Abstract
Extreme temperatures, whether excessively high or low, are critical environmental stressors for organisms, profoundly impacting cellular metabolism and homeostasis. The presented study addresses the effects of varying incubation conditions on the expression of key stress-related genes (HSP70, HSP90, and SOD1) during quail development. Quail eggs were incubated using three distinct methods: standard incubation at 38.2°C and preincubation at either 22°C or 30°C in both in ovo and ex ovo conditions. Results revealed a distinctive contrast in survival rates: embryos cultured ex ovo showed significantly lower viability (26.41 %) compared to the in ovo method (87.66 %). Among ex ovo groups, preincubation at 30°C yielded the highest survival rate (43.48 %), highlighting the critical role of optimal preincubation temperature. The preincubation period resulted in a notable increase in the total weight of embryos in the in ovo group when compared to the ex ovo group. Additionally, preincubation at 30°C resulted in increased weight of the heart and liver in the in ovo group. Gene expression analysis showed clear differences between incubation methods. While in ovo incubation led to uniformly increased gene expression across all examined organs (liver, heart, and breast muscle) at both preincubation temperatures, the ex ovo conditions exhibited mixed results: decreased gene expression in the liver (SOD1) and muscle (HSP70, SOD1) but notable increased in the liver (HSP70) and heart (SOD1). Our findings suggest that preincubating quail eggs at 30°C for 12 h is associated with improved survival under ex ovo conditions, providing insights into optimizing ex ovo incubation practices.
Keywords: Ex ovo, Heat stress, In ovo, Preincubation, Quail embryo, HSP70, HSP90, SOD1
Graphical abstract
1. Introduction
The present study proposes a novel approach by comparing in ovo and ex ovo incubation methods under controlled preincubation temperatures in quail embryos. This research integrates morphological, physiological, and molecular (gene expression) analyses, providing a comprehensive perspective on how incubation methods affect embryo development. The findings of the present study provide valuable insights into the optimization of ex ovo incubation techniques, which have significant potential applications in both developmental biology and biomedical research.
In recent years, there has been an increased emphasis on minimizing the use of experimental animals (Fischer et al., 2022), even though they are undeniably one of the main pillars of biological and biomedical research (Ericsson et al., 2013). The main reasons for considering alternatives are related to ethical issues, cost minimization, time, laws, and public opinion (Davies, 2012; Doke & Dhawale, 2015; Freires et al., 2017; Sung et al., 2018). Consequently, there is a growing effort to replace animals with insentient alternatives, which is currently considered a key aspect of good scientific practice in animal research (Lee et al., 2022; Richter, 2024).
Avian models serve as a promising alternative animal model due to their low cost (no need to feed embryos), easy visualization of embryonic development (Sukparangsi et al., 2022) availability, short generation interval, rapid development, and, finally, a close resemblance to mammals (Nakamura et al., 2019; Wang & White, 2021). The utilization of the quail embryo as an alternative animal model has yielded numerous substantial advantages, thereby propelling substantial conceptual progress in developmental biology, cancer research, virology, endocrinology, and other biomedical fields (Baer et al., 2015; Huss et al., 2008).
Most studies employ the "windowing" technique (in ovo method) to observe morphological alterations after the administration of chemicals during the developmental process of the embryo within the eggshell (Andacht et al., 2004; Farzaneh et al., 2018; Tahara & Obara, 2021). It is used for long-term experiments because its outcomes in fewer developmental abnormalities and allows for natural hatching (Kundeková et al., 2021).
On the other hand, the ex ovo method is widely used in embryology, genetic manipulation, toxicology, regenerative medicine (Tahara & Obara, 2021) or preclinical cancer research (Lenkavska et al., 2019; Rasmussen et al., 2021). This type of culture enables perfect visualization and essentially unlimited access to the chorioallantoic membrane (CAM), (Chen et al., 2021; Schomann et al., 2013) which allows direct monitoring of the effect of pathogens or drugs on the vasculature of the developing embryo (Kundeková et al., 2021; Merlos Rodrigo et al., 2021). To achieve the best results, ex ovo culture must strike a balance between several factors, particularly those relating to oxygen, moisture, and calcium requirements. This is essential to prevent the expected higher mortality rate resulting from the more extensive drying of the embryos or the risk of infection (Sukparangsi et al., 2022).
The need for many embryos has led to the use of cold storage of eggs, which adversely affects the viability of embryos, hatchability of eggs, and poor quality of hatched chicks (Damaziak, 2021). To mitigate these effects, preincubation is used to overcome them (Tesarova et al., 2021). It is possible to use different temperatures and different durations for preincubation, but it is still a subject of debate and research. Various stimuli (incubation temperature, relative humidity, storage before incubation, UV radiation) can affect the development of bird embryos and represent stress factors for the organism that threaten the functionality of cells (Goel et al., 2021). Stress upregulates several genes, such as genes for transcription factors that control cell growth, chromatin structure, cell cycle activation, and enzymes involved in nucleic acid and protein biosynthesis (Feodorova & Sarafian, 2012). The main role in the cellular stress response is attributed to induced heat shock proteins (HSPs), which participate in the folding of denatured or aggregated client proteins, promote the assembly/disassembly of multiprotein complexes or regulate protein degradation (Kaur & Asea, 2019).
In this context, HSP70, HSP90, and superoxide dismutase (SOD) are key players in the stress response. An increase in HSP70 is associated with protection of key protein sensitivity to temperature changes (Zhao et al., 2014). It was found that increased expression of HSP70 protected the spleen against oxidative stress and inflammatory damage induced by cold stress in quail (Ren et al., 2018). Since HSP70 is expressed in internal organs, blood, and feathers, and its synthesis is triggered by a variety of stressors, which include water deprivation, transport, and cold stress, HSP70 could be used to monitor stress levels in many bird species and poultry (Greene et al., 2019). HSP90 is essential for maintaining the folding, structural integrity, and proper regulation of a subset of cytosolic proteins (Prodromou, 2016). It is significantly increased after exposure of cells to heat stress in avian embryos and chicks after hatching. Higher expression was detected in muscle and heart compared to the brain (Al-Zghoul et al., 2015). SOD is an important endogenous antioxidant that scavenges oxygen radicals (Zheng et al., 2023) and is found in the liver, heart, lung, yolk sac membrane, thigh muscle, kidney, and brain of embryos. SOD is particularly relevant in avian embryos, where heat stress leads to decreased antioxidant capacity and altered gene expression. SOD1 manages ROS (reactive oxygen species) primarily in the cytoplasm, where many metabolic reactions produce superoxide. SOD2 handles the majority of ROS generated in mitochondria, which are the primary source of intracellular ROS due to electron transport chain activity. The authors reported that tissue-dependent SOD activity is highest in the heart and decreases in the following order: muscle, yolk sac membrane, kidney, lung, and liver (Truong & King, 2023).
This study aims to evaluate the effects of two preincubation temperatures (22 °C and 30 °C) on embryonic development and the expression of stress-related genes (HSP70, HSP90, and SOD1) in quail embryos incubated using both in ovo and ex ovo techniques. We hypothesize that both preincubation temperature and incubation method significantly influence these developmental and molecular outcomes.
This study addresses a critical challenge in optimizing ex ovo incubation, which, despite its advantages, often results in lower survival rates than traditional in ovo methods. While previous research has primarily focused on in ovo incubation or explored only a narrow range of preincubation temperatures, our study compares both incubation methods under two distinct temperature conditions (22°C and 30°C). This approach provides new insights into embryonic stress responses and supports the refinement of avian alternative models in biomedical research.
