Abstract
Blind mole rats have degenerated subcutaneous eyes that are visually nonfunctional. In this investigation, we have compared the tissue specificity of the small heat shock protein (shsp)/αB-crystallin promoter of the mole rat superspecies, Spalax ehrenbergi, with that of the mouse. Earlier experiments showed that mouse shsp/αB-crystallin promoter/enhancer activity is high in the lens and moderate in the heart and skeletal muscle of transgenic mice. Here, we show in transgenic mouse experiments using the firefly luciferase reporter gene that, despite relatively few changes in sequence, the mole rat shsp/αB-crystallin promoter/enhancer has selectively lost lens activity after 13.5 days of embryogenesis (E13.5). The ratios of mole rat/mouse promoter activity were 0.01 for lens, 1.7 for heart, and 13.6 for skeletal muscle in 8-wk-old transgenic mice. Our data indicate that the shsp/αB-crystallin promoter/enhancer has undergone adaptive changes corresponding to the subterranean evolution of the blind mole rat. We speculate that selective pressures on metabolic economy may have contributed to these tissue-specific modifications of promoter/enhancer function during adaptation to life underground.
Although the eyes of the adult blind mole rat are severely regressed (see Fig. 1), the early embryo develops an optic cup and lens vesicle comparable to that of an embryonic day 10 (E10) mouse eye (1, 2). Further lens development is associated with abnormal primary fiber cell elongation, the appearance of vacuolar spaces, vascularization, and the eventual shriveling of the lens. In addition to the degenerated lens, a hypertrophied and pigmented iris-ciliary body fills the anterior chamber, the optic fissure does not close, and the anterior chamber collapses. The minute, regressed mole rat adult eye, embedded beneath the skin, retains a neuroretina and reduced optic nerve, although retinal projections to thalamic and tectal structures involved in visual perception are reduced by more than 90% (3).
Figure 1.
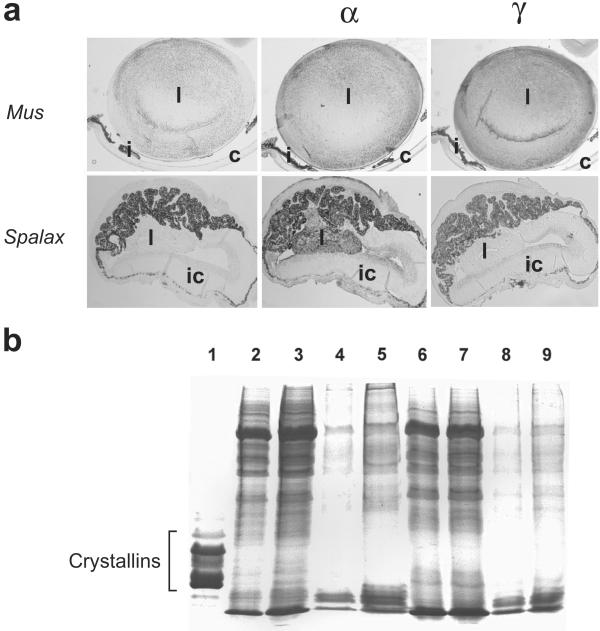
(a) Mouse (Mus) and Spalax eyes. Adult mouse (Upper, Left) and 2-day-postnatal Spalax (Lower, Left). Immunohistochemistry of α-crystallin in the mouse (Upper, Center) and 2-day postnatal Spalax (Lower, Center) eye. Immunohistochemistry of γ-crystallin in the mouse (Upper, Right) and 2-day postnatal Spalax (Lower, Right) eye. l, Lens; c, cornea; i, iris; ic, iris-ciliary body complex. (b) SDS/PAGE of proteins of the eye of the mole rat. Lane 1, Soluble proteins of the bovine lens. Soluble (lanes 2 and 3) and insoluble (lanes 4 and 5) proteins of the adult mole rat eye; soluble (lanes 6 and 7) and insoluble (lanes 8 and 9) proteins of the newborn mole rat eye.
