Abstract
Although organ-specific stem cells possess plasticity that permit differentiation along new lineages, production of endocrine pancreas and insulin-secreting β cells from adult nonpancreatic stem cells has not been demonstrated. We present evidence that highly purified adult rat hepatic oval “stem” cells, which are capable of differentiation to hepatocytes and bile duct epithelium, can trans-differentiate into pancreatic endocrine hormone-producing cells when cultured in a high-glucose environment. These differentiated cells can self-assemble to form three-dimensional islet cell-like clusters that express pancreatic islet cell differentiation-related transcripts detectable by reverse transcription–PCR/nested PCR (e.g., PDX-1, PAX-4, PAX-6, Nkx2.2 and Nkx6.1, insulin I, insulin II, glucose transporter 2, and glucagon) and islet-specific hormones detectable by immunocytochemistry (e.g., insulin, glucagon, and pancreatic polypeptide). In addition, these cells concomitantly lose expression of the hepatocyte protein Hep-par. When stimulated with glucose, these cells synthesize and secrete insulin, a response enhanced by nicotinamide. In a pilot study, the oval cell-derived islet cell-like clusters displayed the ability to reverse hyperglycemia in a diabetic NOD-scid mouse. These results indicate that primary adult liver stem cells can differentiate in a nonlineage-restricted manner. Trans-differentiation into endocrine pancreas could have significant implications for future therapies of diabetes.
During embryogenesis, both the liver and ventral pancreas appear to arise from the same cell population located within the embryonic endoderm (1). Whether these cells differentiate to liver or pancreas tissue is dictated by their locations, local growth factors, and cell adhesion molecule expressions. Thus, it might be assumed that the epithelial cell populations within the pancreas and liver might share common stem cell populations. Evidence suggests that pancreatic stem cells possess the capacity to differentiate into liver cells (2); however, the capacity of liver stem cells to differentiate into pancreatic cells, especially endocrine pancreatic cells, remains unknown.
Liver oval cells, considered to be the hepatic stem cells, have been shown to have bilineage potential, capable of giving rise to both hepatocytes and bile duct cells (3). Oval cell activation, proliferation, and differentiation can be induced in rats exposed first to 2-acetylaminofluorene to suppress hepatocyte proliferation and subsequently partially hepatectomized or treated with carbon tetrachloride (4). Hepatic oval cells can be isolated from these rats by using flow cytometry cell sorting to a purity approaching 97%. These isolated hepatic oval cells express high levels of surface Thy-1.1, cytokeratin-19, OC.2 and OV6, as well as cytoplasmic α-fetoprotein and γ-glutamyl-transpeptidase (2, 3, 5, 6).
It is generally accepted that all endocrine cells of the pancreatic islets of Langerhans, including the glucagon-producing α cells, the insulin-producing β cells, the pancreatic polypeptide-producing γ cells, and the somatostatin-producing δ cells, arise from the same ductal epithelial stem cells through sequential differentiation (7–10). Previous studies have indicated that pancreatic ductal epithelial stem/progenitor cells isolated from prediabetic adult mice can be induced in vitro to produce fully functional islets with the capability to reverse type 1, insulin-dependent diabetes when implanted into diabetic mice (11).
Recently, much attention has focused on the apparent plasticity of adult stem cells, especially the capability of such cells to trans-differentiate into cells of other organs when placed in the environment of a different organ (12–16). In the present study, we have asked whether hepatic oval cells can differentiate into endocrine pancreas and, in particular, cells that can synthesize insulin in response to glucose challenge.
Materials and Methods
Hepatic Oval Cell Isolation and in Vitro Cultures.
Activation of hepatic oval cells was achieved in vivo by using procedures as described by Petersen et al. (4) with the 2-acetylamino-fluorene/hepatic injury model. Activated oval cells were isolated from the intact treated rat livers by using the two-step collagenase perfusion protocol of Seglen (17), then purified by cell sorting for the Thy-1.1-positive cell population (3). The FITC-conjugated anti-rat Thy1.1 antibody was purchased from PharMingen. This technique resulted in hepatic oval cell populations with a purity >95% expressing the hepatic stem cell markers α-fetoprotein, albumin, γ-glutamyl transpeptidase, cytokeratin-19, and OV6 (3).
The purified Thy-1.1-positive hepatic oval cells were cultured in serum-free Iscove's modified DMEM (GIBCO/BRL) supplemented with leukemia inhibitory factor (10 ng/ml), IL-3 (10 ng/ml), stem cell factor (10 ng/ml), and Flt-3 ligand (10 ng/ml). Hepatic oval cell cultures were maintained for at least 6 months before induction of trans-differentiation by switching the cells to RPMI 1640 medium (GIBCO/BRL) supplemented with serum (10% FBS) and high glucose (23 mM, by the addition of 17.5 mM glucose to the medium) (11).
