Abstract
Stable isotope analysis is widely promoted as a practical method for tracing the geographic origins of migratory birds. However, the extent to which geospatial patterns of isotope ratios in avian tissues are influenced by age-specific, altitudinal, and temporal factors remains largely unexplored. We measured carbon (13C/12C) and nitrogen (15N/14N) isotope ratios in feathers of black-throated blue warblers (Dendroica caerulescens) breeding along a relatively steep altitudinal gradient in the Appalachian Mountains to evaluate the effects of altitude and year on the isotopic signatures of yearling (first breeding season) and older males (>2 years). Breeding males (n = 302) collected during 7 consecutive years exhibited significant age-specific and altitudinal effects in δ13C values and age-specific and temporal effects in δ15N values. The δ13C values of older males increased with altitude at the rate of ≈1.3‰ per 1,000 m, suggesting a high degree of year-to-year philopatry to narrow altitudinal zones, if not to breeding territories. In contrast, absence of altitudinal patterns in yearlings most likely reflects natal dispersal. Carbon isotope variation (δ13C = −26.07 to −20.86‰) observed along a single altitudinal transect (755 m) nearly brackets the range of δ13C values recorded in feathers across the North American breeding range of the warbler from Georgia to New Brunswick (11° of latitude) and from New Brunswick to Michigan (22° of longitude). These data indicate that age-specific and altitudinal effects must be considered when using δ13C values to delineate the geographic origin of avian species with large altitudinal and latitudinal ranges.
Recognition that stable-isotope signatures of animal tissues can be used to study the trophic ecology (1–4), nutritional status (5–7), and geographic origins of animals (8–11) has lead to an explosive burst of research in what is arguably a new frontier in animal ecology (12, 13). Of particular interest, the linkage of breeding and wintering ranges of migratory songbird populations has been frustrated by the lack of population-specific morphological and genetic markers, by the infinitesimally low recapture rates of marked birds, and because the majority of species are too small to carry powerful satellite transmitters. Recent analyses have shown that isotopic patterns in the feathers of migratory birds vary systematically across continental areas (8, 14–18), reflecting the natural variation of isotopes in food and water during the period of feather growth (19, 20). Fully grown feathers are metabolically inert, and the isotopic composition of feathers, with the exception of exchangeable hydrogen, is believed to be fixed (8, 11–18). These studies suggest that a combination of isotopic tracers may eventually provide a potent and cost-effective method of linking breeding and wintering populations of most songbirds that breed at temperate latitudes.
Despite the promise of stable isotope analysis in elucidating migratory dynamics and the trophic ecology of animals, ecologists have not critically explored the extent to which geospatial patterns of isotopic signals in tissues are influenced by altitudinal, temporal, and demographic factors. The principal obstacle to testing multifactorial hypotheses for avian species is obtaining sufficient numbers of specimens stratified by sex, age class, year of collection, molt, altitude, and geographic location. Recent analyses of breeding populations of black-throated blue warblers (Dendroica caerulescens) have revealed intriguing continental gradients in δ13C and δD values in feathers, which have been used to link breeding populations from the C3-dominated forests of eastern North America with wintering populations in the Greater Antilles (8, 11). Interestingly, δ13C values appear to be inversely correlated with latitude on the breeding range, with the highest values occurring in high-altitude populations from the southern Appalachian Mountains (<36° N) and the lowest values occurring north of 44° N at relatively low altitudes. This pattern runs counter to the latitudinal trends in δ13C values observed at low altitudes among many species of C3 plants (21) and in monarch butterflies (22), whose larvae feed exclusively on C3 milkweeds. The cause of this empirical paradox is unknown, but it may be due to strong altitudinal influences on photosynthesis (23–28) transmitted through C3-based food webs. Analysis and interpretation of continental gradients of δ13C values may be further complicated by sampling effects linked to year-to-year fluctuations in the isotopic composition of feathers within warbler populations and by pooling data from different age classes of warblers with contrasting dispersal behaviors.