The diverse crystallins comprise 80–90% of the soluble protein of vertebrate lenses (4–6). The ubiquitous crystallins (the α- and the β/γ-crystallins) are represented in all vertebrate lenses whereas the taxon-specific crystallins (also called enzyme-crystallins because they are identical to or derived from metabolic enzymes) are limited to selected species (4, 7). The two α-crystallins (αA and αB) originated by duplication of an ancestral small heat shock protein (shsp) gene (5, 6). αA-Crystallin has specialized for lens expression; by contrast, shsp/αB-crystallin has remained a functional, stress-inducible shsp (4, 5, 8, 9), although it is highly expressed in the lens and to a lesser extent in many other tissues, especially the heart and skeletal muscle (7, 10). Indeed, shsp/αB-crystallin expression commences in the embryonic heart before eye development and in the eye during lens induction (11, 12). Mutations in the human shsp/αB-crystallin gene (CRYAB) cause desmin-related myopathy (13) and dominant congenital posterior polar cataracts (14). In addition, shsp/αB-crystallin is up-regulated in human brain tumors and numerous neurodegenerative diseases including Alexander's disease, Alzheimer's disease, Creutzfeldt-Jakob disease, Parkinson's disease, and multiple sclerosis, among others (9, 15). Although αA-crystallin knockout mice develop lens opacification (16), shsp/αB-crystallin null mice appear to have normal lenses but show various signs of muscle degeneration (17). The lens opacification of the αA-crystallin knockout mice is likely because of the formation of inclusion bodies in the lens composed mainly of shsp/αB-crystallin (16).
Regulatory elements of the mouse αB-crystallin gene that control its expression in the lens, heart, and skeletal muscle have been identified. The lens-specific regulatory (LSR) regions [−147/−118 (LSR1) and −78/−48 (LSR2)] within the −164/+43 proximal promoter can drive lens expression of a reporter gene in transgenic mice (18, 19). LSR1 and LSR2 are transactivated by Pax-6 and retinoic acid receptors (20–22). The −427/−258 enhancer of the mouse shsp/αB-crystallin gene contains at least five regulatory regions that are necessary for expression in skeletal muscle and heart (20, 23, 24). Deletion of this enhancer region also reduces but does not eliminate promoter activity in the lens of transgenic mice (18, 19).
Few studies have explored possible changes in gene expression during eye regression. Despite that the mole rat αA-crystallin cDNA has undergone numerous mutations, consistent with its lack of use as a crystallin in the degenerated lens of this species (25), the sequence of the mole rat αA-crystallin promoter is surprisingly similar to that of the mouse (9, 25). The αA-crystallin promoter sequence of the blind cavefish is also highly similar to the mouse despite that the gene is not expressed in the lens (26). It is not known yet whether the mole rat or blind cavefish αA-crystallin promoter can drive a reporter gene in the lens of transgenic mice. In the present study, we have explored the mole rat shsp/αB-crystallin promoter/enhancer in transgenic mice to test the possibility that lens-specific activity has been down-regulated along with adaptive changes associated with subterranean evolution.
Materials and Methods
Immunohistochemistry of Mouse and Spalax Eyes.
Ten-micrometer eye sections from newborn mole rats Spalax carmeli, diploid chromosome number 2n = 58, from Haifa, and adult mole rats, Spalax judaei, 2n = 60, from Anza, Samaria, both species belonging to the Spalax ehrenbergi superspecies in Israel (27), were deparaffinized, hydrated, postfixed, and treated for endogenous peroxidases as described previously (28). Immunostaining was performed overnight at 4°C with either a rabbit anti-mouse α-crystallin antibody or a rabbit anti-mouse γ-crystallin antibody (both provided by D. Carpenter, National Eye Institute, Bethesda, MD) at a 1:4,000 dilution. Adult mouse lens sections were used as positive controls for the antibodies. The primary antibody was omitted to serve as a negative control. The sections were incubated with biotinylated Ig and avidin-biotin-horseradish peroxidase complex (Vectastain Elite ABC Kit, Vector Laboratories) according to the manufacturer's instructions. Immunoreactivity was visualized with diaminobenzidine (Vector Laboratories). Slides were washed under running tap water to terminate the color-producing reaction and mounted by using Aqua Poly/Mount (Polysciences).
SDS/PAGE of Proteins of Newborn and Adult Mole Rat Eyes.
Newborn and adult mole rat eyes were homogenized in PBS at pH 7.0 and then centrifuged at 15,000 × g for 15 min. The supernatant was saved as the soluble fraction. The pellet was washed twice with PBS buffer and redissolved in 1% SDS as the insoluble fraction. Samples were examined by 12.5% SDS/PAGE; the gel was stained with Coomassie brilliant blue R-250 staining solution (Bio-Rad) and destained with 5% methanol, 7.5% acetic acid destaining solution.
Isolation of the shsp/αB-Crystallin Promoter from S. ehrenbergi.