Reverse Transcription (RT) and PCR.
Total RNA was prepared from primary cultured oval cells and trans-differentiated oval cells by using TRIzol (Life Technologies, Rockville, MD). To eliminate genomic DNA contamination, mRNAs were purified by using oligo(dT) cellulose (MicroFastTrack 2.0 Kit, Invitrogen) according to the manufacturer's protocol. Transcriptional gene expressions related to pancreatic organ genesis from these cultures were determined by RT-PCR or nested PCR according to published protocols (11) with some modifications. In addition, DNA-PCR without RT was performed to determine any DNA contamination. All primers were purchased from Life Technologies. All PCRs were performed by using 30 cycles. PCR products were separated by electrophoresis in 2.5% agarose gel, and the sequences of PCR products were confirmed by Big-Dye DNA sequence analysis using the ABI-377 sequencer (Applied Biosystems) following the manufacturer's protocols. Oligonucleotides used for PCR amplification of the following genes were as follows: insulin I (331 bp), forward 5′-ATGGCCCTGTGGATGCGCTT-3′ and reverse 5′-TAGTTGCAGTAGTTCTCCAGCT-3′; insulin II (209 bp), forward 5′-ATGGCCCTGTGGATCCGCTT-3′, nested forward 5′-CCTGCTCATCCTCTGGGAGCC-3′, and reverse 5′-TGCCAAGGTCTGAAGGTCAC-3′; glucose transporter 2 (Glut-2) (304 bp), forward 5′-TTAGCAACTGGGTCTGCAAT-3′, nested forward 5′-TCCAGTACATTGACTTCC-3′, and reverse 5′-GGTGTAGTCCTACACTCATG-3′; PDX-1 (305 bp), forward 5′-CGGCCACACAGCTCTACAAGG-3′, reverse 5′-CTCCGGTTCTGCTGCGTATGC-3′, and nested reverse 5′-TTCCAGGCCCCCAGTCTCGG-3′; Nkx2.2 (188 bp), forward 5′-CACGCAGGTCAAGATCTG-3′ and reverse 5′-TGCCCGCCTGGAAGGTGGCG-3; Nkx6.1 (205 bp), forward 5′-ATGGGAAGAGAAAACACACCAGAC-3′, nested forward 5′-CGAAGTACTTGGCAGGACC-3′, and reverse 5′-TAATCGTCGTCGTCCTCCTCGTTC-3′; PAX-4 (214 bp), forward 5′-TGGCTTTCTGTCCTTCTGTGAGG-3′ and reverse 5′-TCCAAGACTCCTGTGCGGTAGTAG-3′; PAX-6 (545 bp), forward 5′-AAGAGTGGCGACTCCAGAAGTTG-3′ and reverse 5′-ACCACACCTGTATCCTTGCTTCAGG-3′; glucagon (210 bp), forward 5′-GACCGTTTACGTGGCTGG-3′, nested forward 5′-ACAAGGCAGCTGGCAGCATGC-3′, and reverse 5′-CGGTTCCTCTTGGTGTTCATCAAC-3′; somatostatin (187 bp), forward 5′-GTTTCTGCAGAAGTCTCTGG-3′, nested forward 5′-CAGGAACTGGCCAAGTAC-3′, and reverse 5′-AGTTCTTGCAGCCAGCTTTG-3′; hepatocyte growth factor (HGF) (368 bp), forward 5′-CTACACTCTTGACCCTGACAC-3′ and reverse 5′-GCCACGATAACAATCTTGTCC-3′; c-MET (540 bp), forward 5′-ATGCCTTCGAAAGCAACCATT-3′ and reverse 5′-ATGAATAAGTCGACGCGCTGC-3′.
Measurement of Insulin Content and Secretion by Immunoprecipitation and Western Blotting.
Hepatic oval cell cultures that had achieved confluent growth were cultured in the presence or absence of 10 mM nicotinamide for 7 days. The cells were switched to serum-free medium containing 0.5% BSA and 4.5 mM glucose for 5 h, washed twice with serum-free medium, then stimulated by the addition of 17.5 mM glucose (final concentration of 23 mM) for 2 h. The culture media were collected and frozen at −70°C until assayed for insulin content. The intracellular insulin was extracted with lysis buffer and detected by combination of immunoprecipitation and Western blotting, as described (18). The presence of insulin in the culture media and cell lysates was determined by immunoprecipitation with an anti-rabbit insulin polyclonal antibody (Santa Cruz Biotechnology), followed by separation of the precipitated material by SDS/PAGE using 18% gels, transfer to nylon membranes, and blotting with anti-insulin antibodies. Cell lysates (150 μg) from each culture or culture media (1 ml) were subjected to the initial immunoprecipitation. Normal rabbit serum was used as primary antibody control, and culture medium containing 0.5% BSA was used as a control for secreted insulin measurements. Insulin protein was visualized by chemiluminescence. Three separate experiments to determine insulin content and insulin secretion were performed.