2. Materials and methods
2.1. Preincubation and incubation
Fertilized quail eggs (Coturnix coturnix japonica, n=144) were purchased from the quail farm (Mala Ida, Kosice, Slovakia), no older than three days after laying. Although maternal factors, such as age, health status, and genetics are known to influence embryonic development, these variables were not specifically controlled in this study. Future research should consider these maternal characteristics to better understand their potential impact on embryo viability and development. The eggs were disinfected with 70 % ethanol and divided into two groups: in ovo incubation (65 eggs) and ex ovo incubation (79 eggs). In both the in ovo and ex ovo incubation groups, the fertilized eggs were divided into three groups according to the incubation conditions (Table 1).
Table 1.
Survivability data of quail embryos.
| Incubation method | Condition | Eggs | ED4 (%) | ED6 (%) | ED8 (%) | ED9 (%) |
|---|---|---|---|---|---|---|
| In ovo | Standard incubation | 22 | -c | - | - | 20 (90.91 %) |
| P22a | 21 | - | - | - | 18 (85.71 %) | |
| P30b | 22 | - | - | - | 19 (86.36 %) | |
| Ex ovo | Standard incubation | 27 | 15 (55.56 %) | 11 (40.74 %) | 6 (22.22 %) | 5 (18.52 %) |
| P22 | 29 | 19 (65.52 %) | 14 (48.28 %) | 8 (27.59 %) | 5 (17.24 %) | |
| P30 | 23 | 17 (73.91 %) | 13 (56.52 %) | 10 (43.48 %) | 10 (43.48 %) |
P22 – Preincubation at ±22°C
P30 – Preincubation at 30°C
- – indicates data not available for the given incubation method and embryonic day
2.1.1. In ovo incubation
In ovo incubation was performed in a forced-draft constant-humidity automatic incubator (Brinsea OvaEasy 100 Advance Ex, North Somerset, UK) in a standard condition at the temperature of 38.2 ± 0.5°C and 60 % relative humidity. The first group (22 pcs; 7-8 per replicate) was incubated in a standard condition at the temperature of 38.2°C ± 0.5°C and 60 % relative humidity. The preincubation period of the second and third groups lasted 12 h (Lin et al., 2017; Janikovičová et al., 2019), and subsequently the eggs were incubated under the standard in ovo conditions. The second group of fertilized eggs (21 pcs; 7 per replicate) was preincubated at a room temperature of 22°C (P22). The third group of fertilized eggs (22 pcs; 7-8 per replicate) was preincubated in an automatic incubator (COVINA ET 49, Italy) at 30°C (P30; Lin et al., 2017) and 60 % relative humidity. The embryos were incubated until the 9th embryonic day (ED9) when embryogenesis ends and samples were collected. Quail eggs that were found to be non-fertilized were immediately excluded from the study and removed.
2.1.2. Ex ovo incubation
For ex ovo methodology, the intended eggs were incubated horizontally performed in a forced-draft constant-humidity automatic incubator (Brinsea OvaEasy 100 Advance Ex, North Somerset, UK) in a standard condition at the temperature of 38.2 ± 0.5°C and 60 % relative humidity. After 56 h, the egg content with quail embryo was carefully transferred into a sterile 6-well cell culture plate in laminar box. Quail eggs that were found to be non-fertilized were immediately excluded from the study and removed. The first group (27 pcs; 9 per replicate) was incubated horizontally in a standard condition at the temperature of 38.2°C ± 0.5°C and 70–80 % relative humidity. The preincubation period of the second and third groups lasted 12 h (Lin et al., 2017; Janikovičová et al., 2019), and subsequently the eggs were incubated under the standard ex ovo conditions. The second group of fertilized eggs (29 pcs; 9-10 per replicate) was preincubated horizontally at a room temperature of 22°C (P22). The third group of fertilized eggs (23 pcs; 7-8 per replicate) was preincubated horizontally at 30°C (P30; Lin et al., 2017) in an automatic incubator (COVINA ET 49, Italy) and 60 % relative humidity. We checked the survivability of embryos cultured under ex ovo conditions on days ED4, ED6, and ED8 of incubation. Embryos that were found to be non-viable on these days were promptly extracted and excluded from the study. The embryos were incubated until the ED9 when embryogenesis ends and samples were collected.
The application of the assessment of each group was repeated 3 times for each section in both in ovo and ex ovo methods.
2.2. Macroscopic analysis
Within in ovo methodology, we opened incubated eggs at the blunt end on ED9, and surviving embryos were carefully pulled out. Ex ovo cultured surviving embryos were removed from the culture plates using a retractor, followed by the same procedure as in ovo cultured embryos. The incidence of early embryonic mortality within ex ovo method (ED4, ED6, ED8) and malformations (growth retardation, monophthalmia, anophthalmia, opened body cavity, deformity of the beak, legs, and wings) was evaluated, followed by an assessment of body weight, heart and liver weight as well. After the weighing part, collected samples of heart, liver, and breast muscle were stored at 80°C for RNA extraction.
2.3. Gene expression analysis
Total RNA was extracted from collected tissues (liver, heart and breast muscle) using QIAshredder and total Rneasy Mini Kit from Qiagen (Qiagen, Hilden, Germany) following the manufacturer´s instructions including genomic DNA digestion using the RNase-free Dnase set (Qiagen, Germantown, TN, USA). The RNA purity and yields were analyzed using the NanoDrop Lite Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The application for measurement of RNA reports RNA concentration and two absorbance ratios (A260/280 and A260/230). RNA concentration is reported in ng/µl. A260/280 purity ration represents ration of corrected absorbance at 260 nm to corrected absorbance at 280 nm. An A260/280 purity ratio of ∼2.0 is generally accepted as “pure” for RNA. A260/230 purity ration represents ration of corrected absorbance at 260 nm to corrected absorbance at 230 nm. An A260/230 purity ratio between 1.8 and 2.2 is generally accepted as “pure” for RNA. We used a two-step RT-qPCR approach. In the first step, complementary DNA (cDNA) synthesis was performed using a protocol High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems™, Waltham, MA, USA). A total of 1 µg of total RNA was used to prepare 20 µL of cDNA, which was then used for qPCR. In the second step, the quantification of genes of interest in the cDNA samples was performed using specific primers for HSP90, HSP70 and SOD1 (Bastos et al., 2017; Liu et al., 2019; Mehaisen et al., 2017; Fig. 1). For each gene, SYBR Green Master Mix (Applied Biosystems™, Waltham, MA, USA) was used in a total volume of 10 µL. PCR mixture contained specific primers for each gene (300 nM), SYBR Green PCR Master Mix, and water. cDNA for GAPDH was used as an endogenous control for calculating fold differences in RNA levels by the 2-ΔΔCT method. qPCR was performed under suitable conditions for SYBR Green with the following steps: Initialization at 95°C for 10 min, amplification in 40 cycles at 95°C for 15 s followed by 60°C for 1 min. Each sample was examined as triplicate. Dissociation curve analysis was performed after each completed PCR run to ensure the absence of nonspecific amplifications. The gene expression data were calculated against GAPDH endogenous control and expression levels of selected genes were normalized to untreated samples (control-group without preincubation). For the HSP90 gene, the melting analysis temperature was in the range of 75.85–76.39°C, for HSP70 81.33–81.89°C, and for SOD1 77.22–78°C.
Fig. 1.
Primer sequences used in the study.
As endogenous control, the GAPDH gene was used based on the analysis of gene expression stability using GeNorm algorithm. GAPDH was the most stable in all tested samples. A low M-value 0.3 of GAPDH against 0.4 of beta actin and 0.5 of ubiquitin indicates a stable expression.
