed and stopped directly after 1-hr (lane 1) or 2-hr (lane 2) incubation. In addition, aliquots were removed after 1-hr incubation and heated at 70°C for 10 min before the reaction was stopped (lane 3), or subjected to an increase in salt concentration to 10 mM MgCl2 (lane 4), 0.5 M NaCl (lane 5), or 1 M NaCl (lane 6) and incubated an additional 1 hr at 23°C before stopping the reactions.
Reversibility of the D-loop reactions could also be observed under the conditions stated above (with the exception of the 70°C treatment, which resulted in dissociation of the labeled oligonucleotide, thus not allowing us to track the fate of the cleaved D-loop; data not shown). In addition, we found D-loops could be readily formed on the opposite strands for both plasmids. These D-loops behaved identically with regard to topo IIIβ cleavage and the reversibility treatments (data not shown).
To further rule out the possibility that the cleavage was mediated by a contaminant, we purified a mutant variant of topo IIIβ in which phenylalanine replaced the active-site tyrosine residue (Experimental Procedures). R-loop cleavage was not observed with the Y332F mutant (see Fig. 6, which is published as supporting information on the PNAS web site, www.pnas.org). Furthermore, the cleavage products generated with the wild-type enzyme failed to enter the gel without proteinase K treatment, suggesting a covalent linkage of the enzyme to the cleaved DNA (Fig. 6). These experiments indicate that the cleavage of the R-loops and D-loops is caused by topo IIIβ and not a contaminating nuclease.
Mapping the Cleavage Sites to the Unpaired Strand in the R-Loop and D-Loop Substrates.
The R-loop and D-loop cleavage experiments suggest that the specificity of topo IIIβ lies in its ability to recognize a particular DNA structure rather than a particular nucleotide sequence, because plasmid DNA of identical sequence is not cleaved by topo IIIβ (Fig. 1A). To test whether topo IIIβ cleavage was associated with the loop regions, we used an indirect end-labeling experiment to determine the location of the cleavage sites within the R-loop and D-loop substrates. The strategy for mapping the cleavage sites is shown in Fig. 4. Because the corresponding R-loop and D-loop substrates were formed within the same region of plasmid, each diagram represents both substrates. After cleavage with topo IIIβ, the R-loop and D-loop samples were purified, linearized with BamHI, then electrophoresed under alkaline conditions to separate the two strands of the duplex. The samples were transferred from the gel to nitrocellulose and subjected to Southern blot analysis with radiolabeled probes that anneal within 15–20 bp of the BamHI ends. These probes allow detection of topo IIIβ cleavage along the full length of both strands, as DNA cut by topo IIIβ will migrate at positions ahead of the full-length linear species.
The Southern blots for both pBR322 and pKK405 samples show that topo IIIβ cleavage occurs on the unpaired strand in the region of the loop for both R-loops and D-loops (Fig. 5). The MAP1 blot reveals cleavage within the T4 ori(uvsY) region for pKK405 (lanes 3 and 4), whereas the MAP3 blot shows cleavage of pBR322 within the DUE region (lanes 1 and 2). Equally important is the absence of any major cleavage for the pBR322 samples on the MAP1 blot (lanes 1 and 2) or for the pKK405 samples on the MAP3 blot (lanes 3 and 4).
Figure 5.
Mapping R-loop and D-loop cleavage sites by Southern blotting. The four R-loop and D-loop samples were first incubated with topo IIIβ, purified, and then digested with BamHI before Southern blot analysis. Samples are as follows: pBR322 R-loop (lane 1), pBR322 D-loop (lane 2), pKK405 R-loop (lane 3), and pKK405 D-loop (lane 4). MAP1 and MAP3 probes allow detection of topo IIIβ cleavage along the full length of both strands of the DNA. A section of the gel containing 1-kb DNA ladder was removed and stained with ethidium bromide to determine the size of the fragments on the Southern blot.