We tested this hypothesis by analyzing the carbon (13C/12C) and nitrogen (15N/14N) isotope ratios in feathers of black-throated blue warblers collected over seven consecutive breeding seasons along an altitudinal gradient in the Appalachian Mountains. By restricting the study to territorial males (n = 302) inhabiting a relatively small montane watershed, we were able to focus on within-locality patterns of isotopic variation and factor out variation due to sexual differences in isotope assimilation or dispersal behavior. With a series of regression models, we sought to (i) characterize the significance of year-to-year variation of δ13C and δ15N values in warbler populations; (ii) determine whether yearlings (first breeding season) and older males (≥2 years) differed in isotopic composition; (iii) examine the relationship between body mass and the isotopic signatures of feathers grown the previous year; and (iv) evaluate the correlation between isotope signatures and the altitude of breeding territories. Finally, we address the role of dispersal and philopatry in the resolution of altitudinal and latitudinal patterns in isotopic composition of avian tissues and the usefulness of δ13C data in determining the geographic origins of migratory birds with large altitudinal and latitudinal breeding ranges.
Materials and Methods
Field Site.
Research was conducted in the Big Santeetlah Creek watershed (35° 21′ N, 84° 00′ W; 680–1,689 m above sea level), a heavily forested basin (5,350 ha), on the eastern slope of the Unicoi Mountains, a subdivision of the southern Appalachian Mountains, in Graham County, North Carolina (Fig. 1). This region of the southern Appalachians supports one of the most taxonomically diverse woody floras north of Mexico (29). Forestry practices in the watershed since the 1920s have resulted in a mosaic of even-aged stands (x̄ = 69.8 ± 41.0 years, n = 100 stands) of hardwood-hemlock forest dominated by C3 plants. The watershed is embedded in the largest contiguous tract of montane forest in eastern North America. Small patches of grassland (balds) occur at the summit of peaks (>1,600 m) that rim the watershed. Agricultural crops (e.g., Zea mays and other C4 plants) have not been cultivated in the watershed in more than 70 years.
Figure 1.
The core breeding distribution (A, stippled area) of the black-throated blue warbler (D. caerulescens) in eastern North America showing the location of the Santeetlah Creek watershed (B) in western North Carolina (contour lines in meters). Watershed boundaries are marked with a broken line.
The Focal Species.
Breeding populations of black-throated blue warblers south of 40° N are restricted to higher altitudes in the Appalachian Mountains, where it is one of the most common breeding species (30–32). An estimated 2,000–2,500 pairs breed annually in the Big Santeetlah Creek watershed between 800 and 1,400 m (above sea level). Territories (0.75–3.0 ha) are established by males as early as the first week of May and are defended through early August (30). The diet of nestlings and adults during the breeding season is predominately lepidopteran larvae (30, 33).
Two age classes of black-throated blue warblers can be distinguished by plumage characters in breeding specimens (30, 34): (i) yearlings hatched the previous season and in their first breeding season [first alternate plumage (SY in banding terminology)], and (ii) older individuals in their second or later breeding season [definitive alternate plumage (ASY in banding terminology)]. Warblers undergo a complete molt on or near their breeding or natal territories in July and August before fall migration, so that flank feathers sampled in year t + 1 were grown on the breeding grounds in year t.
Population Sampling.
Territorial males were collected annually along a transect spanning the altitudinal range (790–1,545 m) of the species in the Santeetlah Creek watershed. To ensure that neither migrating nor wandering postbreeding warblers were collected, populations were sampled during the peak of the breeding season and before the annual basic molt: 18–23 June 1995 (n = 39), 12–20 June 1996 (n = 48), 11–19 June 1997 (n = 43), 10–18 June 1998 (n = 35), 10–20 June 1999 (n = 42), 10–18 June 2000 (n = 45), and 9–17 June 2001 (n = 50).
Specimens were packaged and frozen whole in liquid nitrogen immediately after death. Body mass was measured to the nearest 0.1 g. The altitude of territories was determined with a Thommen altimeter calibrated twice daily from landmarks on U.S. Geological Survey 7.5-min topographic maps. Voucher specimens (rounded skins, partial skeletons, and tissue samples) were deposited in the research collections of the National Museum of Natural History, Smithsonian Institution, Washington, DC.
Isotopic Analysis.