2.4. Statistical analysis
Statistical analysis was performed using GraphPad Prism software (version 9.3, USA). A two-way ANOVA test was employed to compare the weights of preincubation in both in ovo and ex ovo groups, with post-hoc analyses conducted using Sidak's multiple comparison test. For analyzing survivability in ex ovo groups and gene expression data, the Tukey's multiple comparison test was applied. The execution of these specific multiple comparison tests was automatically recommended by GraphPad Prism software based on the analysis conducted. The value of statistical significance was considered as p˂0.05 and p˂0.0001. The data are expressed as the mean ± standard deviation (SD), and the assumptions of normality and equal variance were checked prior to analysis.
3. Results
3.1. In ovo incubation
3.1.1. Standard incubation (38.2°C and 60 % relative humidity)
At this incubation, we observed malformations on the pelvic limbs (15 %), and whitish spots on the liver (40 %) of living embryos. Petechial hemorrhages appeared on the head of only one embryo. The mean body weight of the embryos was 1.58 g, while the mean heart weight was 25.68 mg, and the mean liver weight was 40 mg (Table 2). In this cohort, 50 % of the embryos exhibited the formation of black plumage, predominantly in the dorsal region.
Table 2.
Weight of the whole embryo and selected organs on ED9.
|
in ovo | |||
|---|---|---|---|
| Body weight ± SDc (g) |
Liver weight ± SD (mg) |
Heart weight ± SD (mg) | |
| Standard incubation | 1.58 ± 0.159 | 40.00 ± 0.007 | 25.68 ± 0.003 |
| P22a | 1.70 ± 0.202 | 41.61 ± 0.010 | 25.38 ± 0.004 |
| P30b | 1.68 ± 0.199 | 43.47 ± 0.007 | 25.63 ± 0.003 |
| ex ovo | |||
|
Body weight ± SDa (g) |
Liver weight ± SDa (mg) |
Heart weight ± SDa(mg) | |
| Standard incubation | 0.95 ± 0.042d | 28.0 ± 0.006d | 21.6 ± 0.003 |
| P22 | 0.96 ± 0.040d | 30.8 ± 0.009d | 20.4 ± 0.004d |
| P30 | 0.98 ± 0.134d | 26.9 ± 0.005e | 18.2 ± 0.002e |
P22 – Preincubation at ±22°C
P30 – Preincubation at 30°C
SD – standard deviation
p ˂ 0.05
p ˂ 0.0001
3.1.2. Preincubation at room temperature (±22°C)
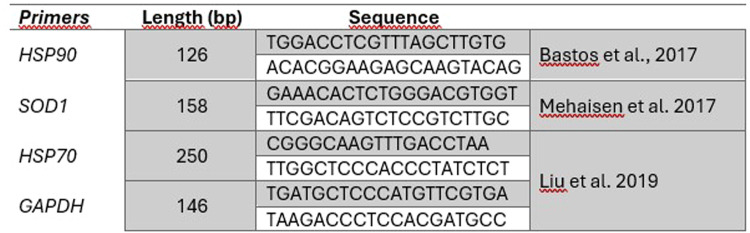
In most cases (66.67 %), living embryos exhibited whitish spots on the liver. In one instance, the entire right lobe of the liver was not developed. Additionally, pelvic limb malformations were observed in 27.78 % of cases. Petechial hemorrhages were observed primarily on the heads. One embryo exhibited fusion, presenting with two pairs of pelvic limbs and two pairs of wings, along with a single head, liver, and heart (Fig. 2). The mean body weight of the embryo was 1.7 g. The mean weight of the heart was 25.38 mg, while the mean weight of the liver was 41.61 mg (Table 2). In 55.56 % of cases, the emergence of incipient black plumage in the dorsal region was observed.
Fig. 2.
Image of an embryo with fusion malformation.
3.1.3. Preincubation at 30°C and 60 % relative humidity
The embryos exhibited a macroscopic increase in size compared to the previous two groups. White spots on the liver were observed in 31.58 % of cases, while petechial hemorrhages were noted on the head in 10.53 % of instances. The mean body weight was 1.68 g, the mean weight of the heart was 25.63 mg, and the mean weight of the liver was 43.47 mg (Table 2). In many live embryos, the onset of black plumage was observed in their dorsal regions, with a significantly higher density compared to previous instances. The incipient plumage was also visible on the wings. Additionally, the presence of colored plumage was observed, with a prevalence of orange hues (42.11 %). This was particularly evident on the tail.
The mean body weight of the entire embryo in the in ovo incubated group was 1.65 g. The in ovo preincubation groups exhibited statistically significant differences in embryo body weight (p˂0.0001) when compared to the ex ovo groups. The in ovo groups exhibited a markedly elevated weight.
3.2. Ex ovo incubation
3.2.1. Standard incubation (38.2°C and 60 % relative humidity)
Following the initial 24-h period of ex ovo incubation (ED4), the mortality rate was recorded at 44.44 %. As the ex ovo incubation period progressed, the mortality rate increased. On ED6 (59.26 %), ED8 (77.78 %), and ED9 (81.48 %), when the samples were collected. On the day of sampling (ED9), the embryos exhibited a reduction in size compared to those incubated in ovo. The mean body weight was 0.95 g, the mean weight of the heart was 21.6 mg, and the mean weight of the liver was 28 mg (Table 2). The plumage had not yet developed, but as with the in ovo incubation, white spots were visible on the liver.
3.2.2. Preincubation at room temperature (±22°C)
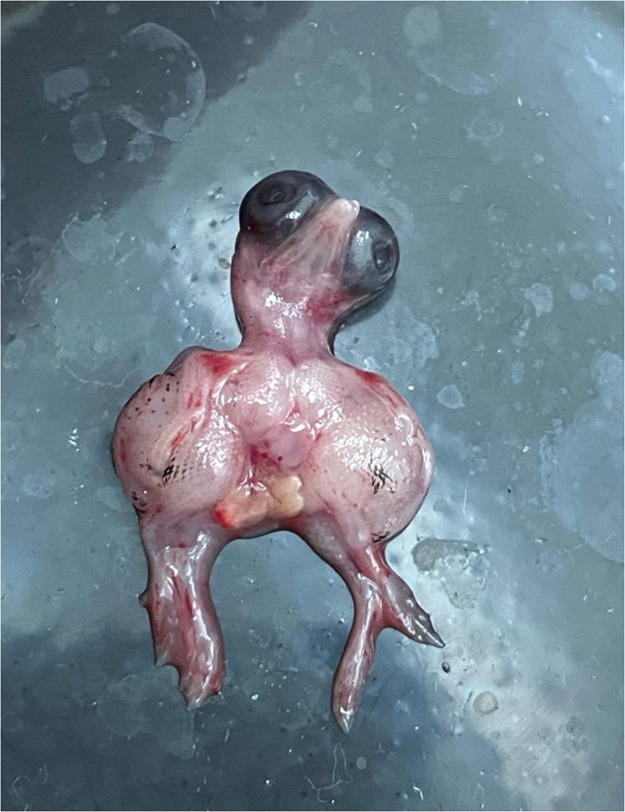
As observed previously, the mortality rate increased with the duration of incubation, even when the samples were preincubated at room temperature. On ED4, the mortality rate was 34.48 %, on ED6 it was 51.72 %, on ED8 it was 72.41 %, and at the time of sampling on ED9 it was 82.76 %. The mean body weight was 0.96 g, the mean weight of the heart was 20.4 mg, and the mean weight of the liver was 30.8 mg (Table 2). Two embryos in this group exhibited incipient black plumage, and white spots on the liver were observed in multiple embryos. In one instance, monophthalmia was observed (Fig. 3), accompanied by petechial hemorrhages that covered the entire body of the embryo.