Immunocytochemistry.
Sterile microscope slide cover slips were coated with fibronectin and placed in tissue culture plate wells. Trans-differentiated hepatic oval cells were grown to confluency and fixed in cold acetone for 10 min, and each slide was frozen at −70°C until assayed. Immunocytochemistry was carried out with primary antibodies reactive against the endocrine hormones (polyclonal guinea pig anti-rat insulin, polyclonal rabbit anti-rat glucagon, and polyclonal rabbit antipancreatic polypeptide), following the manufacturer's protocols (Dako). Positive reactions were visualized with Vector blue (LSAB Kit, Dako). In addition, purified primary hepatic oval cells were isolated, cytocentrifuged to slides, and examined for hepatic oval “stem” cell markers, as described (3).
Transplantation Studies in Mice.
NOD-scid mice received five i.p. injections of streptozotocin (40 μg/g body weight) every other day, according to published procedures (19, 20). The blood glucose levels were monitored daily by using an Accu-CHEK glucose detector (Roche Diagnostics). The NOD-scid mice were determined to have stable hyperglycemia with blood glucose levels >350 mg/dl. Four days after the fifth streptozotocin injection, 30 oval cell-derived insulin-producing clusters were transplanted to the renal subcapsular space of two mice, and one mouse was transplanted with 200 islet-like clusters divided between the renal subcapsular space and a s.c. site. Three control mice received sham surgery without implants. The blood glucose levels were monitored every 2 days after transplantation.
Results
In Vitro Growth of Hepatic Oval Cells and Their Trans-Differentiation to Endocrine Cells.
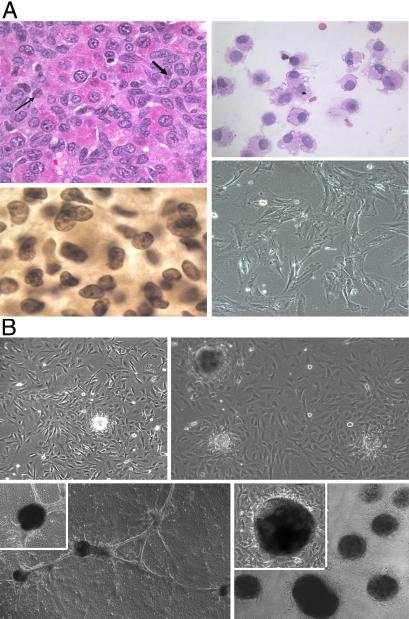
Thy-1+ hepatic oval stem cells were isolated by flow cytometric cell sorting from rat livers after injury with carbon tetrachloride, but blocked from hepatocyte regeneration by treatment with 2-acetylaminofluorene, as described (4). The histology of oval cell proliferation in rat liver is shown in Fig. 1A Upper Left. Isolated oval cells (Fig. 1A Upper Right, hematoxylin/eosin stain) with a purity of >95% were used to establish in vitro cultures that maintained continuous growth for more than 6 months in a serum-free Iscove's modified DMEM supplemented with growth factors. During this culture period, the oval cells maintained their stem-like phenotype (Fig. 1A Lower Right), expressing cytoplasmic α-fetoprotein (Fig. 1A Lower Left), Thy-1, c-kit, and CD34 as determined by immunohistochemistry (data not shown). In addition, they continued to express stem cell associated genes like GATA2 and Tcf-4, as determined by RT-PCR (D. H. Zhang, personal communication). Oval cells 24 h after passage grow in a stellate-like appearance (Fig. 1A Lower Right).
Figure 1.