Before isotopic analysis, two or three flank feathers from each specimen were gently washed in detergent and water and then soaked in a 2:1 chloroform/methanol mixture to remove lipids (2). After drying at room temperature for several days, a feather (1–3 mg) from each specimen was loaded into a clean tin capsule and weighed to the nearest ±1 μg. Capsules were then sealed and placed in the autosampler of a Carlo Erba Elemental Analyzer NA 2500 (Milan), attached to a continuous flow isotope ratio mass spectrometer [Finnigan Delta +XL (Finnigan-MAT, San Jose, CA)] for carbon and nitrogen isotope analysis. Samples were converted to CO2 and N2 in oxidation/reduction furnaces, separated by gas chromatography, and then measured for 13C/12C and 15N/14N ratios on the mass spectrometer. An internal N2(g) working standard was admitted before the introduction of each sample, and a CO2(g) standard was admitted at the conclusion of each combustion for calibration to AIR (nitrogen) and Pee Dee belemnite carbon international standards (35, 36). Stable isotope ratios are reported in per mil units (‰) by using standard δ notation (37). External working standards of dogfish muscle and liver (DORM-2) were reproducible to better than ± 0.2‰ (1 σ SD) for both δ13C and δ15N.
Data Analysis.
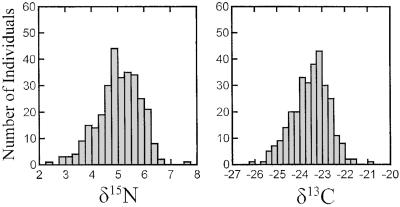
Values of δ13C and δ15N (Fig. 2), partitioned by age class [first alternate plumage (SY) or definitive alternate plumage (ASY)], were normally distributed (Lilliefors test, P > 0.05). Therefore, we used parametric tests throughout. The effects of collection year (entered as categorical variable), age class (SY or ASY), and altitude on δ13C and δ15N values were examined with multivariate general linear models. We used simple least squares linear regression to investigate the relationship between isotope values and altitude and body mass. The relationship between δ13C and δ15N values was explored with bivariate scatterplots and Pearson correlation coefficients.
Figure 2.
Histogram of δ15N and δ13C values of black-throated blue warblers (age classes combined) from the Santeetlah Creek watershed.
Results
Stable Carbon Isotopes.
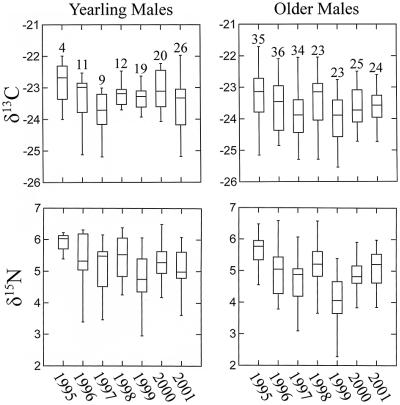
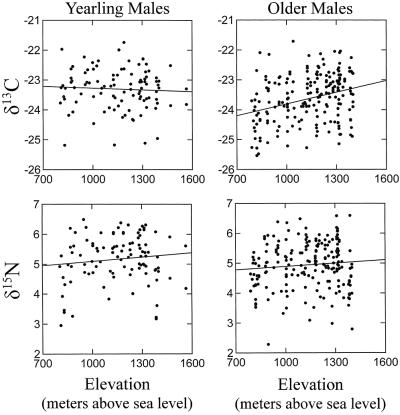
The δ13C values of feathers showed significant age-specific effects (Table 1, Fig. 3). Feathers of yearlings (x̄ = −23.3 ± 0.7; n = 101) exhibited higher δ13C values (+0.4‰) than those of older males (x̄ = −23.7 ± 0.7; n = 200). Analyses of the pooled sample of yearlings and older males revealed marginally significant year effects (P = 0.07) and strongly significant altitudinal effects (P < 0.001) on δ13C values (Fig. 4). We then analyzed each age class separately. Year (F6,93 = 0.82; P = 0.55) and altitudinal effects (F1,93 = 0.09; P = 0.76) were insignificant for yearlings. However, δ13C values of older males were significantly influenced by year of collection (F6,192 = 3.90; P = 0.001) and altitude (F1,192 = 22.30; P < 0.00001). Year-to-year differences (e.g., 1996–1997) in mean δ13C values ranged from 0.00‰ to 0.51‰ in yearlings and from 0.07‰ to 0.56‰ in older males. Based on least squares regression, δ13C values of feathers from older males increased with altitude at the rate of ≈1.3‰ per 1,000 m of altitude (δ13C = 0.00131 [altitude] −25.1; n = 200; R2 = 0.09; P < 0.0001). Body mass (g) was uncorrelated with δ13C values in yearlings (n = 97; R2 = 0.02; P = 0.23) and in older males (n = 194; R2 = 0.01; P = 0.11).
Table 1.