Fig. 3.
Image of an embryo exhibiting monophthalmia.
3.2.3. Preincubation at 30°C and 60 % relative humidity
The lowest mortality rate was observed in this group on ED4 (26.09 %), ED6 (43.48 %), ED8, and ED9 (56.52 %). The mean body weight was 0.98 g, and the mean heart weight was 18.2 mg, representing the lowest mean heart weight observed in all in ovo and ex ovo experimental groups. The mean liver weight was 26.9 mg (Table 2). Prior to removal from the plates, the embryos exhibited the greatest degree of vitality compared to the other ex ovo groups. In four cases, incipient black plumage was observed.
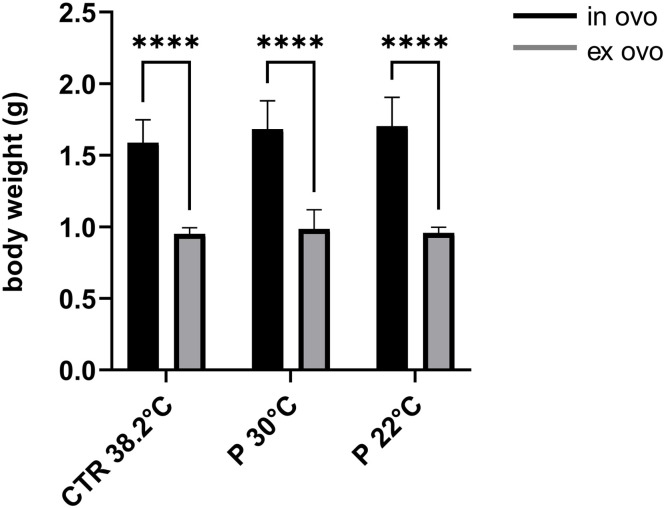
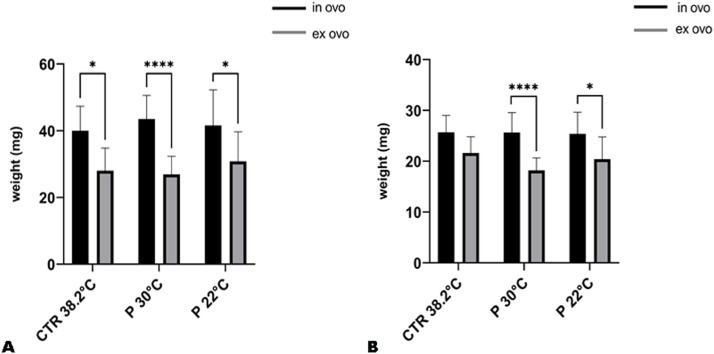
The mean body weight of the ex ovo cultivated group was 0.96 g, which was significantly lower than the mean weight of 1.65 g observed in the in ovo incubation group (Fig. 4). The in ovo group had a mean liver weight of 41.69 mg, significantly lower than the ex ovo group's mean liver weight of 28.57 mg. A similar decrease was observed in the weight of the heart during ex ovo incubation. The average weight in the in ovo group was 26.63 mg; in the ex ovo group, 20.07 mg. Furthermore, it was observed that the weight of the liver and heart was lower in the sub-group that underwent preincubation at 30°C in comparison to the sub-group that underwent preincubation at 22°C in ex ovo groups (Fig. 5).
Fig. 4.
Comparison of the weights of embryos incubated in ovo and ex ovo under different incubation conditions (CTR 38.2°C – standard incubation; P 30°C – preincubation at 30°C; P 22°C – preincubation at ±22°C). Mean (±SEM) values presented; analysis revealed a significant difference between conditions (p < 0.0001).
Fig. 5.
Comparison of liver (A) and heart (B) weights in embryos incubated under different incubation conditions (CTR 38.2°C – standard incubation; P 30°C – preincubation at 30°C; P 22°C – preincubation at ±22°C). Mean (±SEM) values presented; statistical significance indicated as ****p < 0.0001, *p < 0.05.
3.3. Survivability of the embryos
3.3.1. In ovo
In the context of in ovo incubation, it was observed that the overall embryonic survival rate exhibited minimal variation across the experimental groups, with a range of 86.36 % to 90.91 % (Table 1).
3.3.2. Ex ovo
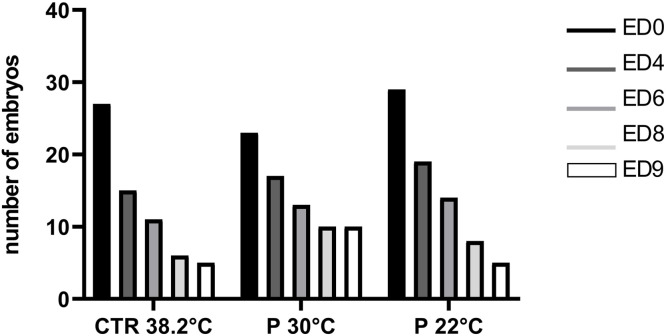
The ex ovo group exhibited a markedly inferior survival rate on ED4, with an average of 65 % across all groups. This suggests the highest mortality rate was reached between ED3, when the embryos were transferred into the cell culture plates, and the first day of control, ED4. The survival of the embryos was also observed on additional control days, which demonstrated that the decline in survival rate was not as rapid as that observed on ED4 (Table 1, Fig. 6). The overall mean survival rate of all groups of ex ovo incubated embryos at ED9 was 26.41 %, while the mean survival rate of in ovo groups reached 87.66 %. These results demonstrate that in ovo incubation, as a natural method of incubation, is an effective approach to maintaining embryo viability.
Fig. 6.
Viability of embryos cultured ex ovo under different incubation conditions (CTR 38.2°C – standard incubation; P 30°C – preincubation at 30°C; P 22°C – preincubation at ±22°C). Mean (±SEM) values presented.
The preincubation period did not have a statistically significant impact on the survival of embryos incubated within the egg. In the ex ovo group, the subgroup that was preincubated at 30°C exhibited the highest survival rate among all the ex ovo cultivated subgroups (43.48 %) on ED9.
3.4. Gene expression analysis of in ovo and ex ovo method
The gene expression profiles of liver, heart and muscle tissue were examined in quail embryos that had been incubated using either the in ovo or ex ovo technique, and with or without a preincubation period.
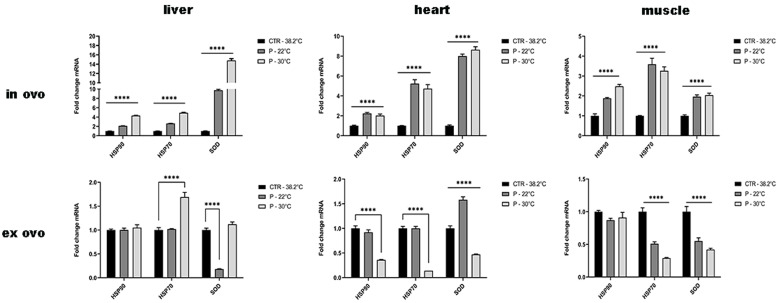
3.4.1. In ovo
The in ovo incubation approach was found to result in a significant upregulation (p<0.0001) of all selected genes involved in stress protection (HSP90, HSP70, and SOD1) in all studied tissues (liver, heart and muscle) for both preincubation conditions (P22 or P30). The highest level was recorded for SOD1 in the liver and then in the heart when preincubation was conducted at 30°C. In the case of muscle tissue, the highest level of HSP70 was recorded at 22°C as the preincubation temperature. Upregulation of SOD1 as the enzyme involved in the first line defense in an antioxidant system should be the response to oxidative stress conditions and higher concentration of reactive oxygen species. Under stressful conditions the upregulation of HSP90 can cause through dissociation of HSF-1 the induction of the transcription of HSP70.
