Abstract
The convergence of scientific capability and technology that generates vast health data at diminishing cost has generated opportunities, challenges, and anticipation surrounding future data-centric healthcare models. Individualized health data spanning biomolecular, physiological, and environmental dimensions comprise a “personal omics profile”. Here we discuss methods and opportunities to bridge genome and dynamic physiology, detect disease at an early stage, and uncover lifestyle and environmental patterns associated with disease. Significant challenges exist to aggregate, integrate, and protect personal omics data to advance our understanding of disease, enable data-driven clinical decisions, and motivate individuals to sustain behavioral change.
Keywords: genomics, transcriptome, metabolomics, proteomics, personal omics profiling, multiomic medicine, N-of-1
Subject Terms: Diabetes: Type 2, Diet and Nutrition, Lifestyle, Genetics, Risk Factors
Introduction
Since the first sequencing of the human genome in 2003, the relationship between genetic variants and phenotypes has remained a central challenge in medicine. Many diseases including coronary atherosclerosis are polygenic or indeed “omnigenic” wherein many variants work together to impact a phenotype1. Potentially confounding factors and small study population size in comparison to the size of the human genome make it challenging to decipher genetic risk for complex and heterogeneous diseases.
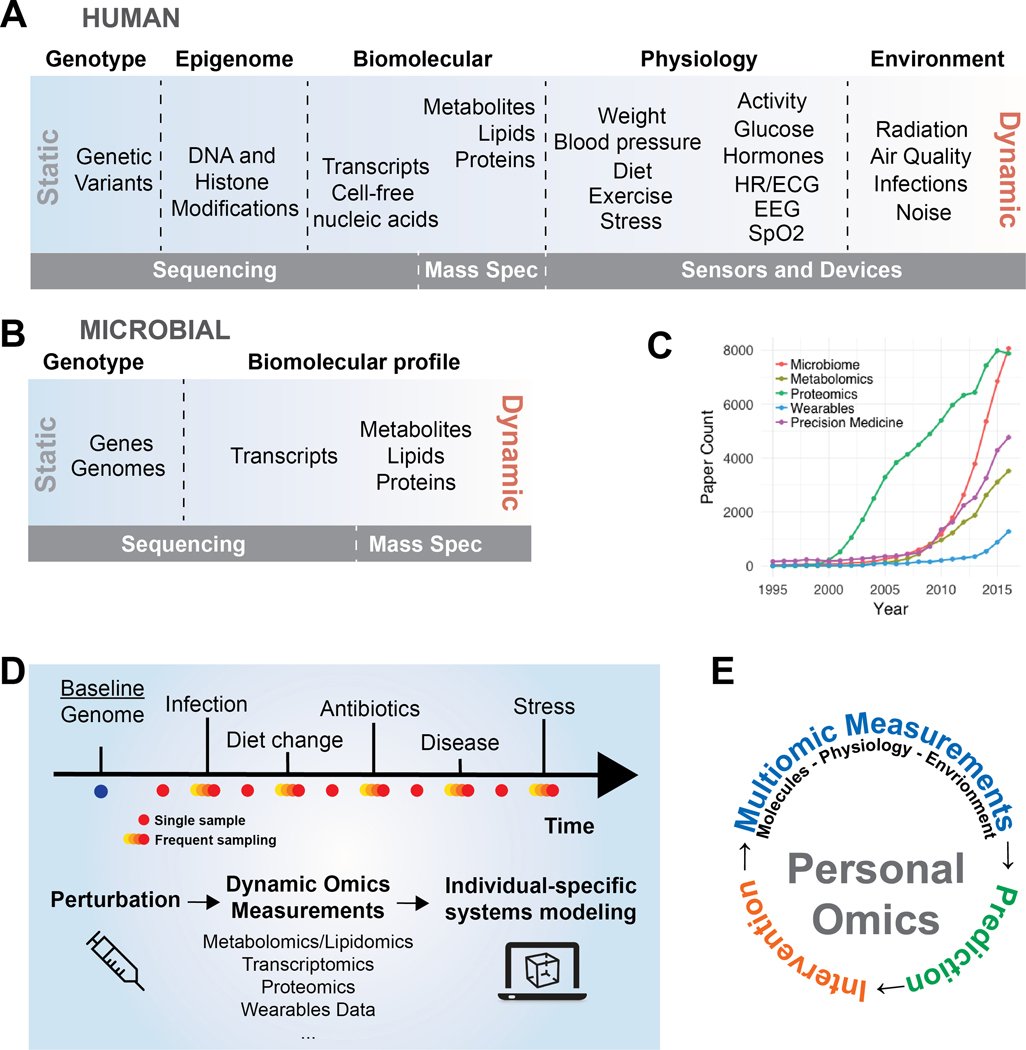
To better understand how genetic variation maps to complex traits, simultaneous measurements that bridge genotype and phenotype are required. This deep phenotyping is the goal of personal omics profiling 2, which combines measures of the genome, epigenome, transcriptome, proteome, metabolome, and additional ‘omes’ (Figure 1A). Rapid advances in sequencing and mass spectrometry drive continued improvement in cost, accuracy, and throughput3,4. Mobile and wearable technologies enable physiological, contextual, and environmental measurements. As we learn more about the symbiotic functions of the microbiome in human health, we also apply multiomic profiling to microbial populations (Figure 1B). Together these measurements provide a holistic profile of dynamic health and facilitate personalized, precision interventions based on predictive models (Figure 1E).