Results from separate analyses of δ13C and δ15N values of feathers of black-throated blue warblers (D. caerulescens) from the Big Santeetlah Creek watershed
| Isotope | Factors | Sum of squares | df | Mean squares | F ratio | P value |
|---|---|---|---|---|---|---|
| δ13C | Age | 8.269 | 1 | 8.269 | 14.397 | 0.0002 |
| Year | 6.737 | 6 | 1.123 | 1.955 | 0.07 | |
| Age by year | 2.351 | 6 | 0.392 | 0.682 | >0.1 | |
| Altitude | 7.534 | 1 | 7.534 | 13.118 | 0.0004 | |
| Error | 164.254 | 286 | ||||
| δ15N | Age | 6.297 | 1 | 6.297 | 12.723 | 0.0004 |
| Year | 27.408 | 6 | 4.568 | 9.229 | <10−6 | |
| Age by year | 2.859 | 6 | 0.476 | 0.963 | >0.1 | |
| Altitude | 1.319 | 1 | 1.319 | 2.665 | 0.1 | |
| Error | 141.553 | 286 |
Figure 3.
Box plots (71) depicting the annual fluctuation of δ13C and δ15N values in yearling and older males. Numbers over boxes represent sample size. Horizontal bars represent median values.
Figure 4.
Relationship between δ13C and δ15N values of feathers of black-throated blue warblers (D. caerulescens) and the altitude of breeding territories (meters above sea level).
Stable Nitrogen Isotopes.
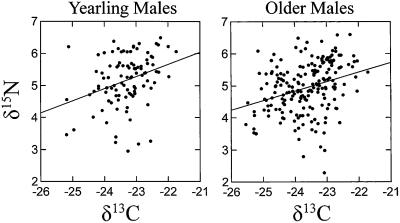
Feathers exhibited significant age-specific effects in δ15N values (Table 1, Fig. 3). Feathers from yearling males (x̄ = 5.2 ± 0.8; n = 101) exhibited higher δ15N values (+0.2‰) than those of older males (x̄ = 5.0 ± 0.8; n = 200). Analyses of the pooled sample of yearlings and older males revealed significant year effects (P < 0.00001) and marginally significant altitudinal effects (P = 0.10). We again partitioned the population sample by age class. In yearlings, the effect of collection year on δ15N values was marginally significant (F6,93 = 1.97; P = 0.08) and altitudinal effects were insignificant (F1,93 = 0.69; P = 0.41). On the other hand, δ15N values in older males were strongly influenced by year of collection (F6,192 = 14.65; P < 0.00001), whereas altitudinal effects were insignificant (F1,192 = 2.03; P = 0.16). Year-to-year differences in mean δ15N values ranged from 0.07 to 0.74‰ in yearlings and from 0.23 to 1.12‰ in older males. Body mass (g) and δ15N values were marginally correlated in yearlings (n = 97; R2 = 0.06; P = 0.02) but were uncorrelated in older males (n = 194; R2 = 0.00; P = 0.66). Finally, there was a positive correlation between δ13C and δ15N values in yearlings (n = 101; R2 = 0.10; P = 0.001) and in older males (n = 200; R2 = 0.10; P < 0.0001) (Fig. 5).
Figure 5.
Relationship between δ15N and δ13C values of feathers of yearling (δ15 n = 0.35 [δ13C] + 13.33) and older male (δ15 n = 0.31 [δ13C] + 12.26) black-throated blue warblers (D. caerulescens).
Discussion
Several environmental and demographic factors correlated with δ13C and δ15N values of feathers in black-throated blue warblers in the Santeetlah Creek watershed. Perhaps the least anticipated finding was that δ13C and δ15N values exhibited significant year-to-year variation. The cause of this temporal fluctuation is unknown, but δ15N values of feathers may be indirectly influenced by factors such as soil nitrogen dynamics (38, 39), water availability (40–44), and plant nitrogen storage strategies (45–49) that affect the isotopic composition of C3-based food webs. These processes are controlled to varying degrees by climatological parameters such as rainfall and temperature. Plants subjected to drought conditions (low soil water availability) often show relative increases of δ15N in biomass (41, 50, 51), and several studies have documented an inverse correlation between rainfall and δ15N values in vertebrate tissues (52–55).
The drought-stress hypothesis cannot be directly addressed because we did not systematically monitor temporal and altitudinal variation in soil moisture and foliar isotope values during the seven seasons of feather growth. Whatever the cause, significant year-to-year fluctuations in nitrogen and carbon isotope signatures in feathers, within a local watershed, indicate that temporal effects may bias analyses of continent-wide isotope gradients if geographically dispersed populations are sampled in different years.