While the major cleavage sites map to the unpaired strand in the loop region, the Southern blots also indicate that both pKK405 R-loop and D-loop exhibit a minor amount of cleavage on the paired strand within the T4 ori(uvsY) region, and that the pKK405 D-loop also exhibits a minor amount of cleavage on both strands within the DUE region as well. The pKK405 plasmid contains two highly A+T-rich regions, T4 ori and DUE. It is possible that fraying of helical ends in the loop regions, as well as incidental unwinding in these A+T-rich regions, may account for the minor cleavage by topo IIIβ. In any event, the indirect end-labeling experiments demonstrate that for all four substrates examined, the major site of cleavage corresponds to the unpaired strand in the region of the loop.
Once the primary location and strand of cleavage were determined, the topo IIIβ and BamHI-cut material was further digested with a restriction enzyme that would cleave within 300–400 bp of the looped region, either PstI for the pBR322 samples or PmlI for the pKK405 samples (see Fig. 4). The samples were similarly electrophoresed through an alkaline agarose gel and transferred to nitrocellulose, then probed with radiolabeled MAP4 (pBR322 samples) or MAP5 (pKK405 samples) oligonucleotide (data not shown). Because the cleavage fragments analyzed were now smaller, these blots provided greater resolution than the first set, allowing us to map the cleavage within a resolution of 30 nucleotides. These Southern blots confirmed that topo IIIβ cleaves primarily in the looped region, on the unpaired strand, for all four R-loop and D-loop samples tested.
Cleavage Occurs at Multiple Sites on the Unpaired Strand of the Looped Substrates.
The topo IIIβ cleavage sites were further refined by DNA sequencing to determine their location on the nucleotide level. R-loop and D-loop samples were first cut with topo IIIβ, purified, and then digested with either HindIII (pKK405 samples) or AlwNI (pBR322 samples) (see Fig. 4). The DNA was subjected to a limit number of cycles of linear PCR using 32P-labeled MAP4 (pBR322) or MAP5 (pKK405) primer, then run on a sequencing gel. In addition, linear PCR was performed on plasmid DNA digested with the appropriate restriction enzyme as a control. For comparison, a ladder was prepared from plasmid DNA and run in the lanes next to the sequenced fragments.
The sequencing results show that cleavage appears to occur along the length of the loop region for both plasmids (see Fig. 7, which is published as supporting information on the PNAS web site). Interestingly, the cleavage profiles are similar for the corresponding R-loop and D-loop samples, whereas the plasmid control does not show any cleavage in the same sequences. For pKK405, some minor cleavage does occur outside the loop region, which may be due to unwinding or fraying in the A+T-rich regions extending just beyond that containing the RNA or DNA hybrid. The DNA sequencing experiment further demonstrates that the specificity of topo IIIβ lies in its ability to recognize a particular DNA structure rather than a particular nucleotide sequence.
Discussion
In this paper, we demonstrate that Drosophila topo IIIβ can cleave both R-loops and D-loops formed within plasmids, but does not cleave the parental plasmids themselves. This structure-specific cleavage activity of topo IIIβ contrasts with the activity of the other type I enzyme in Drosophila, topo I, which is able to fully relax both the R-loop and D-loop substrates as well as the parental plasmids. That topo IIIβ preferentially cleaves R-loops and D-loops is surprising, given its ability to relax hypernegatively supercoiled plasmid DNA. Although we suspect the structures of the substrates are similar in that they all contain single-stranded regions, perhaps the activity of the enzyme depends upon the overall structure of the substrate and not just the presence or absence of a single-stranded region. For example, the active site of the enzyme may not be able to accommodate an RNA⋅DNA or DNA⋅DNA duplex, resulting in cleavage of the R-loops and D-loops without subsequent strand passage. If this is the case, then the in vivo activity of the enzyme (cleavage vs. relaxation) may be modulated by the substrate on which the enzyme acts.