3.4.2. Ex ovo
The use of ex ovo method showed the significant downregulation (p˂0.0001) of SOD1 in the liver at 22°C preincubation temperature. In the heart, significant downregulation was observed in the case of HSP90, HSP70 and SOD1 preincubation at 30°C. In the muscle, significant downregulation was noticed for HSP70 and SOD1 using both preincubation temperatures. The downregulation of SOD1, HSP70 and HSP90 should be the result of high stress when the adaptive function of stress factors is overwhelmed, or the genes are damaged by high concentration of reactive oxygen/nitrogen species.
The results of the gene expression analysis conducted during in ovo and ex ovo incubation with varying preincubation conditions are presented in Table 3 and Fig. 7. These expression patterns were consistent across all biological replicates, reinforcing the reliability of the observed trends.
Table 3.
Gene expression of monitored genes in in ovo and ex ovo groups with preincubation.
| HSP90 | HSP70 | SOD1 | |||||
|---|---|---|---|---|---|---|---|
| P22a | P30b | P22 | P30 | P22 | P30 | ||
| liver | in ovo | +c | + | + | + | + | + |
| ex ovo | nse | ns | ns | + | -d | ns | |
| heart | in ovo | + | + | + | + | + | + |
| ex ovo | ns | - | ns | - | + | - | |
| muscle | in ovo | + | + | + | + | + | + |
| ex ovo | ns | ns | - | - | - | - | |
P22 – Preincubation at ±22°C
P30 – Preincubation at 30°C
+ – Significant up-regulation p < 0.0001
- – Significant down-regulation p < 0.0001
ns – Nonsignificant
Fig. 7.
Gene expression of HSP90, HSP70, and SOD in selected organs during in ovo and ex ovo incubation under different incubation conditions (CTR 38.2°C – standard incubation; P 30°C – preincubation at 30°C; P 22°C – preincubation at ±22°C). Mean (±SEM) values presented; statistical significance indicated as p < 0.0001.
4. Discussion
4.1. Survival rates and incubation methods
The present study demonstrated that the incubation method and preincubation temperature significantly influenced the survival rate, morphological development, and gene expression in quail embryos. In ovo incubation resulted in substantially higher embryo viability, with a mean survival rate of 87.66 % on ED9, compared to only 26.41 % in the ex ovo group. Preincubation at 30°C yielded improved outcomes, particularly in the ex ovo experimental group. Furthermore, gene expression analysis revealed a significant upregulation of stress-related genes (HSP90, HSP70, and SOD1) in in ovo conditions, while ex ovo incubation led to their downregulation, suggesting a higher level of physiological stress.
Embryos cultivated in ex ovo conditions often show much lower survivability compared to chicken embryos cultivated in ovo (Nowak-Sliwinska et al., 2014; Tahara & Obara, 2021), and most of the articles describe only the survival rate of chick embryos. Naik et al. (2018) reported survivability of chick embryos between 15 and 25 % using Petri Dishes and Paper Boats for ex ovo incubation, similar to our experiment (26.41 % on the ED9). However, they also focused on the cup-CAM method, when the entire content of the hatching egg is moved into a stretch wrap stretched over a plastic cup, which achieved 85–95 % survival. Dohle et al. (2009) presented an altered protocol for ex ovo cultivation, and they described survivability of over 50 % even with chicks' fertilized eggs that have been stored for up to one week. A difference in this protocol is that the eggs were opened after 72 h, in contrast to the standard ex ovo protocol for quail eggs, where the eggs are opened and transferred after 52 h. However, this protocol only adjusts values for chicken embryos, whereas we used quail embryos in our experiment. We can only suppose that the survivability rate of quail embryos could be higher if the opening and transfer of eggs into cultivation plates was performed a couple of hours later than after 52 h, still considering the development of CAM and the possibility it could be damaged in later hours of incubation (52–56 h). The other authors Rasmussen et al. (2021) used quail eggs to establish reliable and reproducible ex ovo antitumor drug testing methods in xenografts of the CAM assay. They incubated quail eggs for 72 h and then transferred them to six-well plates. Their study shows that embryo survival drops to 40–50 % by ED10, with most losses occurring between ED4 and ED7.
Another step for improving the protocol of cultivation eggs is the necessity of ensuring constant airflow and ventilation in the incubator and providing an average humidity of at least 60 %, which should not decrease during the entire incubation period. In addition, calcium carbonate supplementation in embryos cultured without the shell can increase hatchability by more than 40 % (Sukparangsi et al., 2022).
These findings point to several physiological limitations that can compromise embryo development outside the shell environment. Ex ovo cultivation presents challenges, including the lack of the protective eggshell, which can lead to dehydration, nutrient loss, and skeletal deformities (Sukparangsi et al., 2022). To improve viability, optimizing humidity, airflow, calcium supplementation, and refining the timing of egg opening and transfer are key steps recommended in the literature (Rasmussen et al., 2021; Sukparangsi et al., 2022).
4.2. Embryonic growth and morphological development
In embryos cultured by the in ovo method, hatchability should exceed 90 %. Romao et al. (2009) observed the effects of different incubation temperatures on hatchability and embryonic mortality of Japanese quail eggs in ovo. At temperatures of 37-38°C, like our standard incubation, they observed lower embryonic death (4-6 %), lower intermediate death (0-1 %), and lower late embryonic death (3–6 %) compared to others observed temperatures. In our experiment, we monitored survivability of 87.66 % at ED9. Piestun et al. (2013) investigated the impact of different preincubation temperatures on several groups of chicken eggs. They used preincubation at 30.2°C for 12 h for eggs stored for 4 days as one option, and the resulting hatchability was 94 %. In our experiment the preincubation with a temperature of 30°C, reached a survivability rate of 86.36 %, which is comparable with chicken embryos. Our ex ovo quail embryos preincubated at 30°C achieved survivability of 43.48 %. It is the highest value among all ex ovo embryos; thus, we can assume that this preincubation temperature can positively influence survivability.
Hanafy and Hegab (2019) observed the influence of two different light sources (florescent light, FL, and incandescent bulbs, INC) during incubation on egg weight, embryonic development, hatchability, and growth after hatching of Japanese quail. They weighed whole embryos and organs on ED14, and they proved that exposure of quail to continuous FL significantly improved embryo growth, increased hatchability and lower percentage of dead embryos compared to continuous INC lighting.
In our study, the average weight of embryos cultivated in ovo reached 1.65 g on ED9. Marks (1975) described the weight of the quail embryo on ED9 as 1.5 g. Thus, the average weight of embryos was almost identical. However, it may be related to the use of a different breed of quail, including the various sizes of the eggs themselves, which was not a monitored or determining parameter in our experiment. The average weight of ex ovo cultivated eggs was only 0.96 g. This significant difference in weight could have occurred because of the ex ovo embryos growing outside of their natural environment (eggshell), which besides providing protection, also ensures an appropriate microenvironment and supply of calcium. Calcium from eggshells is needed for ossification, and its deficiency can result in limb deformity (Sukparangsi et al., 2022). It is possible that in the cultivation plates, the yolk and the egg white could gradually dry out, which could limit the nutrition and growth of the embryo.