The nucleotide region (−943/+45) of the mole rat shsp/αB-crystallin gene (GenBank accession no. AJ427968) was cloned from two species: Spalax galili (2n = 52, Kerem Ben-Zimra population) and S. judaei (2n = 60, Anza population) (27). The region was cloned by employing PCR using a 5′ primer (5′-GTC(T/G)ACACCACCCAAAATAGTGC-3′) derived from the human shsp/αB-crystallin gene (GenBank accession number M28638) and a 3′ primer (5′-GGATCCAGGGGTGGTGGATGGCG-3′) derived from the mole rat shsp/αB-crystallin gene (GenBank accession number AJ293658).
Construction of Mole Rat and Mouse shsp/αB-Crystallin Promoter Constructs.
The −668/+45 mole rat promoter fragment was amplified by PCR using Pfu polymerase (Stratagene). The primers used for the mole rat amplification (5′-GGGGTACCAAGGAGGGGCCTAGTTGAGAC-3′ and 5′-TCCCCCCGGGTGGATGGCGATGTCCA-3′) generated an amplicon with a KpnΙ site at the 5′ end and an XmaΙ site at the 3′ end. The amplicon was inserted into the multiple cloning region of the pGL3-Basic luciferase reporter plasmid (Promega). The −661/+43 promoter fragment of the mouse shsp/αB-crystallin gene was excised from pD96 (7) by using BamHΙ and inserted into the BglΙΙ site in the multiple cloning region of the pGL3-Basic plasmid.
Cell Culture and Transient Transfections.
N/N1003A rabbit lens cell line, αTN4-1 mouse lens cell line, and C2C12 myoblast cell line were cultured as described previously (23). The cells were cotransfected with 1 μg of either mole rat or mouse promoter luciferase reporter plasmid and 100 ng of pRL-SV40 Renilla luciferase vector (Promega) by incubation with Fugene reagent (Roche Molecular Biochemicals) for 48 h. Cells were treated with a cell lysis solution and frozen at −80°C. Luciferase assays were performed by using the Dual Reporter Luciferase Assay Kit (Promega) and Monolight Luminometer (Analytical Luminescence Laboratory, Sparks, MD).
Transgenic Mice and Luciferase Assays.
KpnΙ-SalΙ (2.8-kb) fragments containing either the mouse or mole rat shsp/αB-crystallin promoter fused to the firefly luciferase gene were isolated from their respective plasmids, purified from an agarose gel by using the Geneclean kit (Bio 101), and injected into the pronucleus of a single-celled mouse embryo (FVB/N strain) obtained from superovulated FVB/N females. Injected embryos were transferred into CD1 females. Tissues from transgenic mice were homogenized in lysis buffer by using the luciferase assay system (Promega). Samples were centrifuged at top speed in a microcentrifuge, and firefly luciferase activity of the supernatant fraction was measured by using the Luciferase Assay System (Promega) and the TR717 Microplate Luminometer (Applied Biosystems). The concentration of soluble protein was determined by using the Bio-Rad Protein Assay.
Results
α-Crystallins have been observed previously in lenses of the mole rat, S. ehrenbergi, by immunofluorescence (29). In addition, mole rat shsp/αB-crystallin, which is 90% identical to that of other mammals, has been detected in the eye and other tissues by reverse transcription–PCR (30). Here, we confirm α-crystallin expression in the degenerated newborn Spalax lens by immunocytochemistry (Fig. 1a). Omission of the primary antibody gave negative results, indicating specificity of the α-crystallin antibody in the mole rat lens. We did not observe expression of γ-crystallin in the Spalax lens (Fig. 1a). Furthermore, separate SDS/PAGE of the mole rat eye showed that all crystallins, including shsp/αB-crystallin, are barely detectable at birth or in the adult (Fig. 1b). The actual levels of crystallins within the shriveled mole rat lens cannot be determined because it requires isolation of the minute degenerate lens within the small eye. Nonetheless, although our immunocytochemical results did not distinguish αA- and αB-crystallin, we conclude that shsp/αB-crystallin expression is, at best, relatively low in the lens of the mole rat.