Figure 1. Overview of personal omics.
A) Omic measures span genotype, phenotype, and environment and range from mostly static to highly dynamic. Sequencing, mass spectrometry, and smartphone/wearable sensors are principal technologies driving personal omics. B) Multiomic measures of human-associated microbes that play a vital role in human health. C) The past two decades have seen a dramatic increase in NCBI PubMed records containing personal omics keywords. D) Through regular sampling and high frequency sampling during health perturbations such as disease progression or infection, personal omics reveals the cause, consequence, and course of health changes. E) Personal omics comprises holistic molecular, physiological, and environmental profiling of an individual over time.
Linking genetic variants with dynamic phenotype
Many complex diseases such as type II diabetes involve both genetic and environmental factors. Modeling the interaction of multiple omic data types facilitates dissecting the effects of environmental and genetic factors5. As a first step towards genotype-phenotype analysis, investigators combined genome sequencing (relatively stable) and metabolomics (highly dynamic). Because of the dynamic nature it is challenging to interpret metabolomics data when sampled non-systematically, with examples such as TMAO that fluctuate with dietary intake6. Metabolomics and genomics integration enables discovery of new genetic variants with metabolic consequences, functions for known genetic variants, early signs of disease and drug response (pharmacogenomics), and penetrance of genetic polymorphisms with presumed metabolic impact7,8. Integrating genotype and dynamic physiology through a whole exome sequencing and global metabolomics study on 80 healthy individuals identified subclinical metabolic imbalances and associated metabolic abnormalities with genetic variants 8. Moreover, it was possible to identify novel metabolites for tracking disease risk and health state. With metabolomics now accessible in the clinic, metabolic profiling will complement next generation sequencing (NGS) for disease risk analysis, monitoring, and clinical decision making.
Multiomics integration and detecting the health-disease transition
The explosion of multiomics studies over the last decade (Figure 1C) has led to longitudinal multiomic studies over an extended time period to study transitions between health and disease9. Many complex diseases are highly heterogeneous between patients, and longitudinal profiling increases statistical power by allowing each subject to serve as their own control. We conducted an “N-of-1” study involving deep longitudinal profiling of single individual over 14 months creating an Integrative Personal Omics Profile (iPOP) 2. We performed whole genome sequencing and periodic measurements of transcriptome, proteome, antibodies, metabolites, and clinical biomarkers profiled at higher temporal resolution during events of infection or illness (Figure 1D). Genomic analysis revealed increased risk for type II diabetes. During the course of the study, and following a rhinovirus infection, the participant developed type II diabetes. Complementing genetic risk prediction, comprehensive profiling across transcriptomic, proteomic, and metabolomic layers enabled early detection and dissection of signaling network rewiring during the transitions from health to disease and back.
We expanded the iPOP study to over 100 individuals who we have profiled along many omic dimensions through perturbations including weight gain, infection, and vaccination (iHMP Consortium) 10. We find some consistent changes across individuals in response to particular perturbations but also that individuals vary greatly in several responses. Developing biomarkers to predict how an individual will react to a perturbation provides a basis for personalized risk assessment and development of personalized behavioral and precision therapeutic interventions. The American Heart Association has launched the AHA Precision Medicine Platform to facilitate multiomics data collection and collaboration between physicians and researchers (http://precision.heart.org). Additional ongoing personal omics efforts are summarized in Supplementary Table I.