In addition, we found the cleavage of the R-loop and D-loop substrates could be reversed by subjecting the samples to an increase in monovalent or divalent salt, or to an increase in temperature. Because the DNA nicking can be completely reversed under some conditions, the cleavable complex of topo IIIβ is fully competent in rejoining the transiently broken DNA strands, indicating that no nucleotides are lost because of topo IIIβ cleavage. It is also interesting to note that the products of the reversibility experiments do not appear to have undergone any detectable change in linking number. Therefore, these experiments demonstrate that topo IIIβ possesses a structure-specific reversible DNA cleavage activity that acts independently of its ability to perform strand-passage events.
We were able to map the topo IIIβ cleavage to the unpaired strand within the loop region by Southern blotting and DNA sequencing. Cleavage appears to occur along the length of the unpaired strand, with corresponding D-loops and R-loops being cleaved at similar sites. Thus, the major determinant in the cleavage reaction is the single-stranded region rather than the hybrid duplex that is formed with either RNA or DNA. Because some of the sites in the loop region are cleaved more efficiently than others, there is a minor sequence preference in these cleavage reactions. However, similar sequences are not cleaved in the corresponding plasmid DNA, suggesting that structural specificity plays a dominant role. Earlier experiments also demonstrated cleavage preference in a plasmid DNA containing single-stranded regions for another type IA enzyme, bacterial ω protein (39). However, in this case, the cleavage was mostly located at the single-stranded region near the forks. Furthermore, bacterial topo I can efficiently remove supercoils from such DNA substrates (40), again suggesting that the eukaryotic and prokaryotic type IA enzymes have different biochemical functions.
The biological functions of eukaryotic topo III are intriguing. The mouse knock-out experiments suggest important, nonoverlapping functions for the two isozymes of topo III: the α form is essential at least for embryonic development, whereas the β form is critical for the life span (41, 42). topo III in yeast is required for meiotic growth, where it is apparently involved in resolving recombination intermediates (43). Mitotic growth also requires topo III function, as a top3 mutant grows slowly and has decreased genomic stability (11). Although the exact biochemical activities of topo III underlying these in vivo functions are not yet known, our DNA cleavage results suggest one possible model. topo III may play a role in cleaving DNA with unusual structures, thereby affecting the formation and/or resolution of recombination intermediates. Because topo III cleavage results in a protected, protein-linked 5′ end and a free 3′-hydroxyl end (33), an interacting partner (like a RecQ helicase such as Sgs1) may function at this structure to generate an extended 3′ end that could serve to initiate strand invasion and recombination. Interestingly, a similar role in the initiation of recombination was proposed for the topo II paralog, Spo11, which makes double-strand breaks in DNA to generate recombinogenic 3′ ends (44). Because some of the recombination intermediates may have a structure similar to D-loops and therefore may also be preferred substrates for the topo III cleavage reaction, topo III could play a role in resolving the recombination structures and thus regulating genetic recombination. Through genetic analysis of synthetic lethals of sgs1 mutants in yeast, recent results suggest that the Mms4–Mus81 heterodimeric endonuclease may substitute for the function of the Sgs1–Top3 pair (45). Interestingly, Mms4–Mus81 is a structure-specific endonuclease capable of cleaving at the forked region of DNA (46). The exact functional linkage connecting the structure-specific cleavage of DNA by topo III and its subsequent processing by a RecQ helicase to their biological function in protecting genome stability will be an exciting area to explore in the future.
Supplementary Material
Acknowledgments
We thank Kathy Dudas and Ken Kreuzer for advice on preparing R-loop and D-loop substrates. This work is supported by National Institutes of Health Grant GM29006.