(A) Isolation and proliferation of hepatic oval stem cells. Hematoxylin/eosin section of activated hepatic oval cells present in the liver of a rat at day 13 after 2-acetylaminofluorene treatment and CCL4 injury (Upper Left). Arrows indicate numerous repopulating small oval cells admixed with scattered large polygonal hepatocytes. These oval stem cells were enriched to >95% purity after FACS cell sorting for Thy-1.1-positive cells (Upper Right). Purified hepatic oval cells stained positive for stem cell markers, including α-fetoprotein (Lower Left), which appeared to be present in both nuclear and cytoplasmic regions. Morphology of hepatic oval cells in primary cultures after growth for 6 months in serum-free Iscove's medium supplemented with growth factors (Lower Right). (B) Proliferation and trans-differentiation of hepatic oval cells under conditions to induce endocrine cell phenotypes. Hepatic oval cells cultured in medium containing serum (10% FCS) and high glucose concentration (23 mM) began to form cell clusters (Upper Left). A single cluster was selected and used to seed secondary cultures. After the cultures reached confluent growth, additional cell clusters formed after prolonged culture in high glucose concentrations (Upper Right). As cultures matured, multiple clusters appeared to become linked by a ductal-like network (Lower Left with an Inset shown at ×200 magnification). Incubation of cells with nicotinamide (10 mM) for 7 days promoted increased numbers of clusters as well as a general increase in size (Lower Right with an Inset showing details of one cluster at ×200 magnification). Composite pictures in A are at ×100 magnification; composite pictures in B are at ×40 magnification.
When the hepatic oval stem cells were replated to a RPMI 1640 medium supplemented with 10% FCS and a high glucose content (23 mM), they began to form at about 2 months of culture small spheroid cell clusters on top of the confluent cell monolayer. From these spheroids of tightly aggregated cells, we selected individual spheroids for clonal passage to allow them to repeat the process described above several times (Fig. 1B Upper). After these cells were cultured 5–7 days in the presence of 10 mM nicotinamide, the number and dimension of these spheroid cell clusters are markedly increased (Fig. 1B Lower Right), and they were connected by ductal like structures (Fig. 1B Lower Left). This three-dimensional cell growth resembles morphologically islet-like clusters, as described by Ramiya et al. (11), Zulewski et al. (21), and Bonner-Weir et al. (22) in their cultures of pancreatic stem/progenitor cells. The experimental approach that the high-glucose medium without leukemia inhibitory factor could induce hepatic oval stem cells to differentiate and form spheroid islet-like clusters has been reproduced several times, and it proves to be highly reproducible. These differentiated oval cells have now been maintained in long-term culture for more than a year by serial 1:2 splits into new T-25 flasks, retaining their ability to produce islet-like clusters. The number of clusters obtained per culture in a flask 25 cm2 in area has ranged from 200 to 500 per collection, and cells have been collected from each flask many times. In established cultures, the development of the islet-like clusters takes about 10–20 days. The cells from various passages containing islet-like clusters have been shown to survive storage at liquid nitrogen and retain their ability to produce the islet-like clusters.
Gene Expression of Endocrine Cell Markers and Hormones by Trans-Differentiated Hepatic Oval Cells.
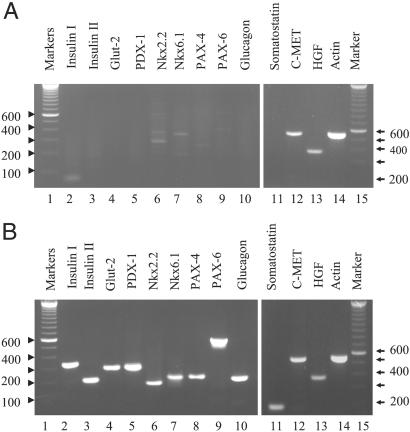
To determine whether the islet-like clusters appearing in the hepatic oval cell cultures may have trans-differentiated to endocrine hormone-expressing cells, the gene expression of a number of endocrine cell differentiation markers and hormones was measured by using nested RT-PCR. All PCR products were sequenced, and each sequence was compared with the published sequences of each gene. Hepatic oval cells cultured for 2 months expressed detectable levels of HGF and its receptor, c-MET, but did not express differentiation markers associated with endocrine pancreas development (i.e., PDX-1, Nkx2.2, Nkx6.1, PAX-4, and PAX-6), Glut-2, or any of the endocrine hormones (Fig. 2A). In contrast, the hepatic oval cell cultures grown in a high concentration of glucose that formed islet-like clusters not only expressed all of the differentiation markers tested (i.e., PDX-1, Nkx2.2, Nkx6.1, PAX-4, and PAX-6) and the cell surface marker, Glut-2, but also the endocrine hormones (i.e., insulin I, insulin II, glucagon, and somatostatin). In addition, these cultures continued to express HGF and c-MET (Fig. 2B). Gene expression studies for both primary cultured hepatic oval cells and trans-differentiated oval cells have been repeated at least three times, and the results showed a similar pattern of gene expression. Interestingly, the hepatic oval cells in the early stage of trans-differentiation expressed much higher levels of PDX-1 than the cells that had differentiated into endocrine hormone expressing, and this down-regulation of PDX-1 appears to correlate with an increased expression of Nkx2.2 (data not shown).