As wearable and smartphone sensors continue to improve in accuracy, usability, and cost, there is increasing appreciation for the potential clinical value of these sensors. We recently studied wearable sensors for managing health and diagnosing disease 11. We found that physiological parameters can indicate health transitions. We developed an algorithm to correct for individual activity patterns, allowing us to detect when an individual deviated from their healthy baseline. Several study participants experienced infection or illness during the course of the study, including one participant who contracted Lyme disease. Changes in heart rate, skin temperature, and blood oxygen saturation from baseline levels preceded infection and inflammation before symptoms developed (Online Figure I) 11.
Detecting environmental and behavioral patterns underlying chronic disease
Combining molecular and sensor measurements can uncover lifestyle patterns that impact disease and reveal biomarkers for personalized optimization of dietary or lifestyle habits. Segal et al. sought to understand intra-individual variability in glucose regulation to personalize diets to minimize blood sugar spikes12. Integrating clinical, anthropometric, and microbiome data allowed the authors to develop a machine learning model to predict individualized glycemic responses. This study illustrates the potential for predictive models based on multiomics data to identify beneficial dietary and lifestyle behaviors.
King et al. developed a mobile platform to understand the interplay between motivation, behavior, and health13. Three motivational frameworks were tested to reduce sedentary behavior in aging adults and health outcomes were explored. This demonstrates how personal omics data can be leveraged to personalize behavioral health interventions.
One can imagine applying a similar methodology in many contexts where complex and individual-specific environmental patterns may trigger symptoms or conditions such as episodes or relapses in autoimmune disease, food allergies, migraines, asthma, chronic fatigue syndrome, fibromyalgia, or psychological or cardiovascular events. Recently researchers used deep learning to detect cardiac arrhythmias in real time with accuracy exceeding that of physicians14. In the future, learning algorithms may also be used to identify triggers of cardiac arrhythmia and other health events that can be addressed to prevent adverse health outcomes. (Online Figure II).
Challenges in aggregation, analysis, integration, and protection of personal omics data
A major challenge facing multiomics integration is the need for large study populations to enable statistical power and machine learning approaches. Recent mobile health studies have demonstrated novel methods of scaling to population levels. Ashley et al. developed the smartphone application MyHeartCounts to gain insights into activity patterns associated with life satisfaction and self-reported disease for 48,000 participants15. The study demonstrated feasibility of consenting and engaging a large population using smartphones and gathering and securely storing data in real time. A related study examined activity patterns in a global study of nearly 800,000 individuals in 111 countries16. Overall, 68 million days of physical activity monitoring revealed city and geosocial features associated with health and obesity16.
With greater reliance on self-collected medical data by individuals using wearable sensors and smartphones, and direct-to-consumer testing services based on, for example, genome and microbiome data (e.g. 23andme, Helix, uBiome) it is becoming increasingly feasible to scale health studies to thousands or even millions of participants to identify geographic, demographic, and other population-scale correlates for disease. Large-scale population health studies like the National Health and Nutrition Examination Survey (NHANES), continue to provide a rich resource for retrospective population-scale health studies (National Center for Health Statistics).
Genomic and wearable sensor data has value in clinical medicine though it is not possible to store such data in electronic health records (EHRs). A next-generation EHR was tested with 37 families and is being rolled out to 1500 patients across the Stanford hospital system. The modern medical record enables assessment of genetic risk, medication personalization, and monitoring of subclinical risk factors based on genomics and wearables data collected over time 17.
Conclusions
Healthcare is undergoing a period of rapid change due to revolutionary new scientific tools, advances in molecular and physiological measurement technologies, and algorithmic and computing advances enabling real-time analysis and pattern discovery in multimodal and high-dimensional health data. Longitudinal “personal omics” leveraging these advances facilitates mapping genomic variants to disease regulatory networks, detecting the health-disease transitions based on molecular and physiological metrics, measuring interaction of environment and health outcomes, uncovering complex behavioral and lifestyle patterns linked to symptoms and progression of chronic disease, and scaling health data collection to participant populations spanning diverse geographies and demographics. Continuing this progress will lead to a preventative care model with engaged patients, interoperable health data, cost savings, and improved wellness.
Supplementary Material
Acknowledgements
We thank Tejaswini Mishra and members of the Snyder lab for thoughtful comments on the article.