4.3. Gene expression and stress response
The two methods of incubating quail eggs afford the researcher different levels of access and handling of the bird embryos. Incubation factors (temperature, humidity, light) causing stress lead to a reaction that can have consequences at various levels. The essential feature of the stress response pathway is the upregulation of multiple heat shock proteins (HSPs), which accumulate at high concentrations in cells (Murshid et al., 2018) and restore cell structure and cellular metabolism (Hu et al., 2022). Murugesan et al. (2017) also suggest that even natural incubation is affected by heat stress due to patchy conditions, as hens move regularly in search of food, resulting in uneven nest insulation. Thus, heat stress can affect developing embryos and increase HSPs even during natural incubation.
HSP90 is essential for eukaryotic viability, and a decrease in the intracellular concentration of HSP90 increases the mortality of mammalian cells at elevated temperatures (Prodromou, 2016). Based on the study of Al-Zghoul et al. (2015), it is apparent that thermal manipulation during incubation leads to a significant increase in HSP90 mRNA expression in developing and post-hatch chickens. HSP90 mRNA expression values varied depending on the analyzed tissue, and the duration of heat manipulation. Higher expression values were presented in muscles and the heart compared to the brain. Santana et al. (2021) observed prenatal ambient temperature affecting the response of Japanese quail. Chicks hatched from heat-stressed mothers showed a higher content of carbonylated proteins and HSP70 expression. Murugesan et al. (2017) spoked about acute heat stress that increased the expression of HSP90, and HSP70 in the liver, the muscle, and the heart of broiler chickens, which confirms our results only for in ovo incubation.
During in ovo incubation, we recorded an increase in all examined organs (liver, heart, and breast muscle) with both types of preincubation (P22 or P30), while a significant increase in these genes during ex ovo incubation was only observed in the liver at P30 for HSP70. Galletta et al. (2022) also mentioned the expression of HSP70 after exposure to temperatures up to the upper limit of tolerance of different species of embryos, including quail (Coturnix coturnix). Late-stage embryos upregulated HSP70 gene expression, followed by a decline before reaching the upper tolerance lethal limits for the embryos in all species. They also suggest that the expression of HSPs genes may vary between developmental stages, as has been demonstrated in most classes of vertebrates. All authors confirm the potential importance of HSPs for the embryo's development and its subsequent ability to survive.
On the other hand, we recorded a significant reduction in the heart for the HSP90 and HSP70, in the muscle for the HSP70 and SOD1 within the ex ovo incubation (P30). We hypothesize that preincubation adversely affected early embryo development, as the expected upregulation of HSPs is generally considered an important part of an integrated physiological response to heat stressors.
An antioxidant-oxidant balance in tissues also supports normal embryonic development and chick viability after hatching during embryonic development. This balance is maintained by several molecules including SOD (Yigit et al., 2014). Heat stress can also threaten quail’s antioxidant system by reducing the overall antioxidant capacity of the liver. In a study by Zhang et al. (2018) broilers exposed to heat stress had lower glutathione levels and SOD. On the contrary, Habashy et al. (2019) investigated enzyme activity affected by heat stress on chickens, and they observed that heat-stressed chicken SOD levels increased in the liver only at 12 days, but there were no changes in SOD levels in P. major muscle.
We observed the most significant increase in expression for SOD1, with the highest values measured in the liver, then the heart, and the lowest (but still statistically significant) in the breast muscle in in ovo incubation. In ex ovo experiment, SOD1 was significantly reduced in the liver and the muscle. It has been observed by numerous authors (Azad et al., 2010; Habashy et al., 2019; Ming et al., 2012) that the response of SOD to thermal stress is contingent upon the duration of the stress (acute or chronic) and the specific tissue in question. These observations align with the findings of our study.
5. Conclusion
This study reveals key differences between in ovo and ex ovo incubation approaches of quail embryos. The conditions of in ovo incubation closely simulate the natural environment required for embryo development, ensuring robust growth supported by the eggshell’s microenvironment and nutrient supply. Several factors such as temperature fluctuations, oxygen supply, hypoxia, oxidative stress and nutrient deficiencies can affect the development of avian embryos. The response of embryos is the increased production of proteins (HSPs, SOD, catalase, glutathione peroxidase) involved in the cellular stress defense system.
Our data show that preincubation at 30 °C for 12 h under in ovo conditions significantly increased both total embryonic and organ weights (liver, heart), suggesting enhanced growth and metabolic support. In contrast, ex ovo incubation exposes embryos to various stressors (e.g., loss of shell protection, altered gas exchange, mechanical manipulation), which appear to elevate cellular stress and reduce gene expression and survival. The uniform upregulation of stress-related genes observed under in ovo conditions likely represents a controlled and protective stress response. In contrast, the mixed or downregulated patterns in ex ovo embryos may indicate a maladaptive response, potentially reflecting cellular overload or an inability to cope with environmental stressors. However, preincubation at 30°C was associated with improved survival rates, suggesting a potential approach to optimize ex ovo incubation. Despite the valuable insights provided by this study, several limitations must be acknowledged. These include small and uneven sample sizes, the lack of sex differentiation, and the absence of post-hatch follow-up. The study also focused on gene expression without protein-level validation, which limits the interpretation of the findings. While these insights offer valuable guidance for enhancing shell-less incubation techniques, further research is needed to confirm these effects and investigate the underlying mechanisms. The potential applications of these findings could benefit both developmental biology and biomedical research, contributing to a more efficient and reliable use of ex ovo incubation in avian models.
As this was a pilot study, the analysis of additional oxidative stress markers such as catalase and glutathione peroxidase was not performed, partly due to the limited availability of validated primers for quail embryos. The initial requirement for these genes is primer design and optimization. However, they represent promising targets for future investigation, to further validate oxidative stress responses observed under different incubation conditions.
Funding
This research was funded by the EU NextGenerationEU through the Recovery and Resilience Plan for Slovakia under the project No. 09I03-03-V05-00017 (IGA-ESGD/04/2024), VEGA 1/0373/24 and APVV-20-0073.
Ethical approval
This research study used avian embryos as experimental ex ovo and in ovo models, which do not fall under the legislation for protecting animals used for scientific purposes (2010/63/EU). No approval of research ethics committees was required to accomplish the goals of this study.
Informed consent
N/A.
Data availability statement
The data is publicly available.
Ethical statement
This research study used avian embryos as experimental ex ovo and in ovo models, which do not fall under the legislation for protecting animals used for scientific purposes (2010/63/EU). No approval of research ethics committees was required to accomplish the goals of this study.
CRediT authorship contribution statement
Bronislava Pokorna: Writing – original draft, Resources, Methodology, Investigation, Funding acquisition, Formal analysis, Conceptualization. Slavomira Stefancova: Methodology, Data curation. Veronika Tauberova: Writing – original draft, Visualization, Resources, Methodology, Investigation. Eva Petrovova: Writing – review & editing, Validation, Supervision, Project administration, Funding acquisition, Conceptualization. Lenka Luptakova: Writing – review & editing, Validation, Supervision, Project administration, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.vas.2025.100486.