Abbreviations
- topo I
topo II, and topo III, topoisomerases I, II, and III
- DUE
DNA-unwinding element
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Wang J C. Annu Rev Biochem. 1996;65:635–692. doi: 10.1146/annurev.bi.65.070196.003223. [DOI] [PubMed] [Google Scholar]
- 2.Champoux J J. Annu Rev Biochem. 2001;70:369–413. doi: 10.1146/annurev.biochem.70.1.369. [DOI] [PubMed] [Google Scholar]
- 3.DiGate R J, Marians K J. J Biol Chem. 1988;263:13366–13373. [PubMed] [Google Scholar]
- 4.Hanai R, Caron P R, Wang J C. Proc Natl Acad Sci USA. 1996;93:3653–3657. doi: 10.1073/pnas.93.8.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim R A, Wang J C. J Biol Chem. 1992;267:17178–17185. [PubMed] [Google Scholar]
- 6.Uematsu N, Eda S, Yamamoto K. Mutat Res. 1997;383:223–230. doi: 10.1016/s0921-8777(97)00005-0. [DOI] [PubMed] [Google Scholar]
- 7.Schofield M A, Agbunag R, Michaels M L, Miller J H. J Bacteriol. 1992;174:5168–5170. doi: 10.1128/jb.174.15.5168-5170.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giaever G N, Wang J C. Cell. 1988;55:849–856. doi: 10.1016/0092-8674(88)90140-7. [DOI] [PubMed] [Google Scholar]
- 9.Wallis J W, Chrebet G, Brodsky G, Rolfe M, Rothstein R. Cell. 1989;58:409–419. doi: 10.1016/0092-8674(89)90855-6. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Di Gate R J, Seeman N C. Proc Natl Acad Sci USA. 1996;93:9477–9482. doi: 10.1073/pnas.93.18.9477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gangloff S, McDonald J P, Bendixen C, Arthur L, Rothstein R. Mol Cell Biol. 1994;14:8391–8398. doi: 10.1128/mcb.14.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng S W, Liu Y, Hasselblatt K T, Mok S C, Berkowitz R S. Nucleic Acids Res. 1999;27:993–1000. doi: 10.1093/nar/27.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimamoto A, Nishikawa K, Kitao S, Furuichi Y. Nucleic Acids Res. 2000;28:1647–1655. doi: 10.1093/nar/28.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu L, Davies S L, North P S, Goulaouic H, Riou J F, Turley H, Gatter K C, Hickson I D. J Biol Chem. 2000;275:9636–9644. doi: 10.1074/jbc.275.13.9636. [DOI] [PubMed] [Google Scholar]
- 15.Harmon F G, DiGate R J, Kowalczykowski S C. Mol Cell. 1999;3:611–620. doi: 10.1016/s1097-2765(00)80354-8. [DOI] [PubMed] [Google Scholar]
- 16.Chakraverty R K, Hickson I D. BioEssays. 1999;21:286–294. doi: 10.1002/(SICI)1521-1878(199904)21:4<286::AID-BIES4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 17.Frei C, Gasser S M. J Cell Sci. 2000;113:2641–2646. doi: 10.1242/jcs.113.15.2641. [DOI] [PubMed] [Google Scholar]
- 18.Karow J K, Wu L, Hickson I D. Curr Opin Genet Dev. 2000;10:32–38. doi: 10.1016/s0959-437x(99)00039-8. [DOI] [PubMed] [Google Scholar]
- 19.Mohaghegh P, Hickson I D. Hum Mol Genet. 2001;10:741–746. doi: 10.1093/hmg/10.7.741. [DOI] [PubMed] [Google Scholar]
- 20.Mohaghegh P, Karow J K, Brosh R M, Jr, Bohr V A, Hickson I D. Nucleic Acids Res. 2001;29:2843–2849. doi: 10.1093/nar/29.13.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett R J, Keck J L, Wang J C. J Mol Biol. 1999;289:235–248. doi: 10.1006/jmbi.1999.2739. [DOI] [PubMed] [Google Scholar]
- 22.van Brabant A J, Ye T, Sanz M, German I J, Ellis N A, Holloman W K. Biochemistry. 2000;39:14617–14625. doi: 10.1021/bi0018640. [DOI] [PubMed] [Google Scholar]