Figure 2.
Expression of mRNA transcripts in cultures of primary oval cell (A) or trans-differentiation cells (B) related to endocrine pancreas development. Total RNA was isolated from cultures of primary and trans-differentiated hepatic oval cell cultures. The mRNA was purified from 100 μg of total RNA by using oligo(dT) columns. cDNA was synthesized from the mRNA by using random hexamer primers. RT-PCR was performed to detect insulin I, Nkx2.2, PAX-6, glucagon, HGF, HGF-receptor (c-MET), and actin, and RT-PCR followed by nested PCR was performed to detect insulin II, Glut-2, PDX-1, Nkx6.1, somatostatin, and PAX-4 mRNA transcripts. The sizes of RT-PCR products are: insulin I, 333 bp; insulin II, 209 bp; Glut-2, 304 bp; PDX-1, 305 bp; Nkx2.2, 188 bp; Nkx6.1, 205 bp; PAX-4, 214 bp; PAX-6, 545 bp; glucagons, 210 bp; somatostatin, 187 bp; HGF, 368 bp; and c-MET, 540 bp.
Endocrine Hormone Synthesis by Trans-Differentiated Hepatic Oval Cells.
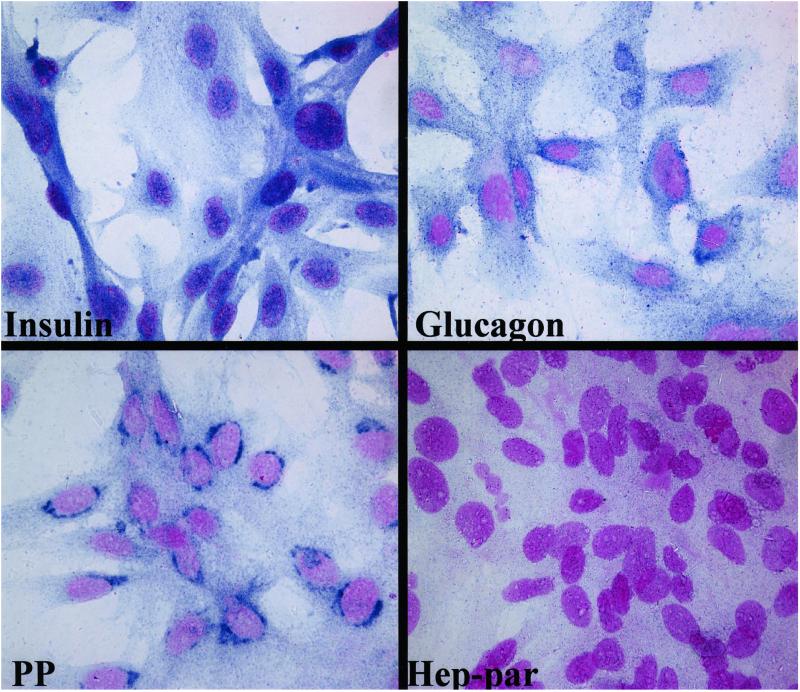
To determine whether the hepatic oval cells subjected to culture conditions inducing transcription of endocrine cell differentiation markers and hormones actually synthesized the endocrine hormone proteins, the trans-differentiated cells were grown on glass coverslips coated with fibronectin until confluence, fixed with acetone, then stained with anti-insulin, antiglucagon, antisomatostatin, and antipancreatic polypeptide antibodies. The cells stained strongly for glucagon and pancreatic polypeptide, but stain for insulin appeared to be more diffused (Fig. 3). A majority of cells stained with both anti-insulin and antiglucagon antibodies, but the cells did not stain with antisomatostatin antibody (data not shown). Furthermore, the trans-differentiated cells did not stain for the hepatocyte protein, Hep-par. Thus, these results are similar to those reported for differentiation of pancreatic ductal stem cells in culture (11). Similar experiments were repeated three times and results obtained were consistent.
Figure 3.
Expression of islet hormones in trans-differentiated oval cells. The presence of the endocrine cell hormones including insulin, glucagon, and pancreatic polypeptide (PP), as well as the hepatocyte marker Hep-par, in the trans-differentiated hepatic oval cells was detected by immunohisto-cytochemical staining. Strong staining was seen with primary antibodies to glucagon and pancreatic polypeptide. Weak staining was occasionally seen with primary antibody to insulin, whereas no staining occurred with antibody to Hep-par. Specific antibody staining was visualized with Vector blue and nuclei were counterstained with Nuclear Fast red. Results shown here represent one of three similar experiments. (Original magnification: ×1,000.)
Insulin Responses After Challenge with Glucose and/or Nicotinamide.