A second unexpected result was that δ13C and δ15N values were greater in yearlings than in older males. Although age-specific differences in isotope signatures have been reported in mammals (56, 57) and marine birds (58), this phenomenon is poorly known in insectivorous songbirds (18, 59). Age-specific variation in black-throated blue warblers may be explained by several interrelated hypotheses involving nutrition. First, fledglings may consume food items from a higher trophic level than those ingested by adults. However, the diets of both fledglings and adults during the period of feather growth are believed to consist primarily of lepidopteran larvae (30, 33) procured from the same sources. A second possibility is that fledglings may disperse to drier or suboptimal habitats and experience a higher degree of nutritional stress during the basic molt, leading to catabolism of proteins and the consequent increases of δ15N in feathers (5–7, 13, 60). A third possibility is that fledglings exhibit a different pattern of isotopic fractionation from that of adults and incorporate a higher proportion of 15N directly into feather keratin, but this would require a fundamental, and unexpected, age-related change in protein synthesis. Our data do not permit us to distinguish among these hypotheses.
The mechanism responsible for the observed positive linear relationship between δ13C and δ15N values in feathers is also unknown. Similar patterns of intraspecific covariation of δ13C and δ15N values have been reported for humans (54), deer (55), elephants (61), bear (62, 63), and cormorants (64). Some authors have suggested that the positive correlation may be a linked response to trophic enrichment of both 13C and 15N (reviewed in ref. 13). However, if trophic enrichment coefficients for δ13C and δ15N values are relatively uniform among individual warblers, intraspecific regression slopes would be relatively unaffected, although slope intercepts would shift. In any case, black-throated blue warblers exhibit one of the more convincing examples of δ13C–δ15N covariation among migratory birds (13).
A third discovery, the positive intraspecific correlation between altitude and δ13C values of feathers, has not been previously reported in birds, nor are we aware of such an altitudinal correlate in the tissues of other animals. A multitude of climatic (e.g., temperature, precipitation, atmospheric pressure) and edaphic (e.g., soil water-holding capacity, nutrient content) factors that covary with altitude are known to affect carbon and nitrogen isotope ratios in terrestrial food webs. In particular, carbon isotope discrimination during photosynthesis in C3 plants decreases with altitude because of greater carboxylation efficiency (21, 23–28). The rate of increase in δ13C values exhibited in feathers of older males (≈1.3‰ per 1,000 m) approximates the average rate of increase reported in several groups of C3 plants (≈1.1‰ per 1,000 m) along altitudinal transects (21).
The positive correlation between altitude and δ13C values in older males suggests a high degree of year-to-year philopatry to a relatively narrow altitudinal zone, if not to particular territories. The modest variation in δ13C values explained by altitude (R2 = 0.09) is especially noteworthy given that annual mortality rates in adult wood warblers may exceed 40% (30, 65, 66). In contrast, the absence of a significant relationship between δ13C values and altitude in yearlings is most likely attributable to natal dispersal. In a previous study, Hobson et al. (17) hypothesized that the increased variance in δD values observed within breeding populations of Bicknell's Thrush, when data from yearling and older birds were pooled, was because of age-specific patterns of dispersal. The postfledging behavior and movements of young black-throated blue warblers (30) are poorly understood, but fledglings of several species of migratory songbirds are known to disperse from natal sites during the molt period (65, 67). In black-throated blue warblers, random scatter in bivariate plots would occur if individuals arbitrarily settled up- or downslope from molting sites occupied during the previous breeding season. Similarly, altitudinal patterns would be obscured if warblers engaged in altitudinal wandering during the molt period, even if they returned to the same breeding territory in subsequent years. Data from the Santeetlah Creek population make it clear that age class must be factored into regression models designed to disentangle the effects of latitude and altitude on isotopic signatures in avian tissues.
Banding studies of black-throated blue warblers in New Hampshire have shown that philopatry increases with age (30). Only one of 800+ nestlings banded on a 70-ha study site was resighted in subsequent years, a female that nested 2.5 km from her natal site. This finding agrees with the overwhelming evidence that natal dispersal in migratory songbirds is leptokurtic; most yearlings breed kilometers from their natal sites, and a small fraction of individuals may settle hundreds of kilometers away (65, 68–70). Philopatry in older songbirds is much higher. Year-to-year return rates of black-throated blue warblers to territories in 10- to 20-ha study plots in New Hampshire ranged from 21 to 45% for males banded as yearlings and from 47 to 61% for males banded in their second or later year, depending on habitat quality (66). Patterns of age-specific variation in δ13C values along altitudinal gradients observed in the present study are consistent with what is known about dispersal and philopatry in banded populations of black-throated blue warblers, suggesting that stable isotope signatures may provide a useful tool for investigating the dispersal dynamics of birds along altitudinal gradients.