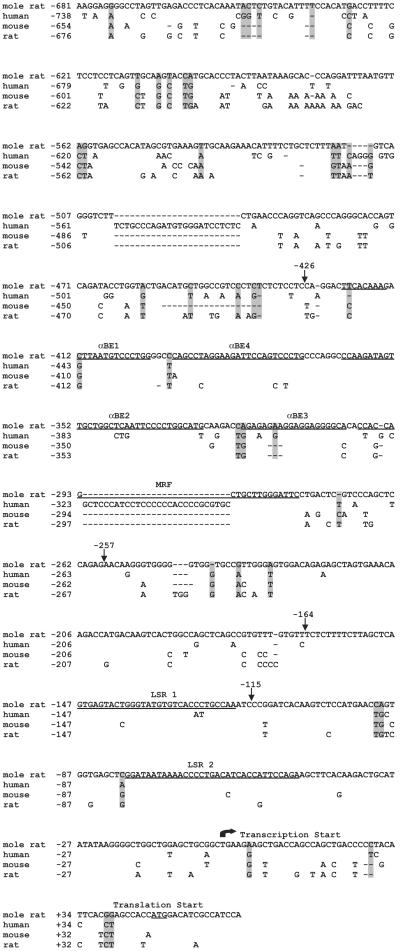
To study promoter structure and function of mole rat shsp/αB-crystallin, we isolated the upstream region of shsp/αB-crystallin gene from two different mole rat species, S. galili and S. judaei (27). The −681/+64 sequences of the shsp/αB-crystallin gene from these mole rats have only two nucleotide differences (data not shown). The S. galili shsp/αB-crystallin upstream sequence is 82%, 83%, and 78% identical to that of the rat, mouse, and human, respectively (Fig. 2). The similarity of the mole rat sequence with the rodent and human sequences, despite rodents and humans having diverged approximately 110 million years ago (31), suggests functional conservation. The sequence similarity is particularly striking in the lens enhancer elements LSR1 and LSR2 within the −164/+43 proximal promoter (18, 20) as well as in the −427/−258 muscle-preferred enhancer of the mouse gene (20, 23), consistent with the conservation of gene regulation.
Figure 2.
Nucleotide sequence of the 5′ flanking region of the Spalax shsp/αB-crystallin gene. The Spalax sequence is compared with those from human, mouse, and rat. Nucleotides that differ from the mole rat nucleotide sequence are given, and nucleotides not present are represented by dashes. The start sites of transcription and translation are noted. Cardiac/skeletal muscle enhancer regions [αBE1, αBE4, αBE2, αBE3, and muscle regulatory factor-binding (MRF)] and lens enhancer regions (LSR1 and LSR2) of mouse αB-crystallin are also underlined. Nucleotides in the Spalax sequence that differ from the nucleotide sequence conserved in rat, mouse, and human nucleotide sequences are shaded in gray.
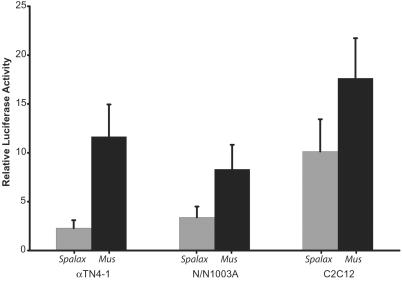
The S. galili −668/+43 promoter fragment was subcloned into the pGL3 firefly luciferase expression vector to compare its activity with the corresponding −661/+43 mouse promoter fragment, shown earlier to be highly active in lens, skeletal muscle, and heart of transgenic mice (7). In transient transfections, the mouse construct was 2.4 and 5 times more active than the Spalax promoter in N/N1003A rabbit lens cells and αTN-4 mouse lens cells, respectively (Fig. 3). By contrast, the mouse construct was only 1.7 times more active than the mole rat promoter in transiently transfected C2C12 mouse myoblasts. The reduced activity of the mole rat promoter in the transfected lens cell lines may be a result of the same changes in transcriptional regulation of shsp/αB-crystallin that cause a reduction of expression in the Spalax eye. To investigate this reduction in promoter activity further, we made transgenic mice with the mole rat promoter construct.
Figure 3.
Activity of the Spalax shsp/αB-crystallin promoter in transient transfection of C2C12 mouse myoblast cell line, N/N1003A rabbit lens cell line, and αTN4-1 mouse lens cell line. Filled gray bars represent the activity of the Spalax shsp/αB-crystallin promoter fragment (−668/+45). Filled black bars represent the activity of the mouse shsp/αB-crystallin fragment (−661/+43). Relative luciferase activity is given as fold stimulation ± SD over the pGL3 vector transfection alone. The differences in activities of the two constructs are statistically significant (P < 0.05).