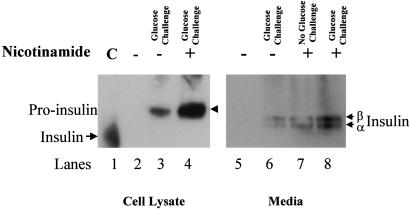
Despite the diffuse cytoplasmic staining for insulin shown in Fig. 3, the trans-differentiated hepatic oval cells apparently synthesize and store detectable levels of insulin in response to nicotinamide treatment and glucose challenge, as determined by immunoprecipitation and Western blot analyses (Fig. 4 Left). However, the anti-insulin antibody staining on Western blots indicates that the cells store insulin in its proinsulin form, which may be one reason insulin has proven difficult to detect by immunohistocytochemistry. When the cells were first treated with 10 mM nicotinamide for 7 days before glucose challenge, there was an increase in detectable (pro)-insulin in the cell lysate. The amount of increased proinsulin in the treated cells was determined by densitometry analysis to be 3.4 times over that in untreated cells. Similar results were obtained when the culture medium was examined for secreted insulin after glucose challenge of the cells untreated versus treated with nicotinamide (Fig. 4 Right).
Figure 4.
Insulin synthesis and secretion in trans-differentiated hepatic oval cells. Trans-differentiated hepatic oval cell cultures were maintained 7 days in the absence or presence of 10 mM nicotinamide, then stimulated for 2 h with glucose (23 mM) in serum-free medium. Insulin contents within the cells and insulin secretions were measured after collection of the media or preparation of cell lysates. The cell lysates and culture medium were pooled from three independent culture flasks. Insulin was immunoprecipitated from either the cell lysates (Left) or media (Right). The precipitated material was separated on SDS/PAGE, transferred to nylon membranes, and developed with anti-insulin antibodies. Insulin control (lane 1), normal rabbit serum (lanes 2 and 5), rabbit anti-rat insulin antibody (lanes 3–4 and 6–8). Growth of the cells in nicotinamide before glucose stimulation increased the levels of insulin detected (lane 4 versus 3; lanes 7 and 8 versus 6).
Preliminary in Vivo Animal Study.
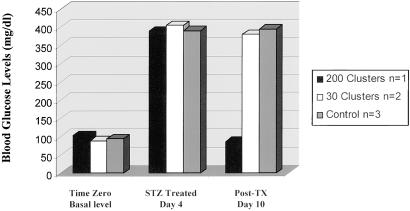
Previous studies with in vitro-grown islets revealed that full functional maturation required implantation into an in vivo environment (11). The ability of oval cell-derived islet-like cell clusters to reverse hyperglycemia was examined in vivo with streptozotocin-induced diabetic NOD-scid mice as recipients. NOD-scid mice were chemically induced into a diabetic state through multiple treatments with low-dose streptozotocin. Thirty oval cell-derived insulin-producing clusters were transplanted to the renal subcapsular space of two mice, and one mouse was transplanted with 200 islet-like clusters divided between the renal subcapsular space and a s.c. site. As shown in Fig. 5, the mouse receiving 200 islet-like clusters exhibited normalized blood glucose levels 10 days after implantation. Control animals that did not receive implants showed a persistent hyperglycemia. In addition, the two diabetic mice that received only renal subcapsular transplantation also exhibited persistent hyperglycemia. Examination of the histologic sections of the implants revealed increased microblood vessel networking. In addition, insulin immunohistochemical staining demonstrated strong insulin staining in the cytoplasm of the implanted clusters (data not presented). These preliminary results provide evidence to support our concept that immature β cells in these clusters matured further in the in vivo environment and functioned in response to the high blood glucose levels. These results are similar to a previously published study (11) demonstrating similar results with pancreatic stem cell-derived islet clusters.
Figure 5.
Reversal of hyperglycemia in diabetic animals. NOD-scid mice 12 weeks of age were treated with streptozotocin (STZ, 40 μg/g weight of the animal) in PBS. The treated mice became hyperglycemic within 4 days, at which time two mice were transplanted with 30 oval cell-derived islet-like clusters into the subrenal capsular space and one mouse was transplanted with 200 oval cell-derived islet-like clusters into the subrenal capsular space and s.c. location. Within 10 days of implantation, the mouse receiving 200 islet-like clusters showed normal blood glucose levels (as shown), whereas mice receiving only 30 islet-like clusters remained hyperglycemic. Control animals that did not receive implants showed a persistent hyperglycemia. Tx, transplantation.