Appendix. Supplementary materials
References
- Al-Zghoul M.-B., Ismail Z.B., Abd Elhafeed S.D., Al-Ramadan A., Althnaian T.A., Al-Ramadan S.Y., Ali A.M., Albokhadaim I.F., Al Busadah K.A., Eljarah A. Hsp90, Hsp60 and HSF-1 genes expression in muscle, heart and brain of thermally manipulated broiler chicken. Research in Veterinary Science. 2015;99:105–111. doi: 10.1016/j.rvsc.2014.12.014. [DOI] [PubMed] [Google Scholar]
- Andacht T., Hu W., Ivarie R. Rapid and improved method for windowing eggs accessing the stage X chicken embryo. Molecular Reproduction and Development. 2004;69(1):31–34. doi: 10.1002/mrd.20155. [DOI] [PubMed] [Google Scholar]
- Azad M., Kikusato M., Maekawa T., Shirakawa H., Toyomizu M. Metabolic characteristics and oxidative damage to skeletal muscle in broiler chickens exposed to chronic heat stress. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2010;155(3):401–406. doi: 10.1016/j.cbpa.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Baer J., Lansford R., Cheng K. Laboratory animal medicine. Elsevier; 2015. Japanese quail as a laboratory animal model; pp. 1087–1108. [Google Scholar]
- Bastos M.S., Del Vesco A.P., Santana T.P., Santos T.S., de Oliveira Junior G.M., Fernandes R.P.M., Barbosa L.T., Gasparino E. The role of cinnamon as a modulator of the expression of genes related to antioxidant activity and lipid metabolism of laying quails. Plos One. 2017;12(12) doi: 10.1371/journal.pone.0189619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaziak K. Preincubation and preheating–two different methods but with one purpose for use in hatchery. Can their interaction be twice as effective? World’s Poultry Science Journal. 2021;77(4):969–981. doi: 10.1080/00439339.2021.1960237. [DOI] [Google Scholar]
- Davies J. John Wiley & Sons; 2012. Replacing animal models: A practical guide to creating and using culture-based biomimetic alternatives. [Google Scholar]
- Dohle D.S., Pasa S.D., Gustmann S., Laub M., Wissler J.H., Jennissen H.P., Dünker N. Chick ex ovo culture and ex ovo CAM assay: How it really works. Journal of Visualized Experiments: JoVE. 2009;(33):1620. doi: 10.3791/1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doke S.K., Dhawale S.C. Alternatives to animal testing: A review. Saudi Pharmaceutical Journal. 2015;23(3):223–229. doi: 10.1016/j.jsps.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson A.C., Crim M.J., Franklin C.L. A brief history of animal modeling. Missouri Medicine. 2013;110(3):201. [PMC free article] [PubMed] [Google Scholar]
- Farzaneh M., Attari F., Khoshnam S., Mozdziak P. The method of chicken whole embryo culture using the eggshell windowing, surrogate eggshell and ex ovo culture system. British Poultry Science. 2018;59(2):240–244. doi: 10.1080/00071668.2017.1413234. [DOI] [PubMed] [Google Scholar]
- Feodorova Y.N., Sarafian V.S. Psychological stress–cellular and molecular mechanisms. Folia Medica (Plovdiv) 2012;54(3):5–13. doi: 10.2478/v10153-011-0091-9. [DOI] [PubMed] [Google Scholar]
- Fischer D., Fluegen G., Garcia P., Ghaffari-Tabrizi-Wizsy N., Gribaldo L., Huang R.Y., Rasche V., Ribatti D., Rousset X., Pinto M.T., Viallet J., Wang Y., Schneider-Stock R. The CAM model-Q&A with experts. Cancers (Basel) 2022;15(1) doi: 10.3390/cancers15010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freires I.A., Sardi J.d.C.O., de Castro R.D., Rosalen P.L. Alternative animal and non-animal models for drug discovery and development: Bonus or burden? Pharmaceutical Research. 2017;34:681–686. doi: 10.1007/s11095-016-2069-z. [DOI] [PubMed] [Google Scholar]
- Galletta L., Craven M.J., Meillère A., Crowley T.M., Buchanan K.L., Mariette M.M. Acute exposure to high temperature affects expression of heat shock proteins in altricial avian embryos. Journal of Thermal Biology. 2022;110 doi: 10.1016/j.jtherbio.2022.103347. [DOI] [PubMed] [Google Scholar]
- Goel A., Ncho C.M., Choi Y.-H. Regulation of gene expression in chickens by heat stress. Journal of Animal Science and Biotechnology. 2021;12:1–13. doi: 10.1186/s40104-020-00523-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene E.S., Rajaei-Sharifabadi H., Dridi S. Feather HSP70: A novel non-invasive molecular marker for monitoring stress induced by heat exposure in broilers. Poultry Science. 2019;98(9):3400–3404. doi: 10.3382/ps/pez120. [DOI] [PubMed] [Google Scholar]
- Habashy W.S., Milfort M.C., Rekaya R., Aggrey S.E. Cellular antioxidant enzyme activity and biomarkers for oxidative stress are affected by heat stress. International Journal of Biometeorology. 2019;63:1569–1584. doi: 10.1007/s00484-019-01769-z. [DOI] [PubMed] [Google Scholar]
- Hanafy A., Hegab I. Effects of egg weight and light sources during incubation period on embryonic development and post-hatch growth of Japanese quail (Coturnix japonica) European Poultry Science/Archiv für Geflügelkunde. 2019;83(268) doi: 10.1399/eps.2019.268. [DOI] [Google Scholar]
- Hu C., Yang J., Qi Z., Wu H., Wang B., Zou F., Mei H., Liu J., Wang W., Liu Q. Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities. Medical Communications. 2022;3(3):e161. doi: 10.1002/mco2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huss D., Poynter G., Lansford R. Japanese quail (Coturnix japonica) as a laboratory animal model. Lab Animal. 2008;37(11):513–519. doi: 10.1038/laban1108-513. [DOI] [PubMed] [Google Scholar]
- Chen L., Wang S., Feng Y., Zhang J., Du Y., Zhang J., Van Ongeval C., Ni Y., Li Y. Utilisation of chick embryo chorioallantoic membrane as a model platform for imaging-navigated biomedical research. Cells (Basel) 2021;10(2):463. doi: 10.3390/cells10020463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janikovičová L., Demčišáková Z., Luptáková L., Petrovová E. Pre-Incubation and its effect on the development and malformations of the chick embryo. Folia Veterinaria. 2019;63:24–31. doi: 10.2478/fv-2019-0004. [DOI] [Google Scholar]
- Kaur P., Asea A.A. Springer; 2019. The Chaperokine activity of heat shock proteins. [Google Scholar]
- Kundeková B., Máčajová M., Meta M., Čavarga I., Bilčík B. Chorioallantoic membrane models of various avian species: Differences and applications. Biology (Basel) 2021;10(4):301. doi: 10.3390/biology10040301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.Y., Kang J.H., Jeong J.W., Kim J.H., Kim H.W., Oh D.H., Kim J.-M., Rhim S.-J., Kim G.-D., Kim H.S. Alternative experimental approaches to reduce animal use in biomedical studies. Journal of Drug Delivery Science and Technology. 2022;68 doi: 10.1016/j.jddst.2022.103131. [DOI] [Google Scholar]
- Lenkavska L., Blascakova L., Jurasekova Z., Macajova M., Bilcik B., Cavarga I., Miskovsky P., Huntosova V. Benefits of hypericin transport and delivery by low-and high-density lipoproteins to cancer cells: From in vitro to ex ovo. Photodiagnosis and Photodynamic Therapy. 2019;25:214–224. doi: 10.1016/j.pdpdt.2018.12.013. [DOI] [PubMed] [Google Scholar]
- Lin Y.M., Druyan S., Yahav S., Brake J. Thermal treatments prior to and during the beginning of incubation affects development of the broiler embryo and yolk sac membranes, and live performance and carcass characteristics. Poultry Science. 2017;96(6):1939–1947. doi: 10.3382/ps/pew467. [DOI] [PubMed] [Google Scholar]