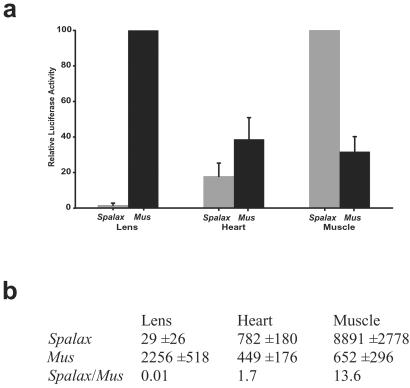
Transgenic mice were made by using the mole rat and mouse shsp/αB-crystallin promoter:luciferase constructs as transgenes, and luciferase activity was determined in different tissues of the 8-wk-old F1 progeny. As expected (18, 32), the highest average values for mouse shsp/αB-crystallin promoter activity were in the lens, heart, and skeletal muscle (gracius leg muscle) of the transgenic mice (Fig. 4a). By contrast, the mole rat promoter showed little lens but high skeletal muscle and moderate heart activity in four lines of transgenic mice. The average luciferase activity found in the lens resulting from the mole rat shsp/αB-crystallin promoter was 77-fold lower than that resulting from the mouse promoter in the transgenic mice (Fig. 4b). This finding was reversed in the muscle, where the mole rat shsp/αB-crystallin promoter was 13 times greater than the mouse promoter. The heart luciferase activity generated by the mole rat promoter was not statistically different from the activity generated by the mouse promoter in the transgenic mice (Fig. 4b). The ratios of mole rat to mouse shsp/αB-crystallin promoter activity were 0.01, 1.7, and 13.6, respectively, in the transgenic mice (Fig. 4b). There were also small elevations of luciferase activity in the cornea, lung, and testis of the transgenic mice containing the mole rat shsp/αB-crystallin promoter that did not occur in the mice containing the mouse promoter (data not shown). Near background levels of mouse and mole rat shsp/αB-crystallin promoter activity were found in the retina, brain, liver, spleen, kidney, and ovary of the transgenic mice (data not shown). Genomic Southern analyses indicated that copy numbers of the shsp/αB-crystallin promoter transgenes of the mouse (between 6 and 10 copies) and mole rat (between 2 and 10 copies) were similar.
Figure 4.
Mole rat and mouse shsp/αB-crystallin promoter activity in 8-wk-old transgenic mice. (a) (Filled gray bars) Average of four lines of transgenic mice with the mole rat promoter. (Filled black bars) Average of three lines of transgenic mice with the mouse promoter. The highest activity using the mole rat construct (muscle) and the highest activity using the mouse construct (lens) were given a value of 100. Setting the mole rat muscle activity to 100 lowered the heart activity relative to the mouse even though the average luciferase activity of the mole rat construct was actually higher than that of the mouse. (b) Average relative luciferase units per μg of soluble protein, with standard deviations of the lens, heart, and skeletal muscle in the transgenic mice. The differences in activity between the mole rat and the mouse promoters were statistically significant in the lens (P < 0.001) and in the muscle (P < 0.02), whereas in the heart the differences were not statistically significant.
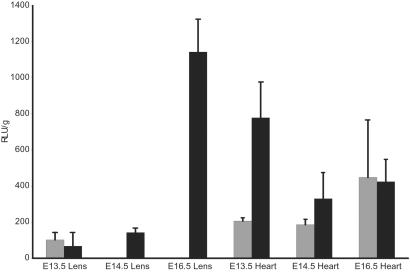
Because the mole rat eye appears normal during early development (1, 2), we investigated whether the mole rat shsp/αB-crystallin promoter functions in the embryonic lens of the transgenic mice (Fig. 5). The results showed higher mole rat shsp/αB-crystallin promoter activity in the lens of the 13.5-day-old transgenic mouse embryos than in the 8-wk-old mice (Fig. 5). Separate whole mount in situ hybridization experiments also showed lens activity of the mole rat shsp/αB-crystallin promoter in E11.5 transgenic mice (data not shown). Lens activity of the mole rat promoter was virtually absent by E14.5, resembling its activity in the 8-wk-old adult lens (Fig. 5). The mole rat shsp/αB-crystallin promoter was active in the heart at E13.5, E14.5, and E16.5 (Fig. 5). In one test, muscle activity of the mole rat shsp/αB-crystallin promoter was ≈3 times greater at E14.5 than the activity of the mouse promoter (data not shown). Thus, there is a developmentally regulated loss of mole rat shsp/αB-crystallin promoter function specifically in the lens of transgenic mice after E13.5, a time that correlates well with the regression of the mole rat eye (1).
Figure 5.