Discussion
The present study strongly suggests that cultures of purified hepatic oval stem cells possess the capacity to trans-differentiate into functional endocrine cells, including insulin-secreting cells, after long-term culture in vitro with culture conditions similar to those permitting pancreatic stem cells to differentiate to insulin-secreting β cells (11). Induction of this trans-differentiation of hepatic oval cells proved to depend on two factors: removal of leukemia inhibitory factor, a factor known to inhibit stem cell differentiation, and a concomitant increase in the concentration of glucose, a factor known to promote growth and differentiation of β cells (10, 23). Of importance, the trans-differentiation of hepatic oval cells observed under the current culture conditions resulted in the appearance of the various endocrine cell types associated with the pancreatic islets.
RT-PCR analyses of the differentiating cells revealed the temporal appearance and increased expression of mRNA transcripts for a number of differentiation markers of endocrine pancreas, including PDX-1, PAX-4, PAX-6, Nkx2.2, and Nkx6.1, as well as the endocrine hormones insulin, glucagon, and somatostatin. Interestingly, the early stages of the trans-differentiation showed much higher levels of PDX-1 than cells expressing the endocrine hormones. This down-regulation of PDX-1 correlated with an increased expression of Nkx2.2 (data not presented), similar to results recently reported by Lumelsky et al. (24). An endocrine pancreas phenotype was supported further by immunocytochemistry and Western blotting that showed the presence of the endocrine hormones in the cytoplasm of the trans-differentiated cells. Lastly, the possibility that hepatic oval cells may give rise to functional β cells is supported by a regulated insulin response to glucose challenge and our pilot in vivo animal study showing reversal of hyperglycemia 10 days after implantation. Interestingly, two diabetic mice receiving only renal subcapsular transplantation exhibited no significant change in blood glucose levels, indicating that the number of implanted islet-like clusters could be important because these two mice only received 30 clusters each. Another possibility for lack of an effect in these two mice may be the implanted time was not sufficient for the implants to grow, expand, and mature. Nevertheless, the immature β cells in these implanted clusters appeared to mature in vivo and express increased insulin content in response to the high blood glucose levels of the diabetic mice.
For the past 40 years, the origin of the hepatic oval cells has been thought to be the bile duct epithelium (25) within the periportal region, presumably in a structure called canals of Hering (26). Recent studies, however, have shown that hepatic oval cells may be derived from hematopoietic stem cells (27). For example, adult male rodent bone marrow contains a subpopulation of cells capable of engraftment into adult female rat livers with liver injury (27), then differentiation to hepatocytes (28, 29). In addition, the potential plasticity of the hepatic oval cells was shown in a recent study in which the hepatic stem-like cell line WB-F344 differentiated into cardiac myocytes (12). Considered together, the hepatic oval cell has now been shown to be able to differentiate to hepatocytes, bile duct epithelium, myocytes, and endocrine pancreas. Thus, based on recent studies showing a bone marrow to oval cells to hepatic pathway, it is possible to hypothesize that bone marrow-derived stem cells may be able to differentiate in vitro into insulin-producing cells.
The current study represents a potential approach in cell therapy-based treatment of insulin-dependent diabetes by generating insulin-producing cells from stem cells derived from nonpancreatic tissue. As such, hepatic oval stem cells and possibly bone marrow-derived stem cells may provide a realistic hope that adult stem cells from one tissue lineage can trans-differentiate along the lineage of other tissues, thus the development of a simple, reliable procedure to obtain autologous stem cells capable of differentiating into functional insulin-producing cells could provide a potentially unlimited source of islet cells for transplantation, thus helping to solve the major problems of availability and allogeneic rejection. It is also critical that this phenomenon be further explored to determine how this trans-differentiation of hepatic stem cells, and possibly bone marrow stem cells, into endocrine pancreas can be best exploited.
Acknowledgments
This work was supported in part by National Institutes of Health Grants DK58614 and DK60015 to B.E.P.