- Liu C., Chaudhry M.T., Zhao D., Lin T., Tian Y., Fu J. Heat shock protein 70 protects the quail cecum against oxidant stress, inflammatory injury, and microbiota imbalance induced by cold stress. Poultry Science. 2019;98(11):5432–5445. doi: 10.3382/ps/pez327. [DOI] [PubMed] [Google Scholar]
- Marks H. Relationship of embryonic development to egg weight, hatch weight, and growth in Japanese quail. Poultry Science. 1975;54(4):1257–1262. doi: 10.3382/ps.0541257. [DOI] [PubMed] [Google Scholar]
- Mehaisen G.M., Ibrahim R.M., Desoky A.A., Safaa H.M., El-Sayed O.A., Abass A.O. The importance of propolis in alleviating the negative physiological effects of heat stress in quail chicks. Plos One. 2017;12(10) doi: 10.1371/journal.pone.0186907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlos Rodrigo M.A., Casar B., Michalkova H., Jimenez Jimenez A.M., Heger Z., Adam V. Extending the applicability of in ovo and ex ovo chicken chorioallantoic membrane assays to study cytostatic activity in neuroblastoma cells. Frontiers in Oncology. 2021;11 doi: 10.3389/fonc.2021.707366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming X., Xian-yi Y., Jin-long L., Yan-hui H., Shu L., Shi-wen X. Effects of acute and chronic cold stress on antioxidant function in intestinal tracts of chickens. Journal of Northeast Agriculture University (English Edition) 2012;19(2):54–61. doi: 10.1016/S1006-8104(13)60038-0. [DOI] [Google Scholar]
- Murshid A., Prince T.L., Lang B., Calderwood S.K. Chaperones: Methods and protocols. 2018. Role of heat shock factors in stress-induced transcription; pp. 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugesan S., Ullengala R., Amirthalingam V. Heat shock proteins in veterinary medicine and sciences. 2017. Heat shock protein and thermal stress in chicken; pp. 179–193. [DOI] [Google Scholar]
- Naik M., Brahma P., Dixit M. A cost-effective and efficient chick ex-ovo CAM assay protocol to assess angiogenesis. Methods and Protocols. 2018;1(2):19. doi: 10.3390/mps1020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Nakane Y., Tsudzuki M. Developmental stages of the blue-breasted quail (Coturnix chinensis) Animal Science Journal. 2019;90(1):35–48. doi: 10.1111/asj.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak-Sliwinska P., Segura T., Iruela-Arispe M.L. The chicken chorioallantoic membrane model in biology, medicine and bioengineering. Angiogenesis. 2014;17:779–804. doi: 10.1007/s10456-014-9440-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piestun Y., Druyan S., Brake J., Yahav S. Thermal treatments prior to and during the beginning of incubation affect phenotypic characteristics of broiler chickens posthatching. Poultry Science. 2013;92(4):882–889. doi: 10.3382/ps.2012-02568. [DOI] [PubMed] [Google Scholar]
- Prodromou C. Mechanisms of Hsp90 regulation. Biochemistry Journal. 2016;473(16):2439–2452. doi: 10.1042/bcj20160005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen S.V., Berlow N.E., Price L.H., Mansoor A., Cairo S., Rugonyi S., Keller C. Preclinical therapeutics ex ovo quail eggs as a biomimetic automation-ready xenograft platform. Scientific Reports. 2021;11(1) doi: 10.1038/s41598-021-02509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J., Liu C., Zhao D., Fu J. The role of heat shock protein 70 in oxidant stress and inflammatory injury in quail spleen induced by cold stress. Environmental Science and Pollution Research. 2018;25:21011–21023. doi: 10.1007/s11356-018-2142-8. [DOI] [PubMed] [Google Scholar]
- Richter S.H. Challenging current scientific practice: How a shift in research methodology could reduce animal use. Lab Animal. 2024;53(1):9–12. doi: 10.1038/s41684-023-01308-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romao J., Moraes T.d., Teixeira R.d.C., Buxade C., Cardoso W. Incubation of Japanese quail eggs at different temperatures: Hatchability, hatch weight, hatch time and embryonic mortality. Archives of Veterinary Science. 2009;14(3):155–162. doi: 10.5380/avs.v14i3.14887. [DOI] [Google Scholar]
- Santana T.P., Gasparino E., de Souza Khatlab A., Brito C.O., Barbosa L.T., Lamont S.J., Del Vesco A.P. Effect of prenatal ambient temperature on the performance physiological parameters, and oxidative metabolism of Japanese quail (Coturnix coturnix japonica) layers exposed to heat stress during growth. Scientific Reports. 2021;11(1):9809. doi: 10.1038/s41598-021-89306-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomann T., Qunneis F., Widera D., Kaltschmidt C., Kaltschmidt B. Improved method for ex ovo-cultivation of developing chicken embryos for human stem cell xenografts. Stem Cells International. 2013;2013(1) doi: 10.1155/2013/960958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukparangsi W., Thongphakdee A., Intarapat S. Avian embryonic culture: A perspective of in ovo to ex ovo and in vitro studies. Frontiers in Physiology. 2022;13 doi: 10.3389/fphys.2022.903491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung J.H., Wang Y.I., Narasimhan Sriram N., Jackson M., Long C., Hickman J.J., Shuler M.L. Recent advances in body-on-a-chip systems. Analytical Chemistry. 2018;91(1):330–351. doi: 10.1021/acs.analchem.8b05293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara Y., Obara K. Ex ovo culture system for avian embryos and its application. Journal of Poultry Science. 2021;58(1):1–4. doi: 10.2141/jpsa.0200016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesarova M.P., Skoupa M., Foltyn M., Tvrdon Z., Lichovnikova M. Research note: Effects of preincubation and higher initiating incubation temperature of long-term stored hatching eggs on hatchability and day-old chick and yolk sac weight. Poultry Science. 2021;100(8) doi: 10.1016/j.psj.2021.101293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong L., King A.J. Lipid oxidation and antioxidant capacity in multigenerational heat stressed Japanese quail (Coturnix coturnix japonica) Poultry Science. 2023;102(11) doi: 10.1016/j.psj.2023.103005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.X., White M.D. Mechanical forces in avian embryo development. Seminars in Cell & Developmental Biology. 2021;120:133–146. doi: 10.1016/j.semcdb.2021.06.001. [DOI] [PubMed] [Google Scholar]
- Yigit A., Panda A., Cherian G. The avian embryo and its antioxidant defence system. World’s Poultry Science Journal. 2014;70(3):563–574. doi: 10.1017/S0043933914000610. [DOI] [Google Scholar]
- Zhang J., Bai K., Su W., Wang A., Zhang L., Huang K., Wang T. Curcumin attenuates heat-stress-induced oxidant damage by simultaneous activation of GSH-related antioxidant enzymes and Nrf2-mediated phase II detoxifying enzyme systems in broiler chickens. Poultry Science. 2018;97(4):1209–1219. doi: 10.3382/ps/pex408. [DOI] [PubMed] [Google Scholar]
- Zhao F.Q., Zhang Z.W., Qu J.P., Yao H.D., Li M., Li S., Xu S.W. Cold stress induces antioxidants and HSPs in chicken immune organs. Cell Stress and Chaperones. 2014;19(5):635–648. doi: 10.1007/s12192-013-0489-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M., Liu Y., Zhang G., Yang Z., Xu W., Chen Q. The applications and mechanisms of superoxide dismutase in medicine, food, and cosmetics. Antioxidants (Basel) 2023;12(9):1675. doi: 10.3390/antiox12091675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data is publicly available.