Mole rat and mouse shsp/αB-crystallin activity in transgenic mouse embryos. (Filled gray bars) Average of five littermates at E13.5, of seven littermates at E14.5, and of six littermates at E16.5 with mole rat promoter. (Filled black bars) Average of four littermates at E13.5, of seven littermates at E14.5, and of six littermates at E16.5 with mouse promoter. Activity is given in relative luciferase units per μg of soluble protein (RLU/μg) ± SD. Differences in the activity of the mole rat promoter in the lens at E14.5 and E16.5 were highly significant (P < 0.01).
Discussion
Our transgenic mouse data suggest that the loss of mole rat shsp/αB-crystallin promoter activity in the lens is accompanied by an increase in promoter activity in the muscle. The relative decrease in mole rat shsp/αB-crystallin promoter activity in the lens was also evident in the transfected αTN4-1 and N/N1003A lens cells, despite that the mole rat promoter activity was greater in the cultured cells than in the transgenic mice. The absence of an increase of the mole rat promoter activity in the transfected C2C12 muscle cells may be due to the fact that these were cultured myoblasts with nonintegrated plasmids rather than differentiated muscle with integrated transgenes as in transgenic mice.
The levels of endogenous shsp/αB-crystallin gene expression in the mole rat lens and muscle have not been determined. The former is complicated by the difficulty of isolating the degenerate mole rat lens. The reverse transcription–PCR data of Avivi et al. (30; Fig. 3) showed that shsp/αB-crystallin gene expression occurs in the mole rat eye and, after normalization for the actin controls, suggest that shsp/αB-crystallin gene expression is indeed higher in skeletal muscle than in the eye and other tissues of the mole rat. Establishing the absolute level of shsp/αB-crystallin expression in the mole rat muscle is complicated by probable variations of shsp/αB-crystallin promoter activity with muscle type, with activity levels, and with age.
Our data showing that the loss of mole rat shsp/αB-crystallin promoter activity in the lens is accompanied by an increase in muscle promoter activity fits with the idea that tissue regression during evolution may be associated with compensating increases, or constructive modifications, in other modalities (2). For example, mole rats have degenerated, subcutaneous eyes that are visually nonfunctional, but are engaged in circadian entrainment and have increases in retinal projections to structures involved in photoperiodic and photo-neuroendocrine regulation (1–3). Moreover, the circadian rhythm transcription factor CLOCK of the blind mole rat has an expanded glutamine-rich domain that may cause a change in its transcriptional activity (33). In addition, the rhodopsin gene of Spalax is functional in retinal cells despite the overall degenerate eye (34), as is true for melanopsin and pituitary adenylate cyclase-activating peptide (PACAP) (J. Hannibal, P. Hindersson, E.N., and J. Fahrenkrug, unpublished results). Blind cavefish, which also show eye regression, have constructive progression of larval jaws, maxillary teeth, taste buds, cranial neuromasts, and telencephalon (35). Cavefish eye degeneration developed over a period of 10,000 to 1 million years (35) vs. the 30–40 million years since mole rats went underground (2).
The relative increase in shsp/αB-crystallin promoter activity in the skeletal muscle of the blind mole rat may have an adaptive role. It seems reasonable to speculate that in the burrowing mole rat the need for shsp/αB-crystallin in muscle would be more adaptively useful than in the lens (2). It is noteworthy that the mole rat muscle regulatory factor-binding (MRF) element (which is critical for muscle activity) of the shsp/αB-crystallin enhancer (23) has more differences than that of the other enhancer elements (see Fig. 2). Further experiments are required to determine whether the apparent adaptive increase in muscle activity of the mole rat promoter occurred independently from the loss in lens expression.
We do not know why the mole rat shsp/αB-crystallin promoter loses activity in the embryonic transgenic mouse lens. One possibility is that mouse shsp/αB-crystallin promoter activity undergoes a mechanistic switch after E13.5 that the mole rat promoter cannot accomplish, such as the binding of an additional transcription factor associated with lens fiber cell differentiation that is aberrant in the mole rat. Another possibility is that the mole rat promoter binds a repressor in the mouse lens after E13.5. In this connection, experiments in our laboratory have revealed the presence of an insulator and putative repressor between the mouse shsp/αB-crystallin enhancer and the MKBP/HSPBP2 gene located ≈1 kb upstream of the shsp/αB-crystallin gene (S. Swamynathan and J.P., unpublished results).