Abbreviations
- RT
reverse transcription
- Glut-2
glucose transporter 2
- HGF
hepatocyte growth factor
References
- 1.Sell S, Ilic Z. Liver Stem Cells. New York: Chapman and Hall; 1997. [Google Scholar]
- 2.Dabeva M D, Hwang S G, Vasa S R, Hurston E, Novikoff P M, Hixson D C, Gupta S, Shafritz D A. Proc Natl Acad Sci USA. 1997;94:7356–7361. doi: 10.1073/pnas.94.14.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen B E, Goff J P, Greenberger J S, Michalopoulos G K. Hepatology. 1998;27:433–445. doi: 10.1002/hep.510270218. [DOI] [PubMed] [Google Scholar]
- 4.Petersen B E, Zajac V F, Michalopoulos G K. Hepatology. 1998;27:1030–1038. doi: 10.1002/hep.510270419. [DOI] [PubMed] [Google Scholar]
- 5.Shiojiri N, Lemire J M, Fausto N. Cancer Res. 1991;51:2611–2620. [PubMed] [Google Scholar]
- 6.Lemire J M, Shiojiri N, Fausto N. Am J Pathol. 1991;139:535–552. [PMC free article] [PubMed] [Google Scholar]
- 7.Gu D, Sarvetnick N. Development (Cambridge, UK) 1993;118:33–46. doi: 10.1242/dev.118.1.33. [DOI] [PubMed] [Google Scholar]
- 8.Swenne I. Diabetologia. 1992;35:193–201. doi: 10.1007/BF00400917. [DOI] [PubMed] [Google Scholar]
- 9.Hellerstrom C, Swenne I. Diabetes. 1991;40, Suppl. 2:89–93. doi: 10.2337/diab.40.2.s89. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg L, Vinik A I. Adv Exp Med Biol. 1992;321:95–104. doi: 10.1007/978-1-4615-3448-8_11. [DOI] [PubMed] [Google Scholar]
- 11.Ramiya V K, Maraist M, Arfors K E, Schatz D A, Peck A B, Cornelius J G. Nat Med. 2000;6:278–282. doi: 10.1038/73128. [DOI] [PubMed] [Google Scholar]
- 12.Malouf N N, Coleman W B, Grisham J W, Lininger R A, Madden V J, Sproul M, Anderson P A. Am J Pathol. 2001;158:1929–1935. doi: 10.1016/S0002-9440(10)64661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kopen G C, Prockop D J, Phinney D G. Proc Natl Acad Sci USA. 1999;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pereira R F, Halford K W, O'Hara M D, Leeper D B, Sokolov B P, Pollard M D, Bagasra O, Prockop D J. Proc Natl Acad Sci USA. 1995;92:4857–4861. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bjornson C R, Rietze R L, Reynolds B A, Magli M C, Vescovi A L. Science. 1999;283:534–537. doi: 10.1126/science.283.5401.534. [DOI] [PubMed] [Google Scholar]
- 16.Krause D S, Theise N D, Collector M I, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis S J. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 17.Seglen P O. J Toxicol Environ Health. 1979;5:551–560. doi: 10.1080/15287397909529766. [DOI] [PubMed] [Google Scholar]
- 18.Yang L J, Rhee S G, Williamson J R. J Biol Chem. 1994;269:7156–7162. [PubMed] [Google Scholar]
- 19.Elliott J I, Dewchand H, Altmann D M. Clin Exp Immunol. 1997;109:116–120. doi: 10.1046/j.1365-2249.1997.4241319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerling I C, Friedman H, Greiner D L, Shultz L D, Leiter E H. Diabetes. 1994;43:433–440. doi: 10.2337/diab.43.3.433. [DOI] [PubMed] [Google Scholar]
- 21.Zulewski H, Abraham E J, Gerlach M J, Daniel P B, Moritz W, Muller B, Vallejo M, Thomas M K, Habener J F. Diabetes. 2001;50:521–533. doi: 10.2337/diabetes.50.3.521. [DOI] [PubMed] [Google Scholar]
- 22.Bonner-Weir S, Taneja M, Weir G C, Tatarkiewicz K, Song K H, Sharma A, O'Neil J J. Proc Natl Acad Sci USA. 2000;97:7999–8004. doi: 10.1073/pnas.97.14.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sjoholm A, Welsh N, Sandler S, Hellerstrom C. Am J Physiol. 1990;259:C828–C833. doi: 10.1152/ajpcell.1990.259.5.C828. [DOI] [PubMed] [Google Scholar]
- 24.Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Science. 2001;292:1389–1394. doi: 10.1126/science.1058866. [DOI] [PubMed] [Google Scholar]
- 25.Sell S. Cancer Res. 1990;50:3811–3815. [PubMed] [Google Scholar]
- 26.Theise N D, Saxena R, Portmann B C, Thung S N, Yee H, Chiriboga L, Kumar A, Crawford J M. Hepatology. 1999;30:1425–1433. doi: 10.1002/hep.510300614. [DOI] [PubMed] [Google Scholar]
- 27.Petersen B E, Bowen W C, Patrene K D, Mars W M, Sullivan A K, Murase N, Boggs S S, Greenberger J S, Goff J P. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- 28.Lagasse E, Connors H, Al Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman I L, Grompe M. Nat Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 29.Theise N D, Badve S, Saxena R, Henegariu O, Sell S, Crawford J M, Krause D S. Hepatology. 2000;31:235–240. doi: 10.1002/hep.510310135. [DOI] [PubMed] [Google Scholar]