Sources of funding
The authors are supported by SNF Early Postdoctoral Mobility fellowship (RK), NIH Big Data To Knowledge Mobilize Center of Excellence Grant U54 EB020405 (JD), and Integrative Human Microbiome Project NIH 8U54DK102556 (MPS).
Footnotes
Disclosures
None.
References
- 1.Boyle EA, Li YI, Pritchard JK. An Expanded View of Complex Traits: From Polygenic to Omnigenic. Cell. 2017;169:1177–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen R, Mias GI, Li-Pook-Than J, Jiang L, Lam HYK, Chen R, Miriami E, Karczewski KJ, Hariharan M, Dewey FE, Cheng Y, Clark MJ, Im H, Habegger L, Balasubramanian S, et al. Personal Omics Profiling Reveals Dynamic Molecular and Medical Phenotypes. Cell. 2012;148:1293–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reuter JA, Spacek DV, Snyder MP. High-Throughput Sequencing Technologies. Molecular Cell. 2015;58:586–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Contrepois K, Jiang L, Snyder M. Optimized Analytical Procedures for the Untargeted Metabolomic Profiling of Human Urine and Plasma by Combining Hydrophilic Interaction ( HILIC ) and Reverse-Phase Liquid Chromatography ( RPLC )– Mass Spectrometry. Molecular & Cellular Proteomics. 2015;14:1684–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yugi K, Kubota H, Hatano A, Kuroda S. Trans-Omics: How To Reconstruct Biochemical Networks Across Multiple “Omic” Layers. Trends in Biotechnology. 2016;34(4):276–290. [DOI] [PubMed] [Google Scholar]
- 6.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nature Medicine. 2013;19:576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Contrepois K, Liang L, Snyder M. Can metabolic profiles be used as a phenotypic readout of the genome to enhance precision medicine? Clinical Chemistry. 2016;62:676–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo L, Milburn MV., Ryals JA, Lonergan SC, Mitchell MW, Wulff JE, Alexander DC, Evans AM, Bridgewater B, Miller L, Gonzalez-Garay ML, Caskey CT. Plasma metabolomic profiles enhance precision medicine for volunteers of normal health. Proceedings of the National Academy of Sciences. 2015;112:E4901–E4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snyder M, Weissman S, Gerstein M. Personal phenotypes to go with personal genomes. Molecular Systems Biology. 2009;5:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Integrative HMP (iHMP) Research Network Consortium. The Integrative Human Microbiome Project: dynamic analysis of microbiome-host omics profiles during periods of human health and disease. Cell host & microbe. 2014;16:276–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Dunn J, Salins D, Zhou G, Zhou W, Sch??ssler-Fiorenza Rose SM, Perelman D, Colbert E, Runge R, Rego S, Sonecha R, Datta S, McLaughlin T, Snyder MP. Digital Health: Tracking Physiomes and Activity Using Wearable Biosensors Reveals Useful Health-Related Information. PLoS Biology. 2017;15:1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan M, Suez J, Mahdi JA, Matot E, Malka G, Kosower N, et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell. 2015;163:1079–1095. [DOI] [PubMed] [Google Scholar]
- 13.King AC, Hekler EB, Grieco LA, Winter SJ, Sheats JL, Buman MP, Banerjee B, Robinson TN, Cirimele J. Harnessing Different Motivational Frames via Mobile Phones to Promote Daily Physical Activity and Reduce Sedentary Behavior in Aging Adults. PLoS ONE. 2013;8:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajpurkar P, Hannun AY, Haghpanahi M, Bourn C, Ng AY. Cardiologist-Level Arrhythmia Detection with Convolutional Neural Networks. 2017. [Google Scholar]
- 15.McConnell MV, Shcherbina A, Pavlovic A, Homburger JR, Goldfeder RL, Waggot D, Cho MK, Rosenberger ME, Haskell WL, Myers J, Champagne MA, Mignot E, Landray M, Tarassenko L, Harrington RA, et al. Feasibility of Obtaining Measures of Lifestyle From a Smartphone App. JAMA Cardiology. 2016;2:67–76. [DOI] [PubMed] [Google Scholar]
- 16.Althoff T, Sosič R, Hicks JL, King AC, Delp SL, Leskovec J. Large-scale physical activity data reveal worldwide activity inequality. Nature. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waggott D, Bog A, Singh E, Batra P, Wright M, Ashley E. The Next Generation Precision Medical Record - A Framework for Integrating Genomes and Wearable Sensors with Medical Records. bioRxiv. 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.