Although the mechanism of eye regression in the mole rat is not known, it is of interest that a reduced domain of Pax-6 expression during the formation of the optic primordia may contribute to the eye degeneration of cavefish (35). By analogy, a Pax-6 involvement in degeneration of the mole rat eye would be consistent with the present lens-specific loss of shsp/αB-crystallin promoter activity because the shsp/αB-crystallin promoter binds and is activated by Pax-6 (21). In any case, the selective loss of the mole rat shsp/αB-crystallin promoter activity in the lens of transgenic mice was surprising in view of its sequence similarity with the mouse promoter. It appears that minor sequence modifications, presumably associated with adaptation to life underground, can be associated with profound changes in promoter activity. These adaptive evolutionary changes may economize metabolic expenses (2, 3), by limiting expression of shsp/αB-crystallin in the degenerated lens and augmenting expression in the muscle, thereby increasing fitness.
Acknowledgments
We thank Dr. Deborah Carper (National Eye Institute, National Institutes of Health, Bethesda, MD) for the rabbit anti-mouse α-crystallin antibody and the rabbit anti-mouse γ-crystallin antibody, the laboratory of Dr. Joseph Horowitz (Jules Stein Eye Institute, University of California School of Medicine, Los Angeles, CA) for technical assistance with the SDS/PAGE of mole rat eye proteins, and the laboratory of Dr. Eric F. Wawrousek (National Eye Institute, National Institutes of Health, Bethesda, MD) for the production of transgenic mice. We thank the Ancell-Teicher Research Foundation for Genetics and Molecular Evolution for financial support.
Abbreviations
- E10
embryonic day 10
- shsp
small heat shock protein
- LSR
lens-specific regulatory
Footnotes
References
- 1.Sanyal S, Jansen H G, de Grip W J, Nevo E, de Jong W W. Invest Ophthalmol Vis Sci. 1990;31:1398–1404. [PubMed] [Google Scholar]
- 2.Nevo E. Mosaic Evolution of Subterranean Mammals Regression, Progression and Global Convergence. Oxford: Oxford Univ. Press; 1999. [Google Scholar]
- 3.Cooper H M, Herbin M, Nevo E. J Comp Neurol. 1993;328:313–350. doi: 10.1002/cne.903280302. [DOI] [PubMed] [Google Scholar]
- 4.Wistow G, Piatigorsky J. Annu Rev Biochem. 1988;57:479–504. doi: 10.1146/annurev.bi.57.070188.002403. [DOI] [PubMed] [Google Scholar]
- 5.de Jong W W, Hendriks W, Mulders W M, Bloemendal H. Trends Biochem Sci. 1989;14:365–368. doi: 10.1016/0968-0004(89)90009-1. [DOI] [PubMed] [Google Scholar]
- 6.Bloemendal H, de Jong W W. Prog Nucleic Acid Res Mol Biol. 1991;41:259–281. doi: 10.1016/s0079-6603(08)60012-4. [DOI] [PubMed] [Google Scholar]
- 7.Dubin R A, Wawrousek E F, Piatigorsky J. Mol Cell Biol. 1989;9:1083–1091. doi: 10.1128/mcb.9.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klemenz R, Fröhli E, Steiger R H, Schäfer R, Aoyama A. Proc Natl Acad Sci USA. 1991;88:3652–3656. doi: 10.1073/pnas.88.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sax C M, Piatigorsky J. Adv Enzymol Relat Areas Mol Biol. 1994;69:155–201. doi: 10.1002/9780470123157.ch5. [DOI] [PubMed] [Google Scholar]
- 10.Bhat S P, Nagineni C N. Biochem Biophys Res Commun. 1989;158:319–325. doi: 10.1016/s0006-291x(89)80215-3. [DOI] [PubMed] [Google Scholar]
- 11.Haynes J I, II, Duncan M K, Piatigorsky J. Dev Dyn. 1996;207:75–88. doi: 10.1002/(SICI)1097-0177(199609)207:1<75::AID-AJA8>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 12.Robinson M L, Overbeek P A. Invest Ophthalmol Visual Sci. 1996;37:2276–2284. [PubMed] [Google Scholar]
- 13.Vicart P, Caron A, Guicheney P, Li Z, Prevost M C, Faure A, Chateau D, Chapon F, Tome F, Dupret J M, et al. Nat Genet. 1998;20:92–95. doi: 10.1038/1765. [DOI] [PubMed] [Google Scholar]