Finally, our results have important implications for tracing the geographic origins of migratory birds. The range of δ13C values in feathers (δ13C = −26.07 to −20.86) observed along the Santeetlah Creek altitudinal transect (vertical rise of 755 m) in the southern Appalachians nearly brackets the range of carbon isotope signatures recorded across the entire breeding range of the black-throated blue warbler (Fig. 1) from Georgia to New Brunswick (11° of latitude) and from New Brunswick to western Michigan (22° of longitude) (8, 11). This observation indicates that δ13C signatures alone are unreliable indicators of geographic origin in black-throated blue warblers because the signature profiles for all known breeding and wintering populations (8, 11) either broadly overlap the range of δ13C values recorded along the Santeetlah Creek transect or, in several cases, form proper subsets of the Santeetlah Creek data set. The altitudinal range of breeding populations of black-throated blue warblers attains its widest amplitude (800+ m) in the southern Appalachians, and it is here that an extreme range of δ13C values appears to be generated by altitudinal effects. Collectively, these data suggest that both latitudinal and altitudinal effects may influence the carbon isotope composition of avian species breeding in C3-dominated ecosystems.
Acknowledgments
We thank Keith Hobson, Jeff Kelly, John Rappole, Noreen Tuross, and Len Wassenaar for reviews of the manuscript. We thank Brian Schmidt, Chris Milensky, Phil Angle, Jim Dean, and Carla Dove for preparing specimens, John Gerwin, Chuck Hunter, and Glen McConnell for help with permits, Joe Bonnette for providing data on the Big Santeetlah Creek watershed, and Lindy Paddock and Jason Enelow for help with isotope measurements. G.R.G. was supported by the Alexander Wetmore Fund and the Biodiversity Surveys and Inventory Program of the National Museum of Natural History, Smithsonian Institution. Specimens were collected under permits issued by the U.S. Fish and Wildlife Service, North Carolina Department of Natural Resources, and the U.S. Forest Service (U.S. Department of Agriculture). C.S.R. was funded in part by the U.S. Department of Energy (Financial Assistance Award DE-FC09-96SR18546) to the Savannah River Ecology Laboratory through the University of Georgia Research Foundation, Inc.
References
- 1.Rau G H, Mearns A J, Young D R, Olson R J, Schafer H A, Kaplan I R. Ecology. 1983;64:1314–1318. [Google Scholar]
- 2.Hobson K A, Welch H E. Marine Progress Ser. 1992;84:9–18. [Google Scholar]
- 3.Koch P L, Heisinger J, Moss C, Carlson R W, Fogel M L, Behrensmeyer A K. Science. 1995;267:1340–1343. doi: 10.1126/science.267.5202.1340. [DOI] [PubMed] [Google Scholar]
- 4.Romanek C S, Gaines K J, Bryan A L, Jr, Brisbin I L., Jr Oecologia. 2000;125:584–594. doi: 10.1007/s004420000471. [DOI] [PubMed] [Google Scholar]
- 5.Hobson K A, Alisaukas R T, Clark R G. Condor. 1993;95:388–394. [Google Scholar]
- 6.Doucett R R, Booth R K, Power G, McKinley R S. Can J Fish Aquat Sci. 1999;56:2172–2180. [Google Scholar]
- 7.Kurle K M, Worthy G A J. Oecologia. 2001;126:254–265. doi: 10.1007/s004420000518. [DOI] [PubMed] [Google Scholar]