- 23.Kogoma T. Microbiol Mol Biol Rev. 1997;61:212–238. doi: 10.1128/mmbr.61.2.212-238.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee D Y, Clayton D A. J Biol Chem. 1998;273:30614–30621. doi: 10.1074/jbc.273.46.30614. [DOI] [PubMed] [Google Scholar]
- 25.Baker T A, Kornberg A. Cell. 1988;55:113–123. doi: 10.1016/0092-8674(88)90014-1. [DOI] [PubMed] [Google Scholar]
- 26.Nossal N G, Dudas K C, Kreuzer K N. Mol Cell. 2001;7:31–41. doi: 10.1016/s1097-2765(01)00152-6. [DOI] [PubMed] [Google Scholar]
- 27.Tian M, Alt F W. J Biol Chem. 2000;275:24163–24172. doi: 10.1074/jbc.M003343200. [DOI] [PubMed] [Google Scholar]
- 28.Tracy R B, Lieber M R. EMBO J. 2000;19:1055–1067. doi: 10.1093/emboj/19.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Drolet M, Phoenix P, Menzel R, Masse E, Liu L F, Crouch R J. Proc Natl Acad Sci USA. 1995;92:3526–3530. doi: 10.1073/pnas.92.8.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masse E, Drolet M. J Biol Chem. 1999;274:16659–16664. doi: 10.1074/jbc.274.23.16659. [DOI] [PubMed] [Google Scholar]
- 31.Masse E, Drolet M. J Mol Biol. 1999;294:321–332. doi: 10.1006/jmbi.1999.3264. [DOI] [PubMed] [Google Scholar]
- 32.Broccoli S, Phoenix P, Drolet M. Mol Microbiol. 2000;35:58–68. doi: 10.1046/j.1365-2958.2000.01671.x. [DOI] [PubMed] [Google Scholar]
- 33.Wilson T M, Chen A D, Hsieh T. J Biol Chem. 2000;275:1533–1540. doi: 10.1074/jbc.275.3.1533. [DOI] [PubMed] [Google Scholar]
- 34.Hotoda N, Hanai R. J Biochem (Tokyo) 2000;127:1109–1113. doi: 10.1093/oxfordjournals.jbchem.a022705. [DOI] [PubMed] [Google Scholar]
- 35.Lee D Y, Clayton D A. J Biol Chem. 1996;271:24262–24269. doi: 10.1074/jbc.271.39.24262. [DOI] [PubMed] [Google Scholar]
- 36.Dudas K C, Kreuzer K N. Mol Cell Biol. 2001;21:2706–2715. doi: 10.1128/MCB.21.8.2706-2715.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mazin A V, Kowalczykowski S C. EMBO J. 1998;17:1161–1168. doi: 10.1093/emboj/17.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaiu W L, Hsieh T S. Mol Cell Biol. 1998;18:4358–4367. doi: 10.1128/mcb.18.7.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirkegaard K, Pflugfelder G, Wang J C. Cold Spring Harbor Symp Quant Biol. 1984;49:411–419. doi: 10.1101/sqb.1984.049.01.047. [DOI] [PubMed] [Google Scholar]
- 40.Kirkegaard K, Wang J C. J Mol Biol. 1985;185:625–637. doi: 10.1016/0022-2836(85)90075-0. [DOI] [PubMed] [Google Scholar]
- 41.Kwan K Y, Wang J C. Proc Natl Acad Sci USA. 2001;98:5717–5721. doi: 10.1073/pnas.101132498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li W, Wang J C. Proc Natl Acad Sci USA. 1998;95:1010–1013. doi: 10.1073/pnas.95.3.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gangloff S, de Massy B, Arthur L, Rothstein R, Fabre F. EMBO J. 1999;18:1701–1711. doi: 10.1093/emboj/18.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keeney S, Giroux C N, Kleckner N. Cell. 1997;88:375–384. doi: 10.1016/s0092-8674(00)81876-0. [DOI] [PubMed] [Google Scholar]
- 45.Mullen J R, Kaliraman V, Ibrahim S S, Brill S J. Genetics. 2001;157:103–118. doi: 10.1093/genetics/157.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaliraman V, Mullen J R, Fricke W M, Bastin-Shanower S A, Brill S J. Genes Dev. 2001;15:2730–2740. doi: 10.1101/gad.932201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.