- 14.Berry V, Francis P, Reddy M A, Collyer D, Vithana E, MacKay I, Dawson G, Carey A H, Moore A, Bhattacharya S S, Quinlan R A. Am J Hum Genet. 2001;69:1141–1145. doi: 10.1086/324158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark J I, Muchowski P J. Curr Opin Struct Biol. 2000;10:52–59. doi: 10.1016/s0959-440x(99)00048-2. [DOI] [PubMed] [Google Scholar]
- 16.Brady J P, Garland D, Douglas-Tabor Y, Robison W G, Jr, Groome A, Wawrousek E F. Proc Natl Acad Sci USA. 1997;94:884–889. doi: 10.1073/pnas.94.3.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brady J P, Garland D L, Green D E, Tamm E R, Giblin F J, Wawrousek E F. Invest Ophthalmol Visual Sci. 2001;42:2924–2934. [PubMed] [Google Scholar]
- 18.Gopal-Srivastava R, Piatigorsky J. Nucleic Acids Res. 1994;22:1281–1286. doi: 10.1093/nar/22.7.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gopal-Srivastava R, Kays W T, Piatigorsky J. Mech Dev. 2000;92:125–134. doi: 10.1016/s0925-4773(99)00341-x. [DOI] [PubMed] [Google Scholar]
- 20.Cvekl A, Piatigorsky J. BioEssays. 1996;18:621–630. doi: 10.1002/bies.950180805. [DOI] [PubMed] [Google Scholar]
- 21.Gopal-Srivastava R, Cvekl A, Piatigorsky J. J Biol Chem. 1996;271:23029–23036. doi: 10.1074/jbc.271.38.23029. [DOI] [PubMed] [Google Scholar]
- 22.Gopal-Srivastava R, Cvekl A, Piatigorsky J. J Biol Chem. 1998;273:17954–17961. doi: 10.1074/jbc.273.28.17954. [DOI] [PubMed] [Google Scholar]
- 23.Gopal-Srivastava R, Piatigorsky J. Mol Cell Biol. 1993;13:7144–7152. doi: 10.1128/mcb.13.11.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gopal-Srivastava R, Haynes J I, II, Piatigorsky J. Mol Cell Biol. 1995;15:7081–7090. doi: 10.1128/mcb.15.12.7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hendriks W, Leunissen J, Nevo E, Bloemendal H, de Jong W W. Proc Natl Acad Sci USA. 1987;84:5320–5324. doi: 10.1073/pnas.84.15.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Behrens M, Wilkens H, Schmale H. Gene. 1998;216:319–326. doi: 10.1016/s0378-1119(98)00346-1. [DOI] [PubMed] [Google Scholar]
- 27.Nevo E, Ivanitskaya E, Beiles A. Adaptive Radiation of Blind Subterranean Mole Rats: Naming and Revisiting the Four Sibling Species of the Spalax ehrenbergi Superspecies in Israel: Spalax galili (2n = 52), S. golani (2n = 54), S. carmeli (2n = 58) and S. judaei (2n = 60) Leiden, The Netherlands: Backhuys Publishers; 2001. [Google Scholar]
- 28.Davis J A, Reed R R. J Neurosci. 1996;16:5082–5094. doi: 10.1523/JNEUROSCI.16-16-05082.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quax-Jeuken Y, Bruisten S, Bloemendal H, de Jong W W. Mol Biol Evol. 1985;2:279–288. doi: 10.1093/oxfordjournals.molbev.a040351. [DOI] [PubMed] [Google Scholar]
- 30.Avivi A, Joel A, Nevo E. Gene. 2001;264:45–49. doi: 10.1016/s0378-1119(00)00603-x. [DOI] [PubMed] [Google Scholar]
- 31.Kumar S, Hedges B. Nature (London) 1998;392:917–920. doi: 10.1038/31927. [DOI] [PubMed] [Google Scholar]
- 32.Dubin R A, Gopal-Srivastava R, Wawrousek E F, Piatigorsky J. Mol Cell Biol. 1991;11:4340–4349. doi: 10.1128/mcb.11.9.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avivi A, Albrecht U, Oster H, Joel A, Beiles A, Nevo E. Proc Natl Acad Sci USA. 2001;98:13751–13756. doi: 10.1073/pnas.181484498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janssen J W H, Bovee-Geurts P H M, Peeters Z P A, Bowmaker J K, Cooper H M, David-Gray Z K, Nevo E, DeGrip W J. J Biol Chem. 2000;275:38674–38679. doi: 10.1074/jbc.M008254200. [DOI] [PubMed] [Google Scholar]
- 35.Jeffery W R. Dev Biol. 2001;231:1–12. doi: 10.1006/dbio.2000.0121. [DOI] [PubMed] [Google Scholar]