- 8.Chamberlain C P, Blum J D, Holmes R T, Feng X, Sherry T W, Graves G R. Oecologia. 1997;109:132–141. doi: 10.1007/s004420050067. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy B P, Folt C L, Blum J D, Chamberlain C P. Nature (London) 1997;387:766–767. [Google Scholar]
- 10.Wassenaar L I, Hobson K A. Proc Natl Acad Sci USA. 1998;95:15436–15439. doi: 10.1073/pnas.95.26.15436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubenstein D R, Chamberlain C P, Holmes R T, Ayres M P, Waldbauer J R, Graves G R, Tuross N C. Science. 2002;295:1062–1065. doi: 10.1126/science.1067124. [DOI] [PubMed] [Google Scholar]
- 12.Hobson K A. Oecologia. 1999;120:314–326. doi: 10.1007/s004420050865. [DOI] [PubMed] [Google Scholar]
- 13.Kelly J F. Can J Zool. 2000;78:1–27. [Google Scholar]
- 14.Hobson K A, Wassenaar L I. Oecologia. 1997;109:142–148. doi: 10.1007/s004420050068. [DOI] [PubMed] [Google Scholar]
- 15.Chamberlain C P, Bensch S, Feng X, Åkesson S, Andersson T. Proc R Soc London Ser B. 2000;267:43–48. doi: 10.1098/rspb.2000.0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wassenaar L I, Hobson K A. Ecol Appl. 2000;10:911–916. [Google Scholar]
- 17.Hobson K A, McFarland K P, Wassenaar L I, Rimmer C C, Goetz J E. Auk. 2001;118:16–23. [Google Scholar]
- 18.Kelly J F, Atudorei V, Sharp Z D, Finch D M. Oecologia. 2002;130:216–221. doi: 10.1007/s004420100789. [DOI] [PubMed] [Google Scholar]
- 19.Hobson K A, Clark R G. Condor. 1992;94:189–197. [Google Scholar]
- 20.Hobson K A, Atwell L, Wassenaar L I. Proc Natl Acad Sci USA. 1999;96:8003–8006. doi: 10.1073/pnas.96.14.8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Körner C, Farquhar G D, Wong S C. Oecologia. 1991;88:30–40. doi: 10.1007/BF00328400. [DOI] [PubMed] [Google Scholar]
- 22.Hobson K A, Wassenaar L I, Taylor O R. Oecologia. 1999;120:397–404. doi: 10.1007/s004420050872. [DOI] [PubMed] [Google Scholar]
- 23.Körner C, Diemer M. Funct Ecol. 1987;1:179–194. [Google Scholar]
- 24.Körner C, Farquhar G D, Roksandic Z. Oecologia. 1988;74:623–632. doi: 10.1007/BF00380063. [DOI] [PubMed] [Google Scholar]
- 25.Vitousek P M, Field C B, Matson P A. Oecologia. 1990;84:362–370. doi: 10.1007/BF00329760. [DOI] [PubMed] [Google Scholar]
- 26.Marshall J D, Zhang J. Ecology. 1994;75:1887–1895. [Google Scholar]
- 27.Sparks J P, Ehleringer J R. Oecologia. 1997;109:362–367. doi: 10.1007/s004420050094. [DOI] [PubMed] [Google Scholar]
- 28.Hultine K R, Marshall J D. Oecologia. 2000;123:32–40. doi: 10.1007/s004420050986. [DOI] [PubMed] [Google Scholar]
- 29.Lorimer C G. Ecology. 1980;61:1169–1184. [Google Scholar]
- 30.Holmes R T. In: The Birds of North America. Poole A, Gill F, editors. Philadelphia, PA, and American Ornithologists' Union, Washington, DC: Academy of Natural Sciences; 1994. , No. 87. [Google Scholar]
- 31.Graves G R. Ecology. 1997;78:2524–2531. [Google Scholar]
- 32.Haney J C, Lee D S, Wilbert M. Condor. 2001;103:268–277. [Google Scholar]
- 33.Rodenhouse N L, Holmes R T. Ecology. 1992;73:357–372. [Google Scholar]
- 34.Graves G R. J Field Ornithol. 1997;68:443–449. [Google Scholar]
- 35.Mariotti A. Nature (London) 1983;303:685–687. [Google Scholar]
- 36.Coplen T B. Geochim Cosmochim Acta. 1996;60:3359–3360. [Google Scholar]
- 37.Craig H. Geochim Cosmochim Acta. 1957;12:133–149. [Google Scholar]
- 38.Johnson D W, Van Miegroet H, Lindberg S E, Harrison R B, Todd D E. Can J For Res. 1991;21:769–787. [Google Scholar]
- 39.Joslin J D, Kelly J M, Van Miegroet H. J Environ Qual. 1992;21:12–30. [Google Scholar]
- 40.Shearer G, Kohl D H, Chien S H. Soil Sci Soc Am J. 1978;42:899–902. [Google Scholar]
- 41.Heaton T H E. Oecologia. 1987;74:236–246. doi: 10.1007/BF00379365. [DOI] [PubMed] [Google Scholar]
- 42.Fry B. Ecology. 1991;72:2293–2297. [Google Scholar]
- 43.Austin A T, Vitousek P M. Oecologia. 1998;113:519–529. doi: 10.1007/s004420050405. [DOI] [PubMed] [Google Scholar]
- 44.Handley L L, Austin A T, Robinson D, Scrimgeour C M, Raven J A, Heaton T H E, Schmidt S, Stewart G R. Aust J Plant Physiol. 1999;26:485–488. [Google Scholar]
- 45.Nadelhoffer K J, Aber J D, Melillo J M. Plant Soil. 1984;80:321–335. [Google Scholar]
- 46.Hill A R, Shakleton M. Biogeochemistry. 1989;8:167–184. [Google Scholar]
- 47.Zak D R, Pregitzer K S. For Sci. 1990;36:367–380. [Google Scholar]
- 48.Handley L L, Scrimgeour C M. Adv Ecol Res. 1997;27:133–212. [Google Scholar]
- 49.Kielland K, Barnett B, Schell D. Can J For Res. 1998;28:485–488. [Google Scholar]
- 50.Dupouey J S, Leavitt S W, Choisnel E, Jourdain S. Plant Cell Environ. 1993;16:939–947. [Google Scholar]
- 51.McNulty S G, Swank W T. Ecology. 1995;76:1581–1586. [Google Scholar]
- 52.Ambrose S H, DeNiro M J. Oecologia. 1986;69:395–406. doi: 10.1007/BF00377062. [DOI] [PubMed] [Google Scholar]
- 53.Heaton T H E, Vogel J C, von la Chevallerie G, Collett G. Nature (London) 1986;322:822–823. [Google Scholar]
- 54.Sealey J C, van Der Merwe N J, Lee Thorp J A, Lanham J L. Geochim Cosmochim Acta. 1987;51:2707–2717. [Google Scholar]
- 55.Cormie A P, Schwarcz H P. Palaeogeogr Palaeoclimatol Palaeoecol. 1994;107:227–241. [Google Scholar]
- 56.Katzenberg M A, Saunders S R, Fitzgerald W R. Am J Phys Anthropol. 1993;90:267–281. doi: 10.1002/ajpa.1330900302. [DOI] [PubMed] [Google Scholar]
- 57.Nelson D E, Angerbjörn A, Lidén K, Turk I. Oecologia. 1998;116:177–181. doi: 10.1007/s004420050577. [DOI] [PubMed] [Google Scholar]
- 58.Schmutz J A, Hobson K A. Condor. 1998;100:119–130. [Google Scholar]
- 59.Hobson K A. Condor. 1999;101:799–805. [Google Scholar]
- 60.Hobson K A, Schell D M. Can J Fish Aquat Sci. 1998;55:2601–2607. [Google Scholar]
- 61.Vogel J C, Talma A S, Hall-Martin A J, Viljoen P J. S Afr J Sci. 1990;86:147–150. [Google Scholar]
- 62.Hilderbrand G V, Farley S D, Robbins C T, Hanley T A, Titus K, Servheen C. Can J Zool. 1996;74:2080–2088. [Google Scholar]
- 63.Hobson K A, McClellan B N, Woods J G. Can J Zool. 2000;78:1332–1339. [Google Scholar]
- 64.Mizutani H, Fukuda M, Kabaya Y, Wada E. Auk. 1990;107:400–403. [Google Scholar]
- 65.Nolan V., Jr . The Ecology and Behavior of the Prairie Warbler Dendroica discolor. Washington, DC: American Ornithologists' Union; 1978. , Ornithol. Monogr. 26. [Google Scholar]
- 66.Holmes R T, Marra P P, Sherry T W. J Anim Ecol. 1996;65:183–195. [Google Scholar]
- 67.Rappole J H, Ballard K. Wilson Bull. 1987;99:475–480. [Google Scholar]
- 68.Greenwood P J, Harvey P H. Annu Rev Ecol Syst. 1982;13:1–21. [Google Scholar]
- 69.Moore W S, Dolbeer R A. Condor. 1989;91:242–253. [Google Scholar]
- 70.Payne R B. Evolution(Lawrence, KS) 1991;45:49–62. [Google Scholar]
- 71.Tukey J W. Exploratory Data Analysis. Reading, MA: Addison–Wesley; 1977. [Google Scholar]